Role of the Aryl Hydrocarbon Receptor in Colon Neoplasia
Abstract
:1. Introduction
1.1. Colorectal Cancer is a Major Health Concern
1.2. The Aryl Hydrocarbon Receptor
1.3. AhR Ligands
2. Role of AhR in Colon Tumorigenesis
2.1. AhR Is a Tumor Suppressor in Mouse Models of CRC
2.2. AhR Expression in Colon Tumors
2.3. AhR Target Genes and Their Roles in CRC
2.4. AhR Cross-Talks with Multiple Signaling Pathways
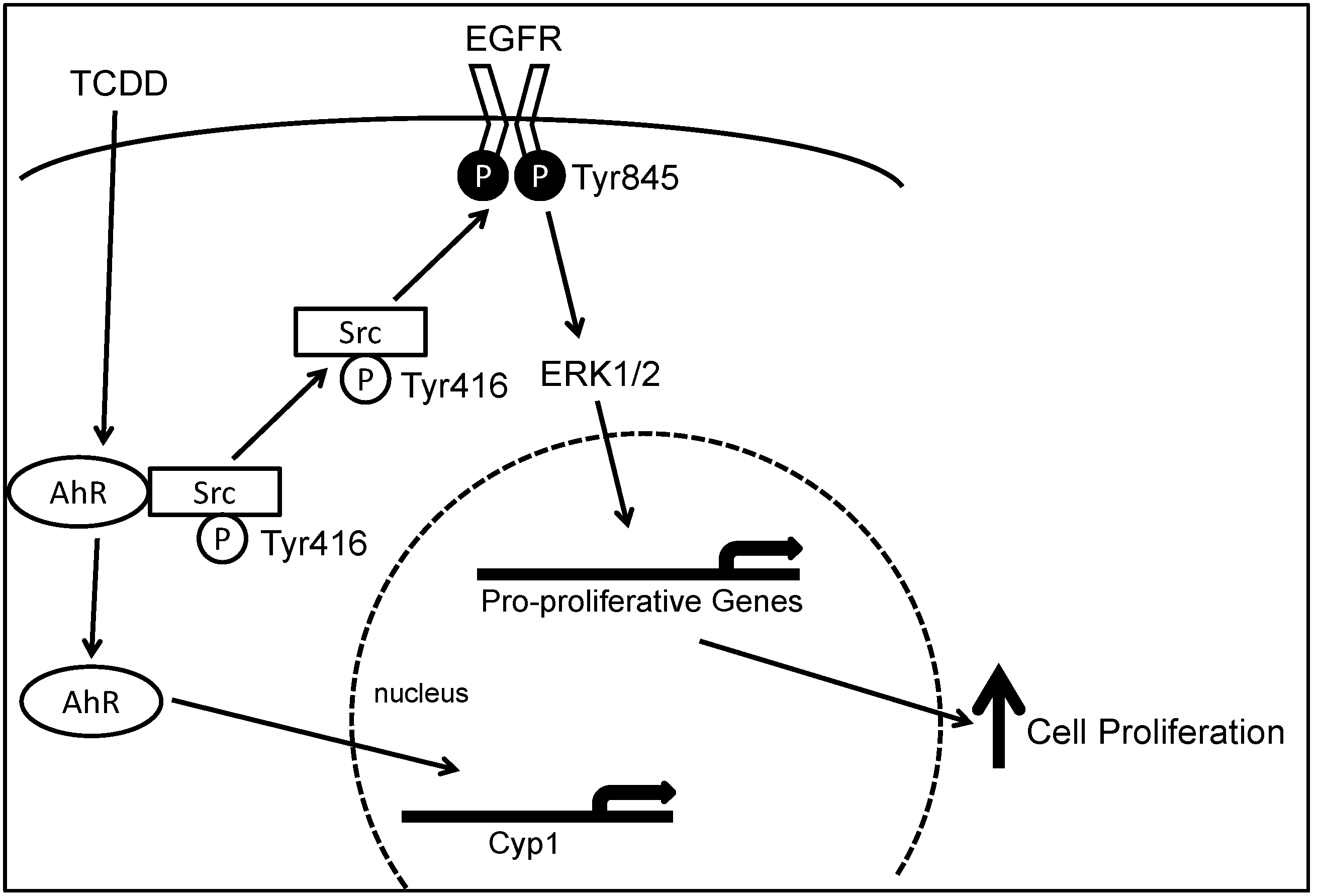
2.5. Role of AhR in Chronic Intestinal Inflammation
2.6. Role of AhR in Inflammation-Associated Colon Neoplasia
2.7. Role of AhR in Circadian Clock Circuitry and CRC
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the united states: 2012 update. Gastroenterology 2012, 143. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Hewett, D.G.; Rex, D.K. The big picture: Does colonoscopy work? Gastrointest. Endosc. Clin. N. Am. 2015, 25, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Pignone, M.; Saha, S.; Hoerger, T.; Mandelblatt, J. Cost-effectiveness analyses of colorectal cancer screening: A systematic review for the U.S. Preventive services task force. Ann. Intern. Med. 2002, 137, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A. The future of colon cancer screening: What do we recommend and will it be too much, too little, or just right? Gastroenterology 2011, 141, 1956–1958. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Sandler, R.S.; Bresalier, R.S.; Quan, H.; Riddell, R.; Lanas, A.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Loftus, S.; et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology 2006, 131, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Solomon, S.D.; Kim, K.; Tang, J.; Rosenstein, R.B.; Wittes, J.; Corle, D.; et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 2006, 355, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Helin-Salmivaara, A.; Saarelainen, S.; Gronroos, J.M.; Vesalainen, R.; Klaukka, T.; Huupponen, R. Risk of upper gastrointestinal events with the use of various nsaids: A case-control study in a general population. Scand. J. Gastroenterol. 2007, 42, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.J.; Dunn, J.A.; Langman, M.J.; Smith, J.L.; Midgley, R.S.; Stanley, A.; Stokes, J.C.; Julier, P.; Iveson, C.; Duvvuri, R.; et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 360–369. [Google Scholar] [CrossRef] [PubMed]
- McGettigan, P.; Henry, D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006, 296, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, H.; Essapen, S. Epidermal growth factor receptor inhibitors in cancer treatment: Advances, challenges and opportunities. Anticancer Drugs 2009, 20, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Hoff, P.M. Egfr-targeted therapies in colorectal cancer. Dis. Colon Rectum 2007, 50, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P. Epidermal growth factor receptor-targeted treatment for advanced colorectal carcinoma. Cancer 2005, 103, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Sipples, R. Common side effects of anti-egfr therapy: Acneform rash. Semin. Oncol. Nurs. 2006, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, F.; Fujii-Kuriyama, Y.; Kato, S. AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions. Biochem. Pharmacol. 