An Analytical Model of Leakage Neutron Equivalent Dose for Passively-Scattered Proton Radiotherapy and Validation with Measurements
Abstract
:1. Introduction
2. Methods
2.1. Analytical Model

2.2. Monte Carlo Simulated H/D Values for General Purpose Beamline at 100 to 250 MeV
2.3. Measured H/D for Ocular Beamline at 75 MeV
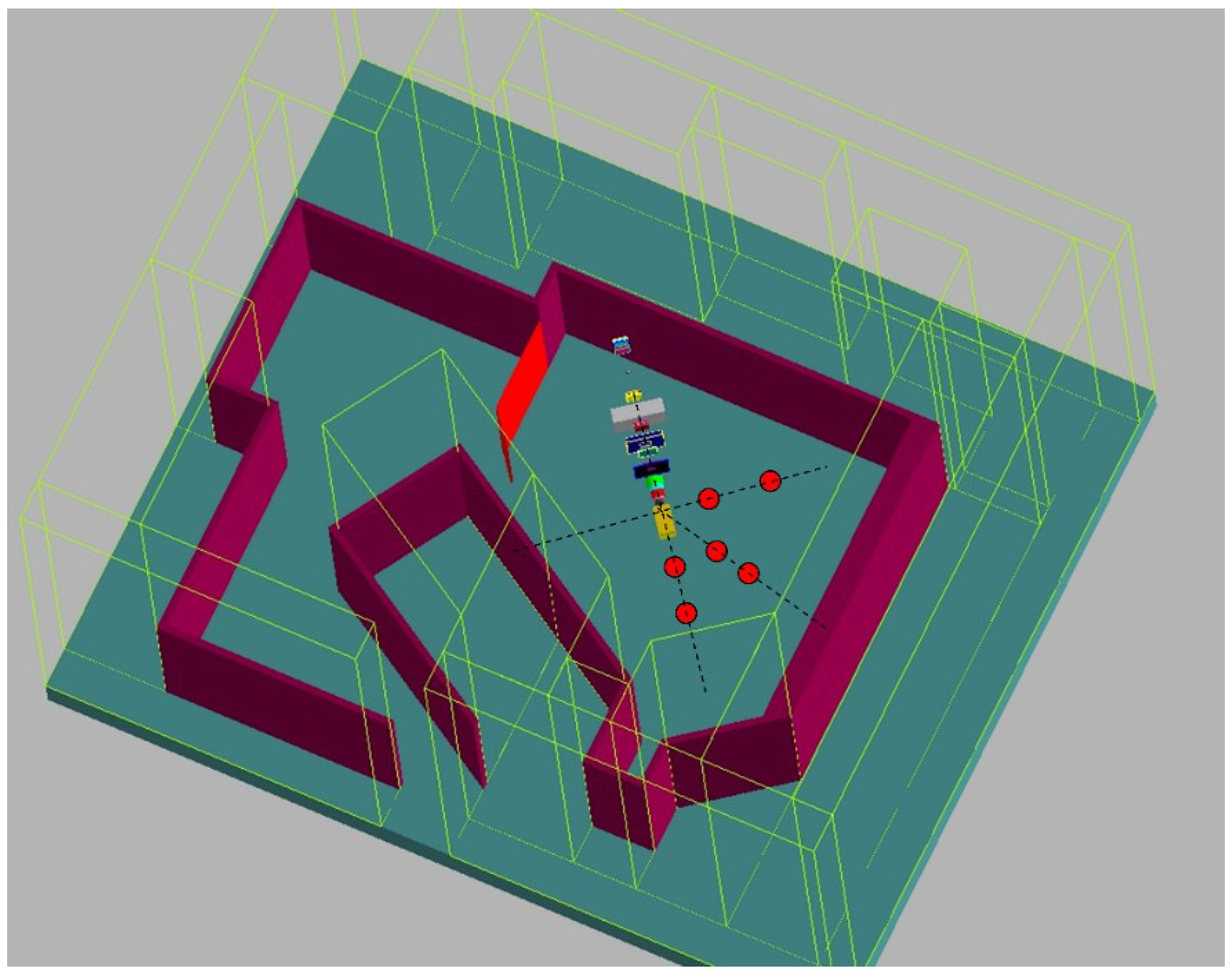
2.4. Model Training
3. Results
3.1. Model Agreement with Monte Carlo Data at 100 to 250 MeV




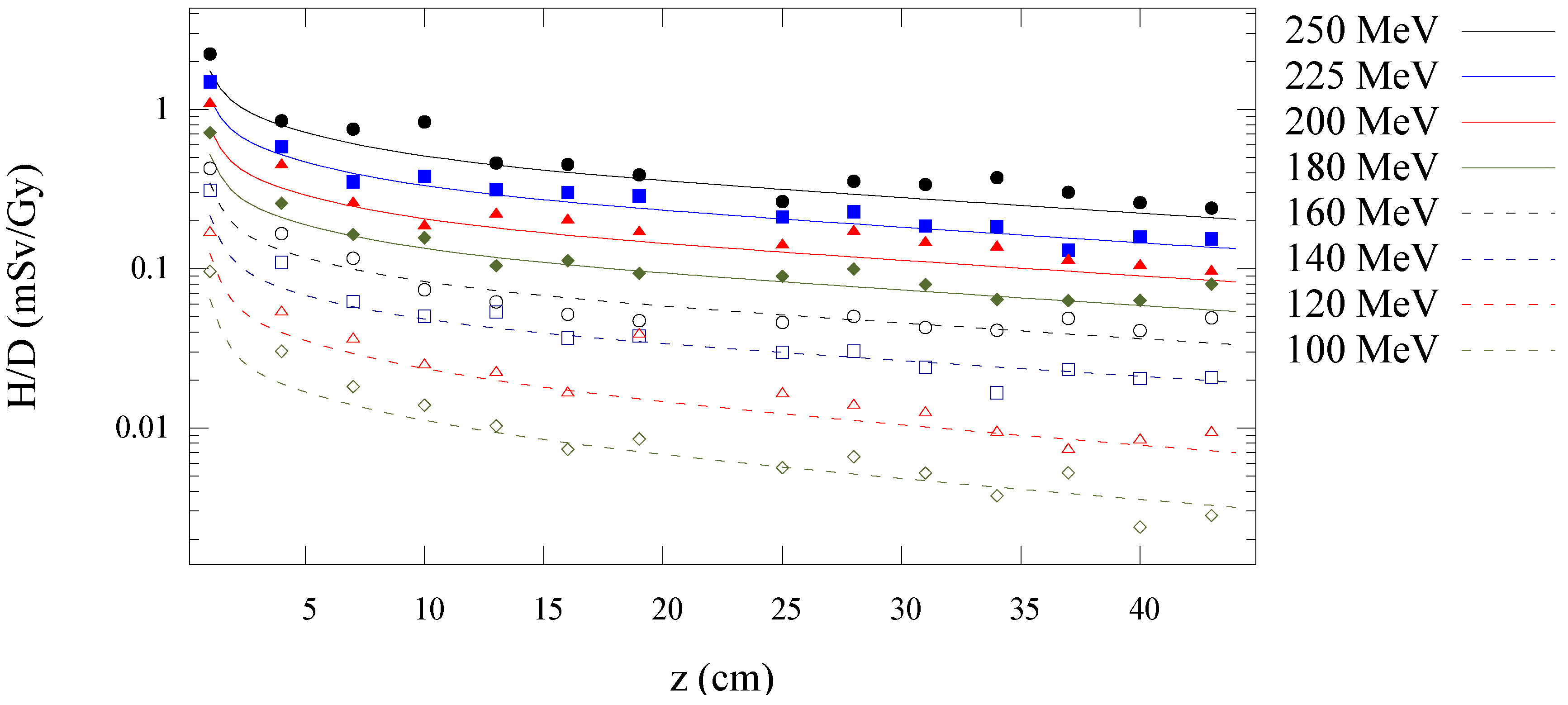
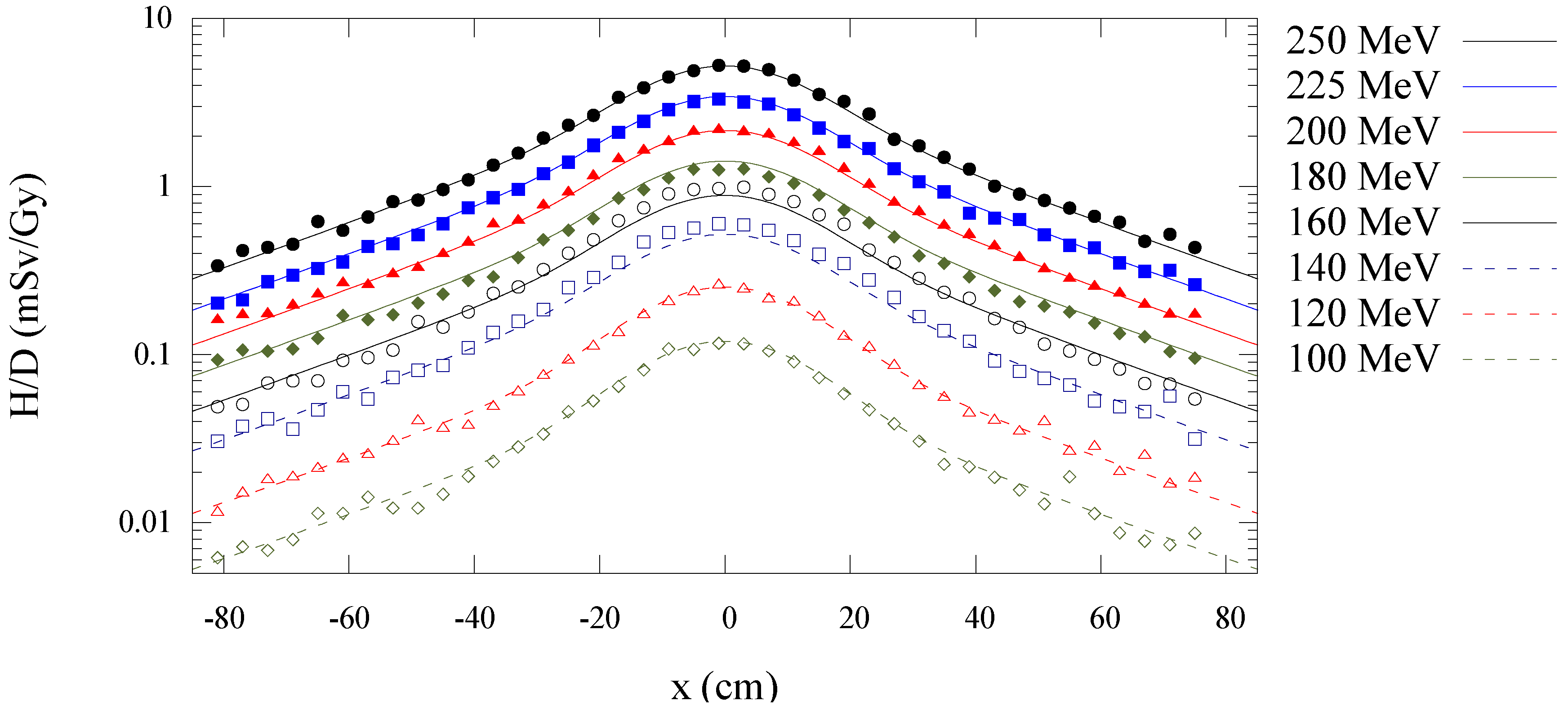
| (Sv/Gy) | ||
|---|---|---|
| 8.0 × 10−9 | 4.1 × 100 | 1.2 × 10−4 |
| Neutron Energy Regime | ||||
|---|---|---|---|---|
| Intranuclear Cascade | −4.8 × 10−4 | 6.0 × 10−1 | N/A | N/A |
| Evaporation | 1.2 × 10−1 | 1.3 × 102 | 5.0 × 100 | −1.3 × 10−11 |
