Neuroendocrine Tumors of the Lung
Abstract
:1. Introduction
- Foregut: Thymus, lung, bronchi, trachea,
- Midgut: Small intestine, gallbladder, pancreas,
- Hindgut: Colon, excluding appendix, rectum,
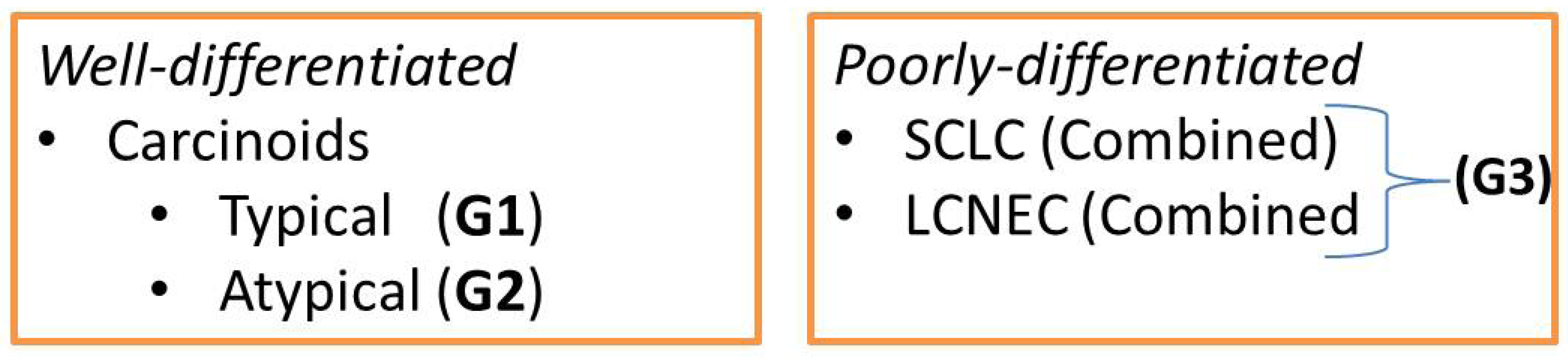
- Carcinoid tumors (typical (TC)/atypical (AC)),
