Quality of Life in Patients with NSCLC Receiving Maintenance Therapy
Abstract
:1. Introduction
2. Methods
3. Results
| First Author | Induction n | Maintenance n | % Maintenance | Regimen Used | Maintenance | Survival Difference | HRQoL (PRO) |
|---|---|---|---|---|---|---|---|
| Brodowicz [13] | 352 | 206 | 58.5% | Gemcitabine/cisplatin followed by gemcitabine or BSC | Continuation gemcitabine | TTP p < 0.001 | LCSS trend favours better control of haemoptysis, cough and pain in maintenance arm |
| OS 13.0 vs. 11.0 mon | |||||||
| p = 0.195 | |||||||
| Ciuleanu [4,9,14] | Na | 663 | na | Four cycles platinum based doublet followed by pemetrexed | Switch Pemetrexed | PFS 4.4 vs. 2.6 mon | LCSS published separately significant longer time to worsening pain and haemoptysis |
| HR 0.50 p < 0.0001 | |||||||
| OS 13.4 vs. 10.6 | |||||||
| HR 0.79 p = 0.012 | |||||||
| Cappuzzo [3,15] | 1949 | 889 | 45.6% | Four cycles platinum based doublet followed by erlotinib or placebo | Switch Erlotinib | PFS 12.3 vs. 11.1 weeks | FACT-L time to deterioration no difference in HRQoL |
| HR 0.71 p < 0.0001 | |||||||
| OS 12.0 vs. 11.0 mon | Time to pain improved by maintenance erlotinib but not time to cough or dyspnoea | ||||||
| HR 0.81 p = 0.009 | |||||||
| Paz-Ares [6,16,17,18] | 939 | 539 | 57.4% | Pemetrexed/cisplatin followed by pemetrexed or placebo | Continuation Pemetrexed | PFS 4.4 vs. 2.8 mon | EQ5-D published separately |
| HR 0.60 p < 0.01 | |||||||
| OS 13.9 vs. 11.0 | No significant differences, but Qol improvement by induction-cycles | ||||||
| HR 0.78 p = 0.02 | |||||||
| Westeel [19] | 573 | 227 | 39.6% | Four cycles of MIC followed by vinorelbine maintenance or observation | Switch Vinorelbine | PFS 5.0 vs. 3.0 | Not published |
| HR 0.77 p = 0.11 | |||||||
| OS 12.3 vs. 12.3 | |||||||
| HR 1.08 p = 0.65 | |||||||
| Sculier [20] | 485 | 281 | 59.6% | Three cycles of GIP gemcitabine/ifosfamid/cisplatin followed by paclitaxel or GIP | Switch | PFS 4.0 vs. 4.4 | Not published |
| Paclitaxel | p = 0.56 | ||||||
| Continuation | OS 9.7 (Pacli) vs. 11.9 (GIP) | ||||||
| Gemcitabine | HR 0.81 p = 0.10 | ||||||
| Barlesi [5,21,22] | 376 | 253 | 67.3% | Pemetrexed/cisplatin/bevacizumab followed by pemetrexed/bevacizumab or bevacizumab alone | Continuation | PFS 7.4 vs. 3.7 mon | EORTC QLQ-C30 and QLQ-LC13 Published separately no significant differences but trend to better pain and dyspnoea control in combination arm |
| HR 0.48, p < 0.001 | |||||||
| Pemetrexed and bevacizumab | OS 17.1 vs. 13.2 | ||||||
| HR 0.87 p = 0.29 | |||||||
| Perol [23] | 834 | 464 | 55.6% | Gemcitabine/cisplatin followed by gemcitabine or erlotinib maintenance compared to BSC | Continuation | Gem PFS 3.8 vs. 1.9 mon | Not published |
| Gemcitabine | HR 0.56 p < 0.01 | ||||||
| Switch | OS 12.1 vs. 10.8 | ||||||
| Erlotinib | HR 0.89 p = 0.39 | ||||||
| Zhang [24,25] | Na | 296 | Na | Four cycles platinum based doublet followed by gefitinib or placebo | Switch | PFS 4.8 vs. 2.6 | FACT-L compliance rate 47% (gefitinib) and 33% (placebo) only 10% known EGFR-Mutation status |
| HR 0.42 p < 0.0001 | |||||||
| Gefitinib | OS 18.7 vs. 16.9 | ||||||
| HR 0.84 p = 0.26 | |||||||