| Epithermal | 4.0 × 10−1 | N/A | N/A | N/A |
| Thermal | 1.2 × 10−7 | −6.6 × 10−5 | 1.1 × 10−2 | N/A |
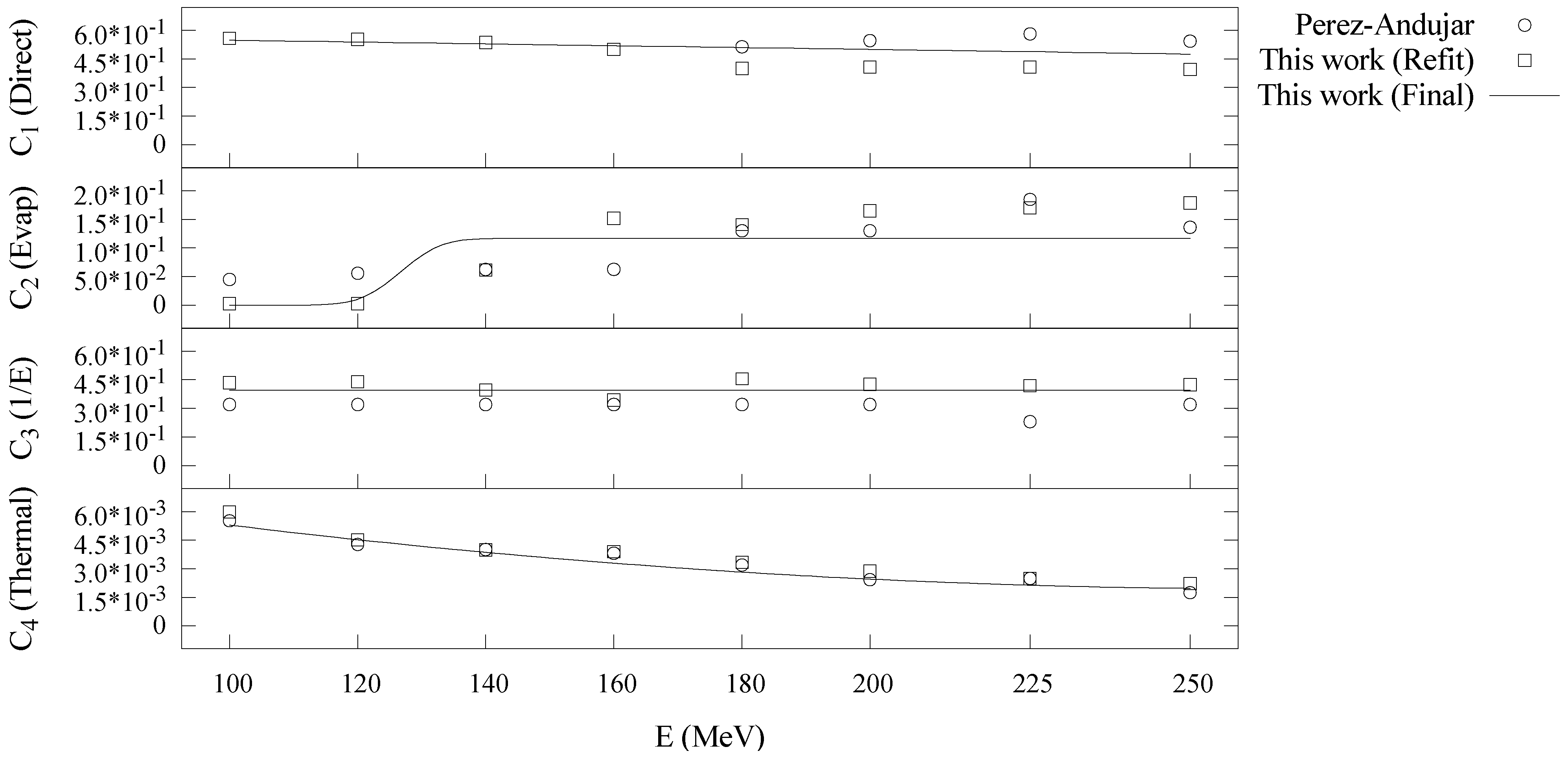
| Direct | Evaporation | Epithermal | Thermal | ||||
|---|---|---|---|---|---|---|---|
| (cm−1) | (cm) | (cm−1) | (cm) | (cm−1) | (cm) | (cm−1) | (cm) |
| 1.3 × 10−2 | 1.4 × 101 | 1.3 × 10−2 | 7.7 × 101 | 3.2 × 10−2 | 3.9 × 103 | 3.3 × 10−1 | 3.9 × 103 |
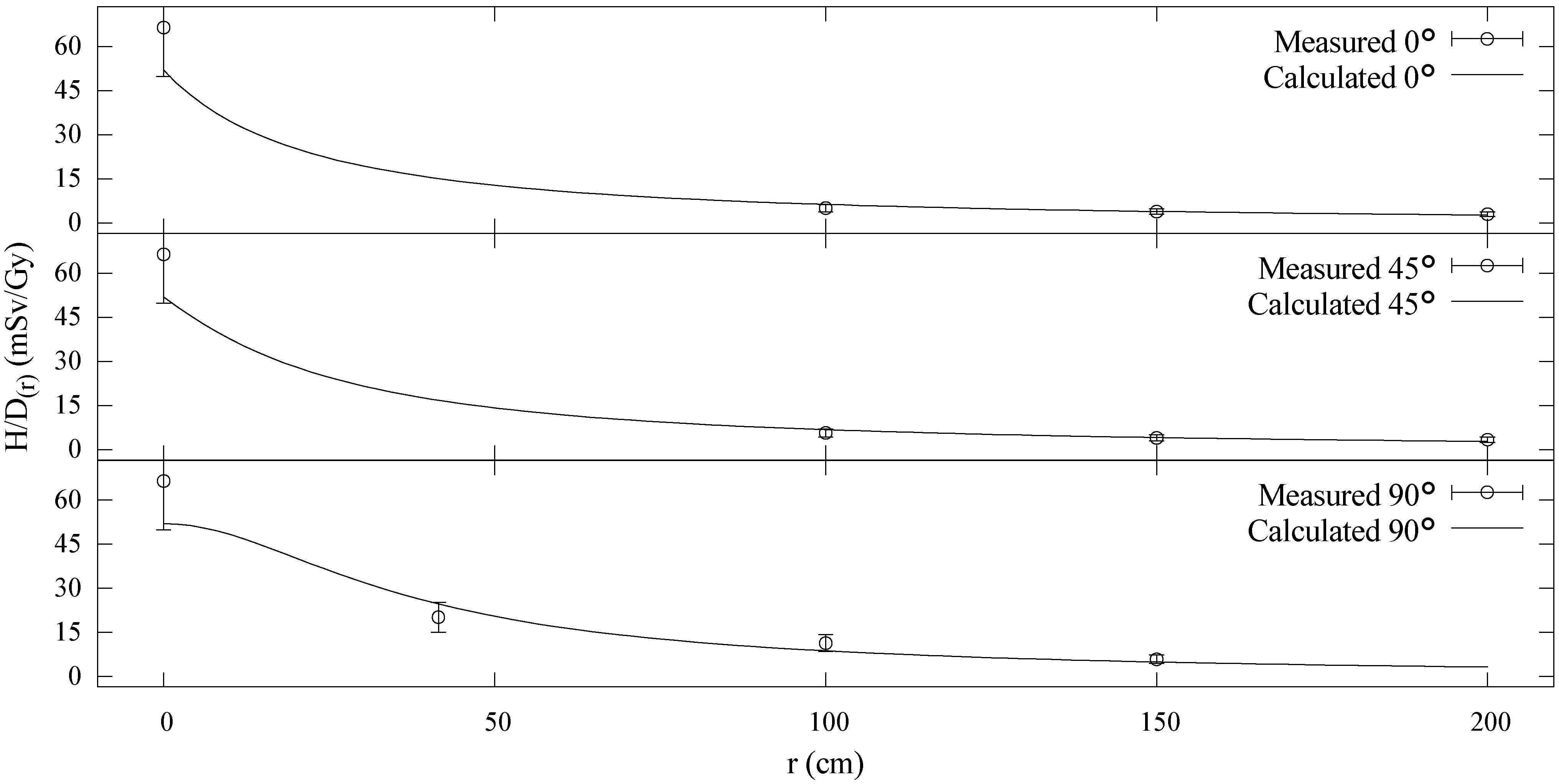
3.2. Model Agreement with Measured Data at 75 MeV
| Proton Energy (MeV) | This Work | Perez-Andujar et al. [14] | ||
|---|---|---|---|---|
| All | 10 | 60 | 10 | 76 |
| 250 | 7 | 39 | 7 | 30 |
| 225 | 7 | 29 | 6 | 29 |
| 200 | 7 | 31 | 6 | 34 |
| 180 | 11 | 31 | 7 | 34 |
| 160 | 11 | 33 | 10 | 45 |
| 140 | 11 | 45 | 10 | 46 |
| 120 | 11 | 60 | 15 | 61 |