- Large cell neuroendocrine carcinomas (LCNEC),
- Small cell carcinomas (SCLC).
- Well-differentiated low grade (G1) typical and intermediate grade (G2) atypical carcinoids,
- Poorly-differentiated high grade (G3) LCNEC and SCLC.
2. Preneoplastic/Precursor Lesions
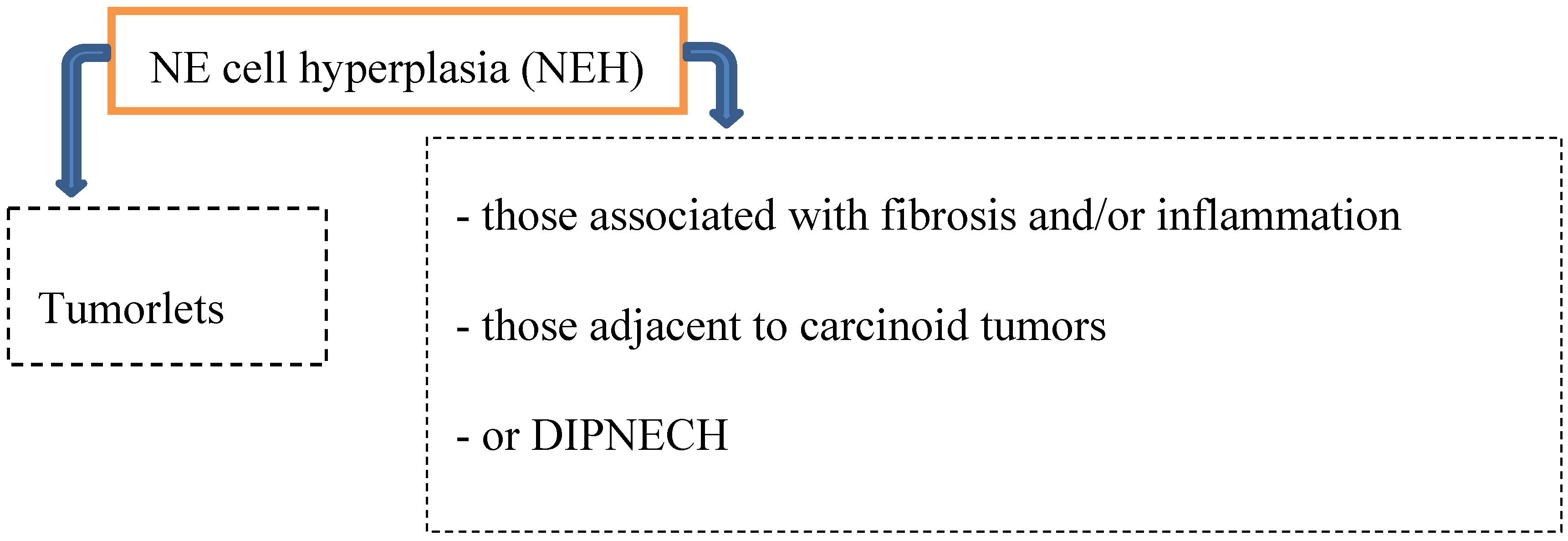
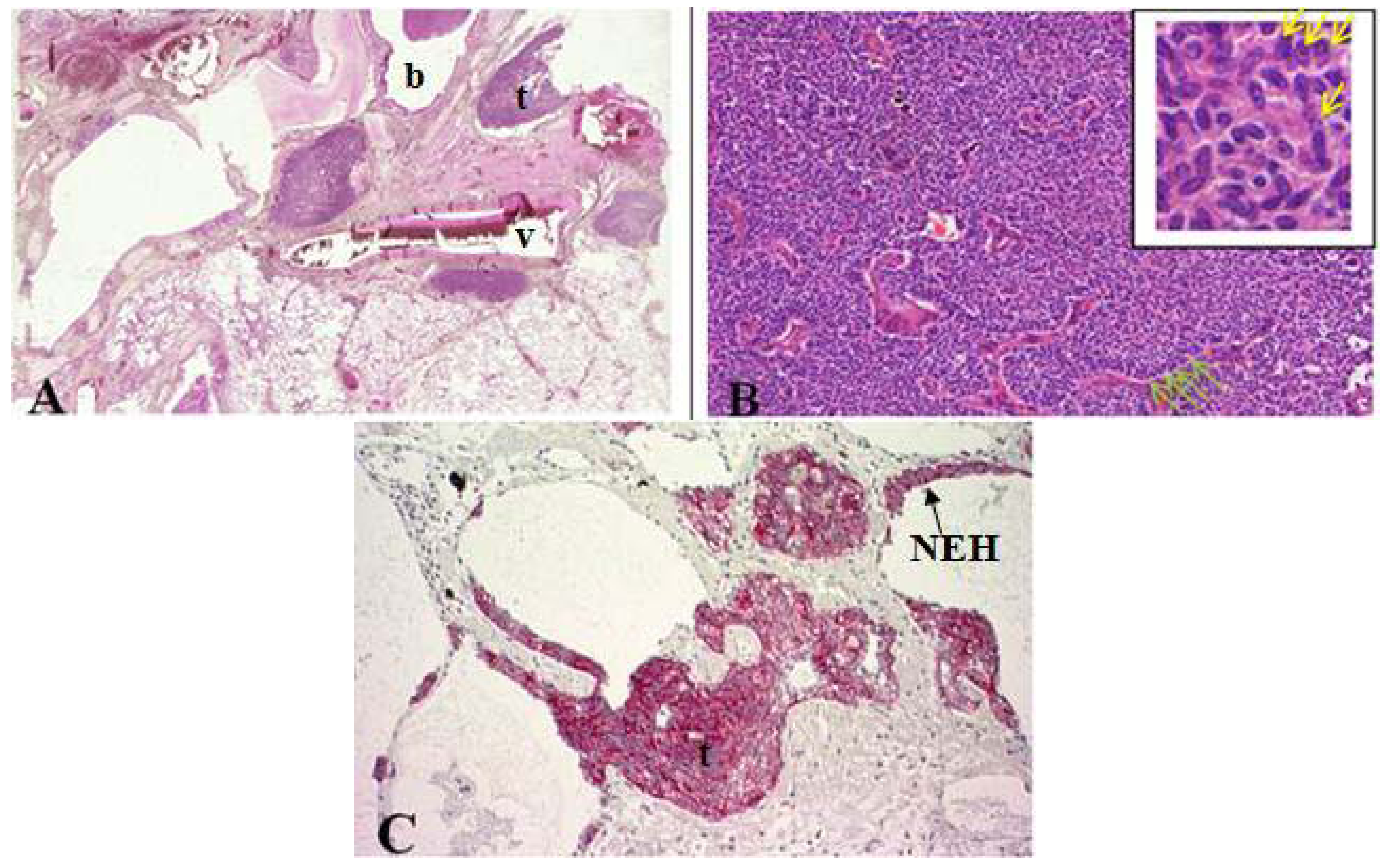
2.1. Tumorlet
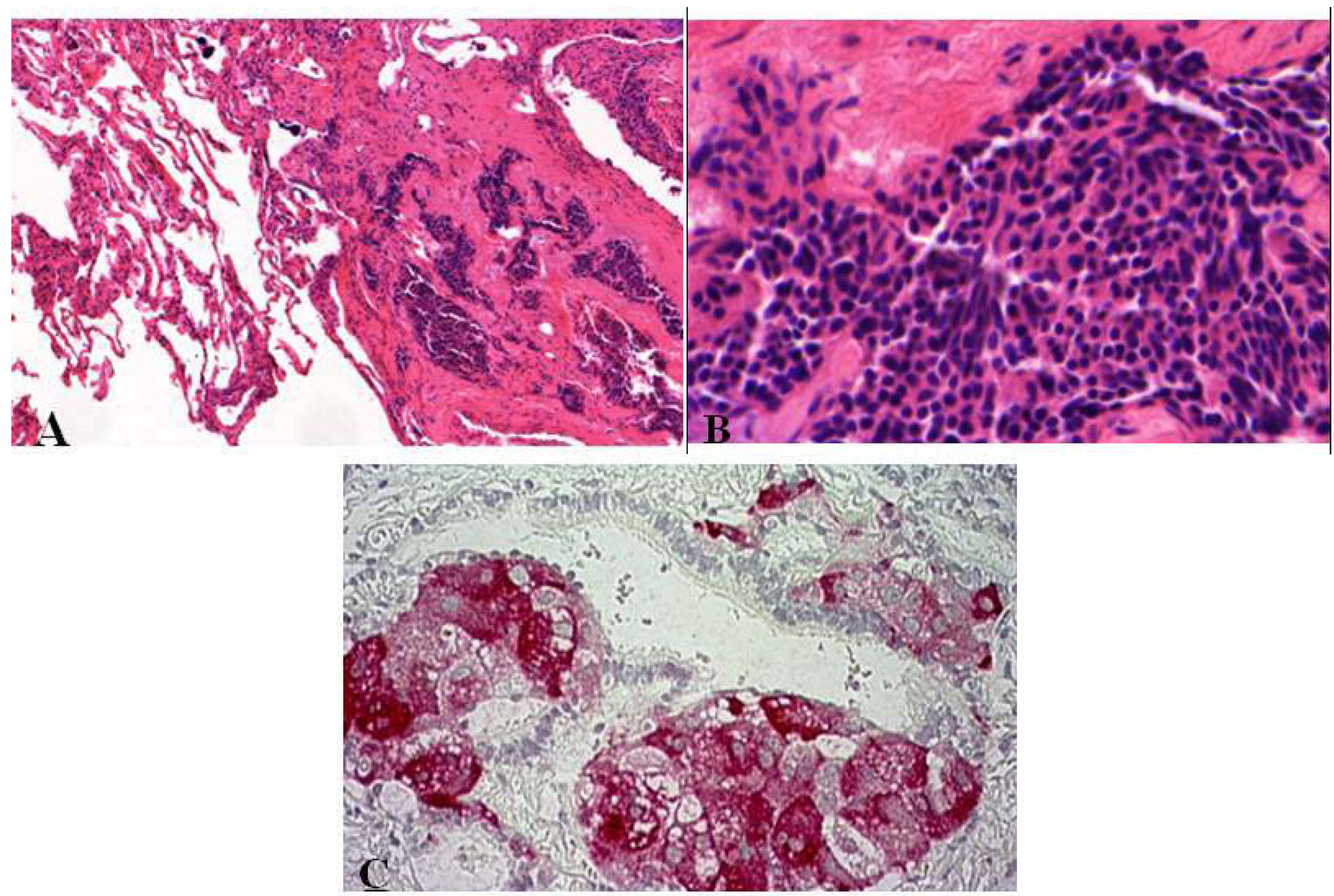
2.2. DIPNECH
- Chronic lung injury in case of bronchiectasis or fibrosis → progression to carcinoid tumors is typically not seen.