| Patel [26,27] | Na | 939 | Na | Pemetrexed/carboplatin/bevacizumab followed by pemetrexed/bevacizumab vs. paclitaxel/carboplatin/ bevacizumab followed by bevacizumab alone | Continuation | PFS 6.0 vs. 5–6 mon | FACT-L without differences between both arms |
| Bevacizumab alone | HR 0.83, p = 0.012 | ||||||
| Continuation | OS 12.6 vs. 13.4 mon | FACT-Ntx favouring pemetrexed containing arm regarding neurotoxicity | |||||
| Pemetrexed and bevacizumab | HR 1.0 p = 0.95 |
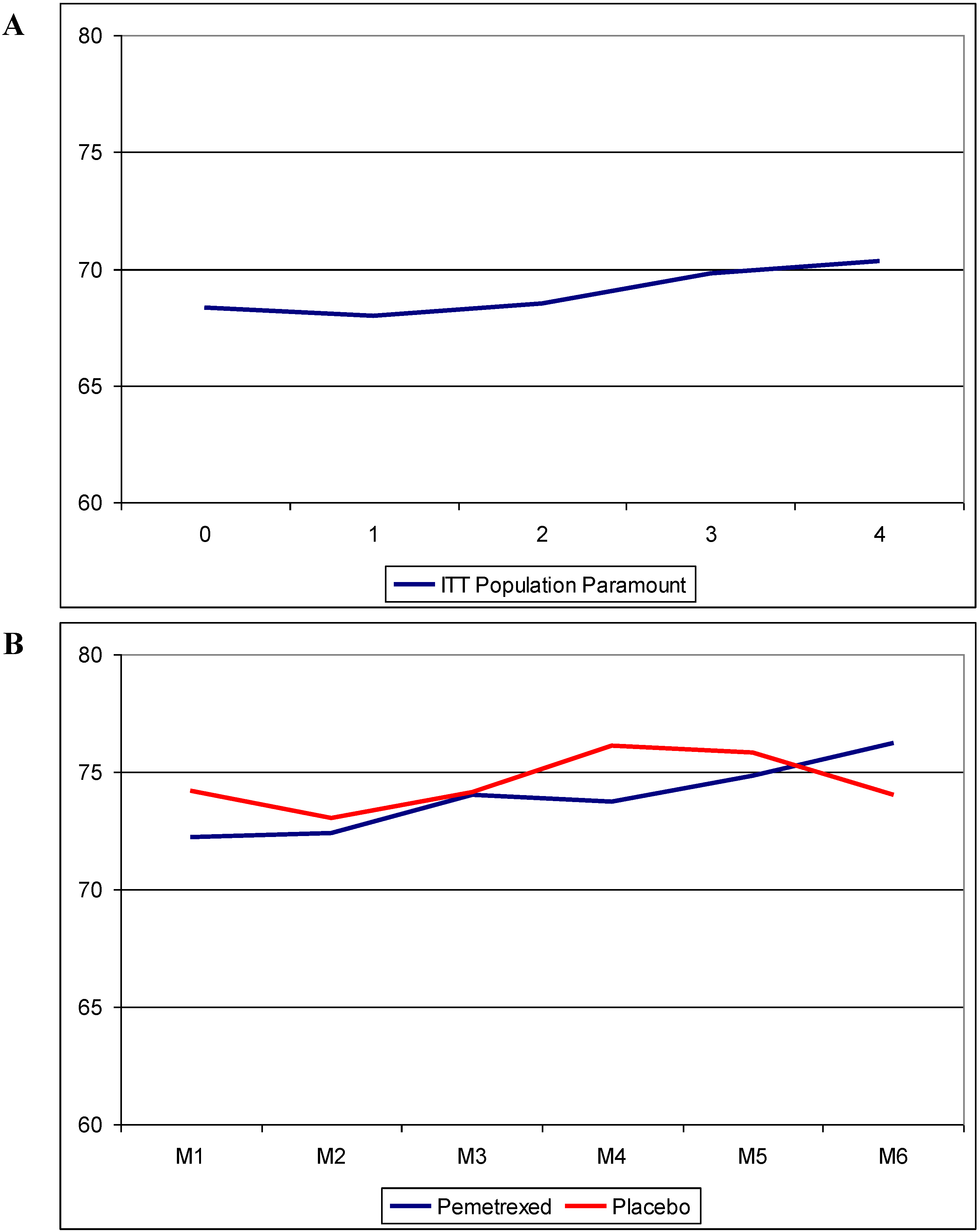


4. Discussion
4.1. Keep it Short and Simple! Different Tools to Assess QoL
4.2. Some Symptoms Can Be Improved and Controlled for a Longer Period of Time by Maintenance Therapy
4.3. Not All Symptoms Are Equal
4.4. PS and Symptoms Can Be Used to Select Patients Who Benefit from Maintenance Therapy
4.5. QoL Should Be Assessed Much Longer
5. Conclusions
Conflicts of Interest
References
- Reck, M.; Heigener, D.F.; Mok, T.; Soria, J.C.; Rabe, K.F. Management of non-small-cell lung cancer: Recent developments. Lancet 2013, 382, 709–719. [Google Scholar] [CrossRef]
- Lee, J.E.; Chung, C.U. Update on the evidence regarding maintenance therapy. Tuberc. Respir. Dis. 2014, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzo, F.; Ciuleanu, T.; Stelmakh, L.; Cicenas, S.; Szczésna, A.; Juhász, E.; Esteban, E.; Molinier, O.; Brugger, W.; Melezínek, I.; et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010, 11, 521–529. [Google Scholar] [CrossRef]
- Ciuleanu, T.; Brodowicz, T.; Zielinski, C.; Kim, J.H.; Krzakowski, M.; Laack, E.; Wu, Y.L.; Bover, I.; Begbie, S.; Tzekova, V.; et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet 2009, 374, 1432–1440. [Google Scholar] [CrossRef]
- Barlesi, F.; Scherpereel, A.; Rittmeyer, A.; Pazzola, A.; Ferrer, T.N.; Kim, J.H.; Ahn, M.J.; Aerts, J.G.; Gorbunova, V.; Vikström, A.; et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J. Clin. Oncol. 2013, 31, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; de Marinis, F.; Dediu, M.; Thomas, M.; Pujol, J.L.; Bidoli, P.; Molinier, O.; Sahoo, T.P.; Laack, E.; Reck, M.; et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Chevalier, T.L.; Soria, J.C. Maintenance therapy and advanced non-small-cell lung cancer: A skeptic’s view. J. Thorac. Oncol. 2012, 7, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, T.E.; Cella, D. Does maintenance pemetrexed maintain quality of life? Lancet Oncol. 2012, 13, 224–225. [Google Scholar] [CrossRef]
- Obasaju, C.; Bowman, L.; Wang, P.; Shen, W.; Winfree, K.B.; Smyth, E.N.; Boye, M.E.; John, W.; Brodowicz, T.; Belani, C.P.; et al. Identifying the target NSCLC patient for maintenance therapy: An analysis from a placebo-controlled, phase III trial of maintenance pemetrexed (H3E-MC-JMEN). Ann. Oncol. 2013, 24, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Maio, D.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardiello, F.; de Placido, S.; et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 2015. [Google Scholar] [CrossRef]
- Reck, M.; von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Brodowicz, T.; Krzakowski, M.; Zwitter, M.; Tzekova, V.; Ramlau, R.; Ghilezan, N.; Ciuleanu, T.; Cucevic, B.; Gyurkovits, K.; Ulsperger, E.; et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: A phase III trial. Lung Cancer 2006, 52, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Brodowicz, T.; Ciuleanu, T.E.; Krzakowski, M.; Yang, S.H.; Franke, F.; Cucevic, B.; Madhavan, J.; Santoro, A.; Ramlau, R.; et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): Results from a randomised, double-blind, phase 3 study. Lancet Oncol. 2012, 13, 292–299. [Google Scholar] [CrossRef]