| 100 | 16 | 54 | 18 | 76 |
| 1.1 × 10−2 | 7.8 × 10−1 | 1.0 × 10−1 | 9.6 × 10−2 | 9.4 × 100 | 1.0 × 103 | 4.2 × 103 | 4.2 × 103 | 5.2 × 10−5 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miralbell, R.; Lomax, A.; Cella, L.; Schneider, U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Fontenot, J.D.; Mahajan, A.; Kornguth, D.; Stovall, M.; Zheng, Y.; Taddei, P.J.; Mirkovic, D.; Mohan, R.; Cox, J.D.; et al. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys. Med. Biol. 2009, 54, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Howell, R.M.; Giebeler, A.; Taddei, P.J.; Mahajan, A.; Newhauser, W.D. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys. Med. Biol. 2013, 58, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Agosteo, S.; Birattari, C.; Caravaggio, M.; Silari, M.; Tosi, G. Secondary neutron and photon dose in proton therapy. Radiother. Oncol. 1998, 48, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Nuclear interactions in proton therapy: Dose and relative biological effect distributions originating from primary and secondary particles. Phys. Med. Biol. 2002, 47, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, F.; Durante, M. Proton radiobiology. Cancers Basel 2015, 7, 353–381. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Durante, M. Assessing the risk of second malignancies after modern radiotherapy. Nat. Rev. Cancer 2011, 11, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Polf, J.C.; Newhauser, W.D. Calculations of neutron dose equivalent exposures from range-modulated proton therapy beams. Phys. Med. Biol. 2005, 50, 3859–3873. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Newhauser, W.; Fontenot, J.; Taddei, P.; Mohan, R. Monte Carlo study of neutron dose equivalent during passive scattering proton therapy. Phys. Med. Biol. 2007, 52, 4481–4496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Newhauser, W.; Fontenot, J.; Koch, N.; Mohan, R. Monte Carlo simulations of stray neutron radiation exposures in proton therapy. J. Nucl. Mater. 2007, 361, 289–297. [Google Scholar] [CrossRef]
- Zhang, R.; Perez-Andujar, A.; Fontenot, J.D.; Taddei, P.J.; Newhauser, W.D. An analytic model of neutron ambient dose equivalent and equivalent dose for proton radiotherapy. Phys. Med. Biol. 2010, 55, 6975–6985. [Google Scholar] [CrossRef] [PubMed]
- Anferov, V. Analytic estimates of secondary neutron dose in proton therapy. Phys. Med. Biol. 2010, 55, 7509–7522. [Google Scholar] [CrossRef] [PubMed]
- Perez-Andujar, A.; Zhang, R.; Newhauser, W. Monte Carlo and analytical model predictions of leakage neutron exposures from passively scattered proton therapy. Med. Phys. 2013. [Google Scholar] [CrossRef]
- Newhauser, W.; Fontenot, J.; Zheng, Y.; Polf, J.; Titt, U.; Koch, N.; Zhang, X.; Mohan, R. Monte Carlo simulations for configuring and testing an analytical proton dose-calculation algorithm. Phys. Med. Biol. 2007, 52, 4569–4584. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.S.; McKinney, G.W.; Durkee, J.W.; Finch, J.P.; Fensin, M.L.; James, M.R.; Johns, R.C.; Pelowitz, D.B.; Waters, L.S.; Gallmeier, F.X. MCNPX, Version 26c; Los Alamos National Laboratory: Los Alamos, NM, USA, 2006.