2009, 77, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Yamamoto, H.; Itoh, F.; Hinoda, Y.; Okada, Y.; Imai, K. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut 1999, 45, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Carver, L.A.; Bradfield, C.A. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J. Biol. Chem. 1997, 272, 11452–11456. [Google Scholar] [PubMed]
- Chen, H.S.; Perdew, G.H. Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. J. Biol. Chem. 1994, 269, 27554–27558. [Google Scholar] [PubMed]
- Ma, Q.; Whitlock, J.P., Jr. A novel cytoplasmic protein that interacts with the ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Biol. Chem. 1997, 272, 8878–8884. [Google Scholar] [PubMed]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Bradfield, C.A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008, 21, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Lee, S.O.; Jin, U.H. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol. Sci. 2013, 135, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W.; Kohle, C. Ah receptor- and TCDD-mediated liver tumor promotion: Clonal selection and expansion of cells evading growth arrest and apoptosis. Biochem. Pharmacol. 2005, 69, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Knerr, S.; Schrenk, D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 2006, 50, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Thun, M.J.; Connell, C.J.; McCullough, M.L.; Jacobs, E.J.; Flanders, W.D.; Rodriguez, C.; Sinha, R.; Calle, E.E. Meat consumption and risk of colorectal cancer. JAMA 2005, 293, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, H.; Johnsen, N.F.; Christensen, J.; Frederiksen, K.; Overvad, K.; Tjonneland, A. Association of adherence to lifestyle recommendations and risk of colorectal cancer: A prospective danish cohort study. BMJ 2010, 341. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Bjeldanes, L.F.; Kim, J.Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar] [CrossRef] [PubMed]
- Bonnesen, C.; Eggleston, I.M.; Hayes, J.D. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001, 61, 6120–6130. [Google Scholar] [PubMed]
- Kim, Y.S.; Milner, J.A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.D.; Steinmetz, K. Vegetables, fruit and phytoestrogens as preventive agents. IARC Sci. Publ. 1996, 139, 61–90. [Google Scholar] [PubMed]
- Jin, U.H.; Lee, S.O.; Sridharan, G.; Lee, K.; Davidson, L.A.; Jayaraman, A.; Chapkin, R.S.; Alaniz, R.; Safe, S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014, 85, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; de Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Kyle, J.; Cetnarskyj, R.; Farrington, S.M.; Tenesa, A.; Barnetson, R.; Porteous, M.; Dunlop, M.; Campbell, H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Tsutsumi, T.; Nakamura, M.; Kitagawa, H.; Fujino, J.; Sasaki, K.; Toyoda, M.; Yoshida, T.; Maitani, T. Activation of the aryl hydrocarbon receptor by some vegetable constituents determined using in vitro reporter gene assay. Biol. Pharm. Bull. 2003, 26, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, C.; Safe, S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003, 111, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, S.; Kautenburger, T.; Habermann, N.; Sauer, J.; Dietrich, H.; Will, F.; Pool-Zobel, B.L. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol. Carcinog. 2006, 45, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T.; Kohn, A.D.; de Ferrari, G.V.; Kaykas, A. Wnt and beta-catenin signalling: Diseases and therapies. Nat. Rev. Genet. 2004, 5, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Kobayashi, Y.; Ohtake, F.; Ikuta, T.; Matsushima, Y.; Mimura, J.; Pettersson, S.; Pollenz, R.S.; Sakaki, T.; Hirokawa, T.; et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in apcmin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 13481–13486. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Boivin, G.P.; Knudsen, E.S.; Nebert, D.W.; Xia, Y.; Puga, A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010, 70, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Dolwick, K.M.; Schmidt, J.V.; Carver, L.A.; Swanson, H.I.; Bradfield, C.A. Cloning and expression of a human ah receptor cdna. Mol. Pharmacol. 1993, 44, 911–917. [Google Scholar] [PubMed]
- Hayashi, S.; Watanabe, J.; Nakachi, K.; Eguchi, H.; Gotoh, O.; Kawajiri, K. Interindividual difference in expression of human ah receptor and related p450 genes. Carcinogenesis 1994, 15, 801–806. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.F.; Kopparapu, P.R.; Koch, D.C.; Jang, H.S.; Phillips, J.L.; Tanguay, R.L.; Kerkvliet, N.I.; Kolluri, S.K. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS ONE 2012, 7, e40926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome p450 cyp1a1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Spyrou, I.; Ploumidis, A.; Papalampros, A.E.; Kyriakakis, M.; Delakas, D.; Spandidos, D.A.; Tsatsakis, A.M. Expression profile of cyp1a1 and cyp1b1 enzymes in colon and bladder tumors. PLoS ONE 2013, 8, e82487. [Google Scholar] [CrossRef] [PubMed]
- Le Ferrec, E.; Lagadic-Gossmann, D.; Rauch, C.; Bardiau, C.; Maheo, K.; Massiere, F.; le Vee, M.; Guillouzo, A.; Morel, F. Transcriptional induction of cyp1a1 by oltipraz in human caco-2 cells is aryl hydrocarbon receptor- and calcium-dependent. J. Biol. Chem. 2002, 277, 24780–24787. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, L.M.; Li, H.; Li, L.; Lynch, C.; Xie, Y.; Nakanishi, T.; Ross, D.D.; Wang, H. A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem. Pharmacol. 2010, 80, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Villard, P.H.; Caverni, S.; Baanannou, A.; Khalil, A.; Martin, P.G.; Penel, C.; Pineau, T.; Seree, E.; Barra, Y. PPARalpha transcriptionally induces ahr expression in Caco-2, but represses AhR pro-inflammatory effects. Biochem. Biophys. Res. Commun. 2007, 364, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Haarmann-Stemmann, T.; Bothe, H.; Abel, J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem. Pharmacol. 