- Juhasz, E.; Kim, J.H.; Klingelschmitt, G.; Walze, R.S. Effects of erlotinib first-line maintenance therapy versus placebo on the health-related quality of life of patients with metastatic non-small-cell lung cancer. Eur. J. Cancer 2013, 49, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Maione, P.; Rossi, A. The PARAMOUNT trial: A phase III randomized study of maintenance pemetrexed versus placebo immediately following induction first-line treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. Rev. Recent Clin. Trials 2013, 8, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; de Marinis, F.; Dediu, M.; Thomas, M.; Pujol, J.L.; Bidoli, P.; Molinier, O.; Sahoo, T.P.; Laack, E.; Reck, M.; et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012, 13, 247–255. [Google Scholar] [PubMed]
- Reck, M.; Paz-Ares, L.G.; de Marinis, F.; Molinier, O.; Sahoo, T.P.; Laack, E.; John, W.; Zimmermann, A.H.; Visseren-Grul, C.; Gridelli, C. PARAMOUNT: Descriptive subgroup analyses of final overall survival for the phase III study of maintenance pemetrexed versus placebo following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Westeel, V.; Quoix, E.; Moro-Sibilot, D.; Mercier, M.; Breton, J.L.; Debieuvre, D.; Richard, P.; Haller, M.A.; Milleron, B.; Herman, D.; et al. Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 2005, 97, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sculier, J.P.; Lafitte, J.J.; Lecomte, J.; Berghmans, T.; Thiriaux, J.; Florin, M.C.; Efremidis, A.; Alexopoulos, C.G.; Recloux, P.; Ninane, V.; et al. A three-arm phase III randomised trial comparing combinations of platinum derivatives, ifosfamide and/or gemcitabine in stage IV non-small-cell lung cancer. Ann. Oncol. 2002, 13, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Scherpereel, A.; Gorbunova, V.; Gervais, R.; Vikstr, A.; Chouaid, C.; Chella, A.; Kim, J.H.; Ahn, M.J.; Reck, M.; et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann. Oncol. 2014, 25, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Gorbunova, V.; Vikström, A.; Scherpereel, A.; Kim, J.H.; Ahn, M.J.; Chella, A.; Chouaid, C.; Campbell, A.K.; Barlesi, F. Health-related quality of life in patients with advanced nonsquamous non-small-cell lung cancer receiving bevacizumab or bevacizumab-plus-pemetrexed maintenance therapy in AVAPERL (MO22089). J. Thorac. Oncol. 2013, 8, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Perol, M.; Chouaid, C.; Pérol, D.; Barlési, F.; Gervais, R.; Westeel, V.; Crequit, J.; Léna, H.; Vergnenègre, A.; Zalcman, G.; et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, S.; Song, X.; Han, B.; Cheng, Y.; Huang, C.; Yang, S.; Liu, X.; Liu, Y.; Lu, S.; et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): A multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012, 13, 466–475. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, Y.; Ma, S.; Song, X.; Han, B.; Cheng, Y.; Huang, C.; Yang, S.; Liu, X.; Liu, Y.; et al. Final overall survival results from a phase III, randomised, placebo-controlled, parallel-group study of gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804). J. Thorac. Oncol. 2015, 10, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Socinski, M.A.; Garon, E.B.; Reynolds, C.H.; Spigel, D.R.; Olsen, M.R. PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 4349–4357. [Google Scholar] [PubMed]