- Farah, J.; Martinetti, F.; Sayah, R.; Lacoste, V.; Donadille, L.; Trompier, F.; Nauraye, C.; de Marzi, L.; Vabre, I.; Delacroix, S.; et al. Monte Carlo modeling of proton therapy installations: A global experimental method to validate secondary neutron dose calculations. Phys. Med. Biol. 2014, 59, 2747–2765. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Newhauser, W.D.; Titt, U. Design tools for proton therapy nozzles based on the double-scattering foil technique. Radiat. Prot. Dosim. 2005, 116, 211–215. [Google Scholar] [CrossRef]
- Herault, J.; Iborra, N.; Serrano, B.; Chauvel, P. Monte Carlo simulation of a protontherapy platform devoted to ocular melanoma. Med. Phys. 2005, 32, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Newhauser, W.D.; Titt, U.; Gombos, D.; Coombes, K.; Starkschall, G. Monte Carlo calculations and measurements of absorbed dose per monitor unit for the treatment of uveal melanoma with proton therapy. Phys. Med. Biol. 2008, 53, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Tayama, R.; Fujita, Y.; Tadokoro, M.; Fujimaki, H.; Sakae, T.; Terunuma, T. Measurement of neutron dose distribution for a passive scattering nozzle at the Proton Medical Research Center (PMRC). Nucl. Instrum. Methods Phys. Res. Sect. A 2006, 564, 532–536. [Google Scholar] [CrossRef]
- Tayama, R.; Handa, H.; Hayashi, K.; Nakano, H.; Sasamoto, N.; Nakashma, H.; Masukawa, F. Benchmark calculations of neutron yields and dose equivalent from thick iron target for 52–256 MeV protons. Nucl. Eng. Des. 2002, 213, 119–131. [Google Scholar] [CrossRef]
- Burgkhardt, B.; Fieg, G.; Klett, A.; Plewnia, A.; Siebrt, B.R.L. The neutron fluence and H*(10) response of the new LB 6411 REM counter. Radiat. Prot. Dosim. 1997, 70, 361–364. [Google Scholar] [CrossRef]
- Silari, M.; Agosteo, S.; Beck, P.; Bedogni, R.; Cale, E.; Caresana, M.; Domingo, C.; Donadille, L.; Dubourg, N.; Exposito, A.; et al. Intercomparison of radiation protection devices in a high-energy stray neutron field. Part III: Instrument response. Radiat. Meas. 2009, 44, 673–691. [Google Scholar] [CrossRef]
- Olsher, R.H.; Hsu, H.H.; Beverding, A.; Kleck, J.H.; Casson, W.H.; Vasilik, D.G.; Devine, R.T. WENDI: An improved neutron rem meter. Health Phys. 2000, 79, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Lasdon, L.S.; Fox, R.L.; Ratner, M.W. Nonlinear Optimization Using the Generalized Reduced Gradient Method; National Technical Information Service: Springfield, VA, USA, 1973.
- Farah, J.; Bonfrate, A.; de Marzi, L.; de Oliveira, A.; Delacroix, S.; Martinetti, F.; Trompier, F.; Clairand, I. Configuration and validation of an analytical model predicting secondary neutron radiation in proton therapy using Monte Carlo simulations and experimental measurements. Phys. Med. 2015, 31, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Eley, J.; Newhauser, W.; Homann, K.; Howell, R.; Schneider, C.; Durante, M.; Bert, C. Implementation of an analytical model for leakage neutron equivalent dose in a proton radiotherapy planning system. Cancers 2015, 7, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fontenot, J.; Taddei, P.; Mirkovic, D.; Newhauser, W. Monte Carlo simulations of neutron spectral fluence, radiation weighting factor and ambient dose equivalent for a passively scattered proton therapy unit. Phys. Med. Biol. 2008, 53, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Bues, M.; Newhauser, W.D.; Titt, U.; Smith, A.R. Therapeutic step and shoot proton beam spot-scanning with a multi-leaf collimator: A Monte Carlo study. Radiat. Prot. Dosim. 2005, 115, 164–169. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Zhang, R.; Jones, T.G.; Giebeler, A.; Taddei, P.J.; Stewart, R.D.; Lee, A.; Vassiliev, O. Reducing the cost of proton radiation therapy: The feasibility of a streamlined treatment technique for prostate cancer. Cancers Basel 2015, 7, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Hyer, D.E.; Hill, P.M.; Wang, D.; Smith, B.R.; Flynn, R.T. A dynamic collimation system for penumbra reduction in spot-scanning proton therapy: Proof of concept. Med. Phys. 2014. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, C.; Newhauser, W.; Farah, J. An Analytical Model of Leakage Neutron Equivalent Dose for Passively-Scattered Proton Radiotherapy and Validation with Measurements. Cancers 2015, 7, 795-810. https://doi.org/10.3390/cancers7020795
Schneider C, Newhauser W, Farah J. An Analytical Model of Leakage Neutron Equivalent Dose for Passively-Scattered Proton Radiotherapy and Validation with Measurements. Cancers. 2015; 7(2):795-810. https://doi.org/10.3390/cancers7020795
Chicago/Turabian StyleSchneider, Christopher, Wayne Newhauser, and Jad Farah. 2015. "An Analytical Model of Leakage Neutron Equivalent Dose for Passively-Scattered Proton Radiotherapy and Validation with Measurements" Cancers 7, no. 2: 795-810. https://doi.org/10.3390/cancers7020795