- DIPNECH and the progression to carcinoid tumors (both AT and TC) has been documented predominantly in women → small airway obliteration or respiratory failure (rare).
- Coexistence of hyperplasia and tumorlets (often seen in patients with resected carcinoid tumors; 46–76%).
3. Epidemiology
4. Clinical Features
5. Clinical Diagnostics
- CT Scan;
- Chest X-ray;
- Bronchoscopy, Endosonography and Biopsy;
- Octreotide Scintigraphy;
- Somatostatin Receptor PET.
6. General Morphological Diagnostic Tools for Identifying and Classifying Pulmonary Neuro-endocrine Lesions
6.1. Biopsies and Cytology
6.2. Histopathological Features of Pulmonary Neuroendocrine Tumors
| NEs | Macroscopy | Histology/Cytology | |||
|---|---|---|---|---|---|
| Characterization | Necrosis | Mitosis/10HPF * | Azzopardi effect * | ||
| TC (G1) |
|
| No | <2 | no |
| AC (G2) |
|
| possible focal | 2–10 | no |
| Neuroendocrine carcinoma of small cell/intermediate or large cell type (G3) |
|
| large areas | >10; LCNEC: often ≥10; SCLC: often >50 | LCENC: uncommo n; SCLC: occasional |
6.3. Immunohistochemistry
| Carcinoids | SCLC | LCNEC |
|---|---|---|
| Synaptophysin, Chromogranin A, CD56/NCAM, TTF1 (50% are positive + staining weak and focal), Estrogen receptor (50% of carcinoids) * | Synaptophysin, Chromogranin A (only weak), CD56 (most are positive), cytokeratins (AE1/AE3 or CAM 5.2), 34ßE12, TTF-1 (90%) | Synaptophysin, Chromogranin A (coexpressed in 70%), TTF-1 (50%) |
6.4. Current Staging
- – Absence or minimal presence of tumor regression(Regression grade I),
- – Incomplete tumor regression (Regression grade II),
- – Greater than 10% residual viable tumor (Regression grade IIA),