- Spigel, D.R.; Patel, J.D.; Reynolds, C.H.; Garon, E.B.; Hermann, R.C.; Govindan, R.; Olsen, M.R.; Winfree, K.B.; Chen, J.; Liu, J.; et al. Quality of Life Analyses from the Randomized, Open-Label, Phase III PointBreak Study of Pemetrexed-Carboplatin-Bevacizumab followed by Maintenance Pemetrexed-Bevacizumab versus Paclitaxel-Carboplatin-Bevacizumab followed by Maintenance Bevacizumab in Patients with Stage IIIB or IV Nonsquamous Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 353–359. [Google Scholar] [PubMed]
- Socinski, M.A.; Schell, M.J.; Peterman, A.; Bakri, K.; Yates, S.; Gitten, R.; Unger, P.; Lee, J.; Lee, J.H.; Tynan, M.; et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. 2002, 20, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; de Marinis, F.; Pujol, J.L.; Reck, M.; Ramlau, R.; Parente, B.; Pieters, T.; Middleton, G.; Corral, J.; Winfree, K.; et al. Safety, resource use, and quality of life in paramount: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Cella, D. The Functional Assessment of Cancer Therapy-Lung and Lung Cancer Subscale assess quality of life and meaningful symptom improvement in lung cancer. Semin. Oncol. 2004, 31, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.; Aaronson, N.K.; Ahmedzai, S.; Kaasa, S.; Sullivan, M. The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur. J. Cancer 1994, 30, 635–642. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 65–76. [Google Scholar] [CrossRef]
- Shaw, J.W.; Johnson, J.A.; Coons, S.J. US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Med. Care 2005, 43, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Maringwa, J.T.; Quinten, C.; King, M.; Ringash, J.; Osoba, D.; Coens, C.; Martinelli, F.; Vercauteren, J.; Cleeland, C.S.; Flechtner, H.; et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer 2011, 19, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Hollen, P.J.; Gralla, R.J.; Kris, M.G.; McCoy, S.; Donaldson, G.W.; Moinpour, C.M. A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptom Scale (LCSS): Does format affect patient ratings of symptoms and quality of life? Qual. Life Res. 2005, 14, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Hollen, P.J.; Gralla, R.J.; Cox, C.; Eberly, S.W.; Kris, M.G. A dilemma in analysis: issues in the serial measurement of quality of life in patients with advanced lung cancer. Lung Cancer 1997, 18, 119–136. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittmeyer, A. Quality of Life in Patients with NSCLC Receiving Maintenance Therapy. Cancers 2015, 7, 950-962. https://doi.org/10.3390/cancers7020817
Rittmeyer A. Quality of Life in Patients with NSCLC Receiving Maintenance Therapy. Cancers. 2015; 7(2):950-962. https://doi.org/10.3390/cancers7020817
Chicago/Turabian StyleRittmeyer, Achim. 2015. "Quality of Life in Patients with NSCLC Receiving Maintenance Therapy" Cancers 7, no. 2: 950-962. https://doi.org/10.3390/cancers7020817