2009, 77, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Ma, C.; Marlowe, J.L. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009, 77, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Duran, A.; Carvajal-Gonzalez, J.M.; Mulero-Navarro, S.; Santiago-Josefat, B.; Puga, A.; Fernandez-Salguero, P.M. Fitting a xenobiotic receptor into cell homeostasis: How the dioxin receptor interacts with tgfbeta signaling. Biochem. Pharmacol. 2009, 77, 700–712. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.K.; Smolenski, A. Phosphodiesterases link the aryl hydrocarbon receptor complex to cyclic nucleotide signaling. Biochem. Pharmacol. 2009, 77, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, J.C.; Gustafsson, J.A. Aryl hydrocarbon receptor-mediated signal transduction. Crit. Rev. Toxicol. 1997, 27, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Peng, Z.; Raufman, J.P. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1006–G1015. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Kaina, B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis 2010, 31, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; MacDonald, T.T.; Pallone, F.; Monteleone, G. The aryl hydrocarbon receptor in inflammatory bowel disease: Linking the environment to disease pathogenesis. Curr. Opin. Gastroenterol. 2012, 28, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate il-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, K.; Nishiumi, S.; Kawano, Y.; Ooi, M.; Yoshie, T.; Shiomi, Y.; Kutsumi, H.; Ashida, H.; Fujii-Kuriyama, Y.; Azuma, T.; et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig. Dis. Sci. 2011, 56, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Xu, C.; Sun, L.; Yu, M.; Peng, K.; Qiu, Y.; Xiao, W.; Yang, H. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig. Dis. Sci. 2015, 60, 1958–1066. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; Kobayashi, Y.; Kitazawa, M.; Shiizaki, K.; Itano, N.; Noda, T.; Pettersson, S.; Poellinger, L.; Fujii-Kuriyama, Y.; Taniguchi, S.; et al. Asc-associated inflammation promotes cecal tumorigenesis in aryl hydrocarbon receptor-deficient mice. Carcinogenesis 2013, 34, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Beischlag, T.V.; Vinciguerra, M.; Mazzoccoli, G. The circadian clock circuitry and the AhR signaling pathway in physiology and pathology. Biochem. Pharmacol. 2013, 85, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Vinciguerra, M.; Papa, G.; Piepoli, A. Circadian clock circuitry in colorectal cancer. World J. Gastroenterol. 2014, 20, 4197–4207. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, G.; Raufman, J.-P. Role of the Aryl Hydrocarbon Receptor in Colon Neoplasia. Cancers 2015, 7, 1436-1446. https://doi.org/10.3390/cancers7030847
Xie G, Raufman J-P. Role of the Aryl Hydrocarbon Receptor in Colon Neoplasia. Cancers. 2015; 7(3):1436-1446. https://doi.org/10.3390/cancers7030847
Chicago/Turabian StyleXie, Guofeng, and Jean-Pierre Raufman. 2015. "Role of the Aryl Hydrocarbon Receptor in Colon Neoplasia" Cancers 7, no. 3: 1436-1446. https://doi.org/10.3390/cancers7030847