- – Less than 10% residual viable tumor (Regression grade IIB),
- – Complete tumor regression, without viable tumor tissue (Regression grade III).
7. Carcinoid (Typical/Atypical) Tumors
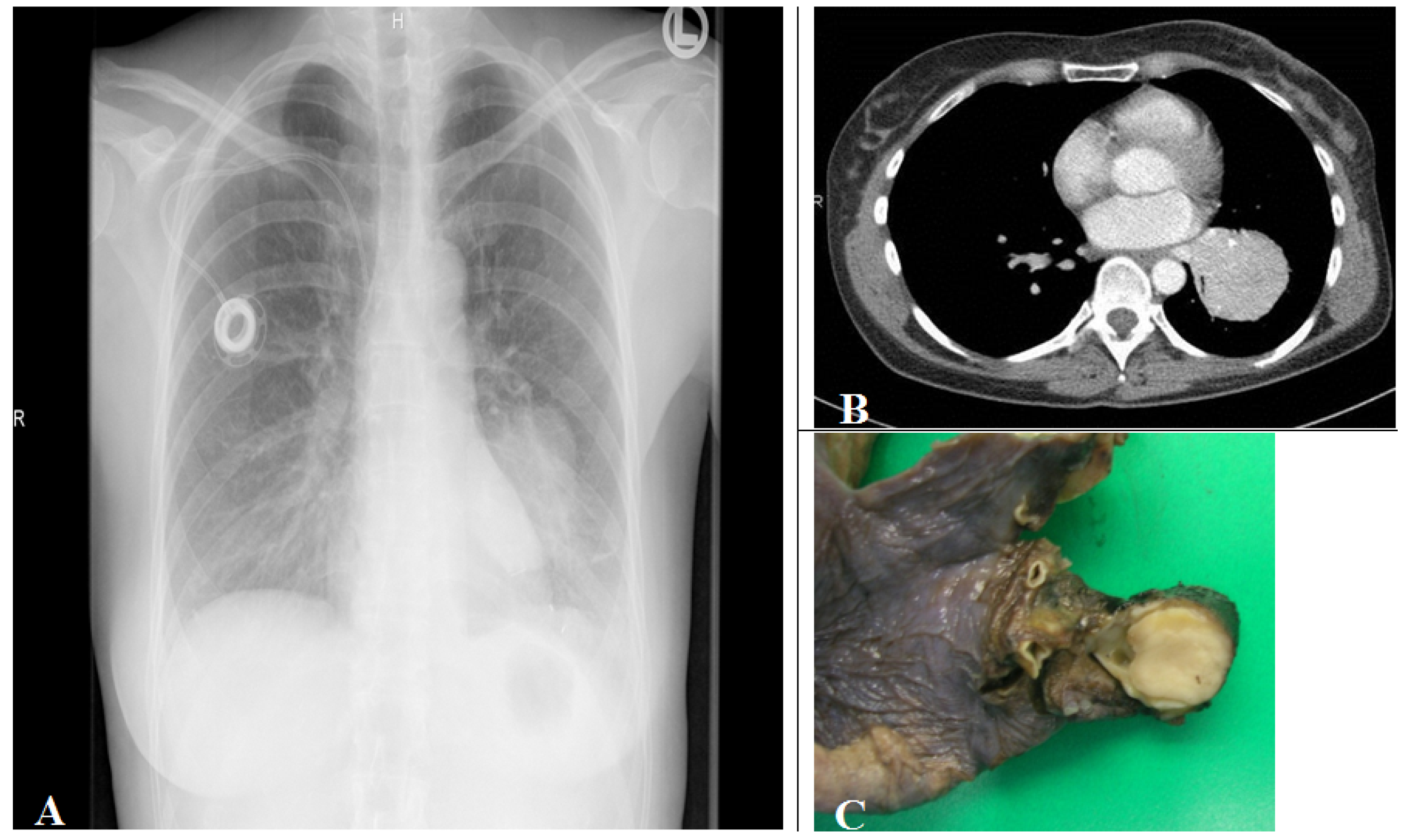
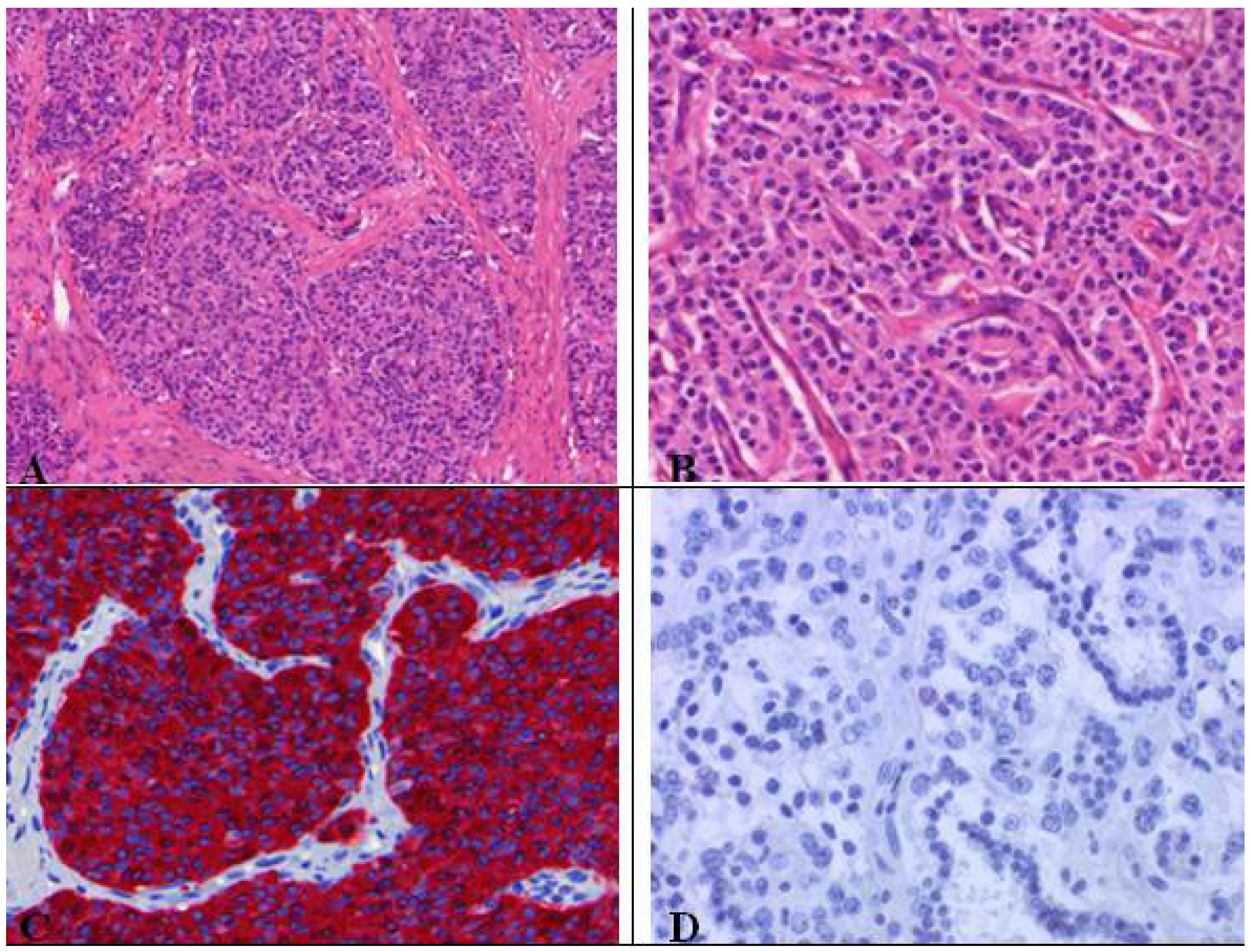
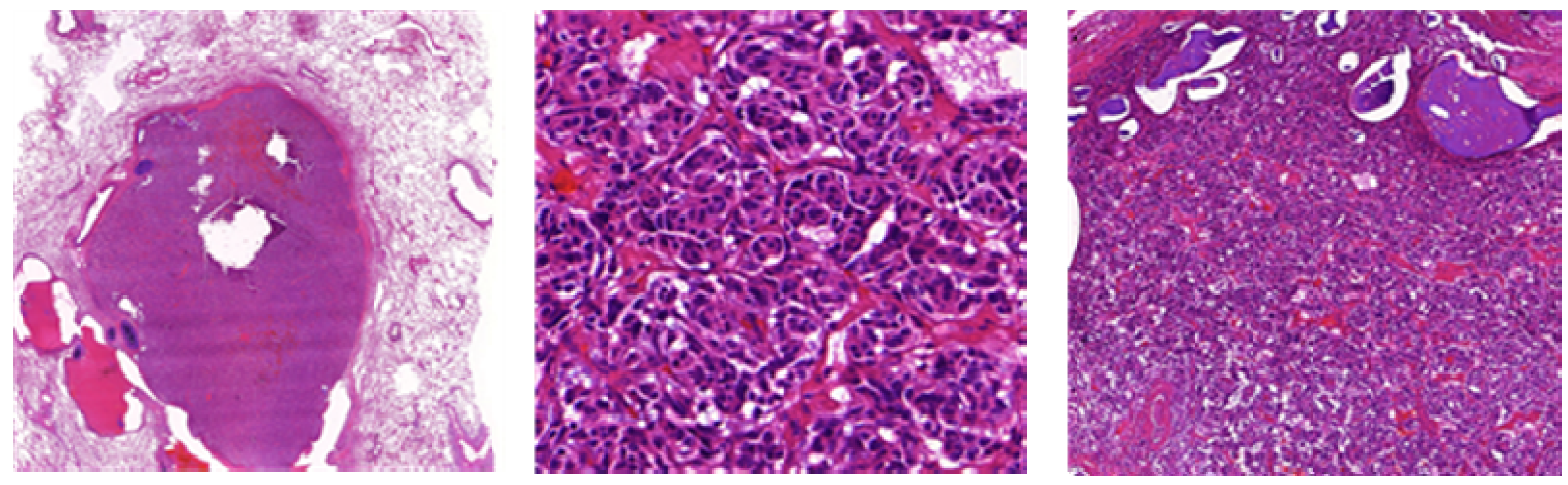
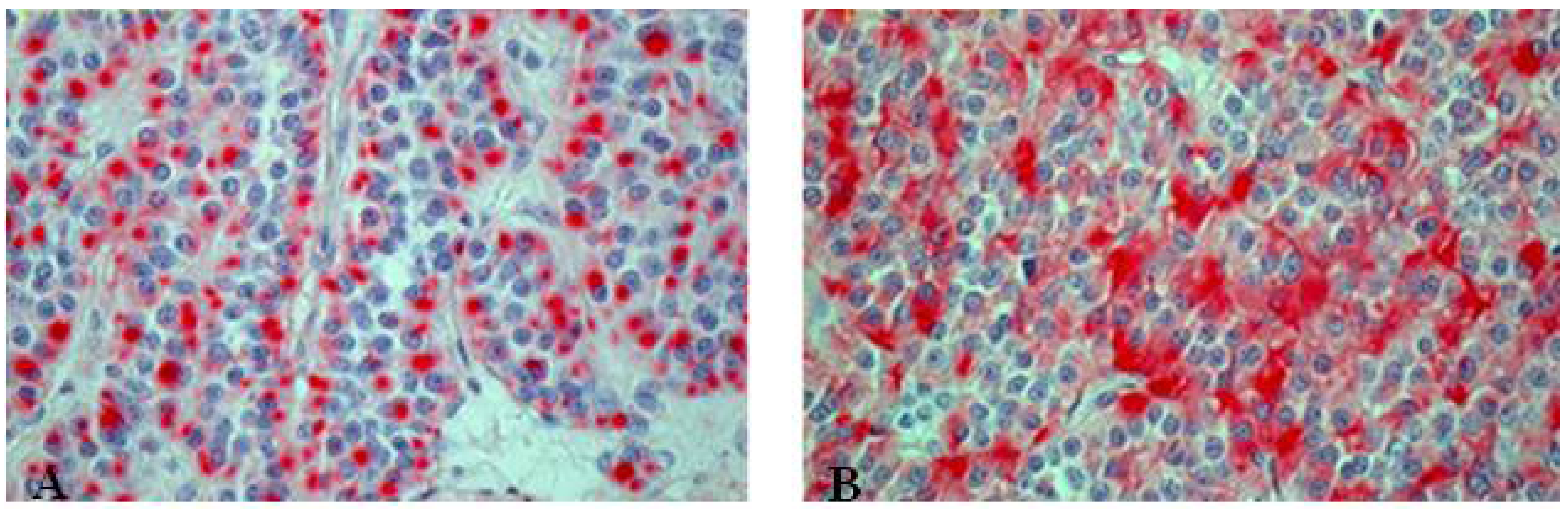
- Carcinoid tumorlets;
- High grade neuroendocrine tumors:
- Large cell neuroendocrine carcinoma,
- Small cell carcinoma;
- Adenocarcinoma;
- Mucoepidermoidcarcinoma (glandular pattern) adenoidcystic carcinoma;
- Paraganglioma;
- Glomus tumor.
8. LCNEC
- → peripherally (~84%) (not accessible to the bronchoscope);
- → centrally (~16%) with endobronchial tumor growth (tan-colored polypoid mass infiltrating the airway lumen).
- → Organoid nesting, trabecular growth, rosettes and perilobular (peripheral) palisading pattern, large zones of necrosis (comparable to SCLC), large tumor cells, usually abundant eosinophilic cytoplasm, prominent and frequent nucleoli (contrast to SCLC, but not always, therefore the cell size should not be used alone to differentiate between SCLC and LCNEC), mitotic counts are typically ≥11 per 2 mm² of viable tumor, at least one neuroendocrine marker should be positive. 50% of LCNEC are positive for TTF1 [8].

- Large cell carcinomas have to be differentiated from SCLC, typical carcinoids and NSCLCs, which has an influence on therapeutic treatment options and prognosis.
8.1. Combined Large Cell Neuroendocrine Carcinoma—What Does This Mean?
- → adenocarcinoma,
- → squamous cell carcinoma,
- → giant cell carcinoma and/or spindle cell carcinoma.
8.2. Differentiation LCNEC Versus NSCLC with NE Differentiation (NSCLC-NED)—Always Possible?

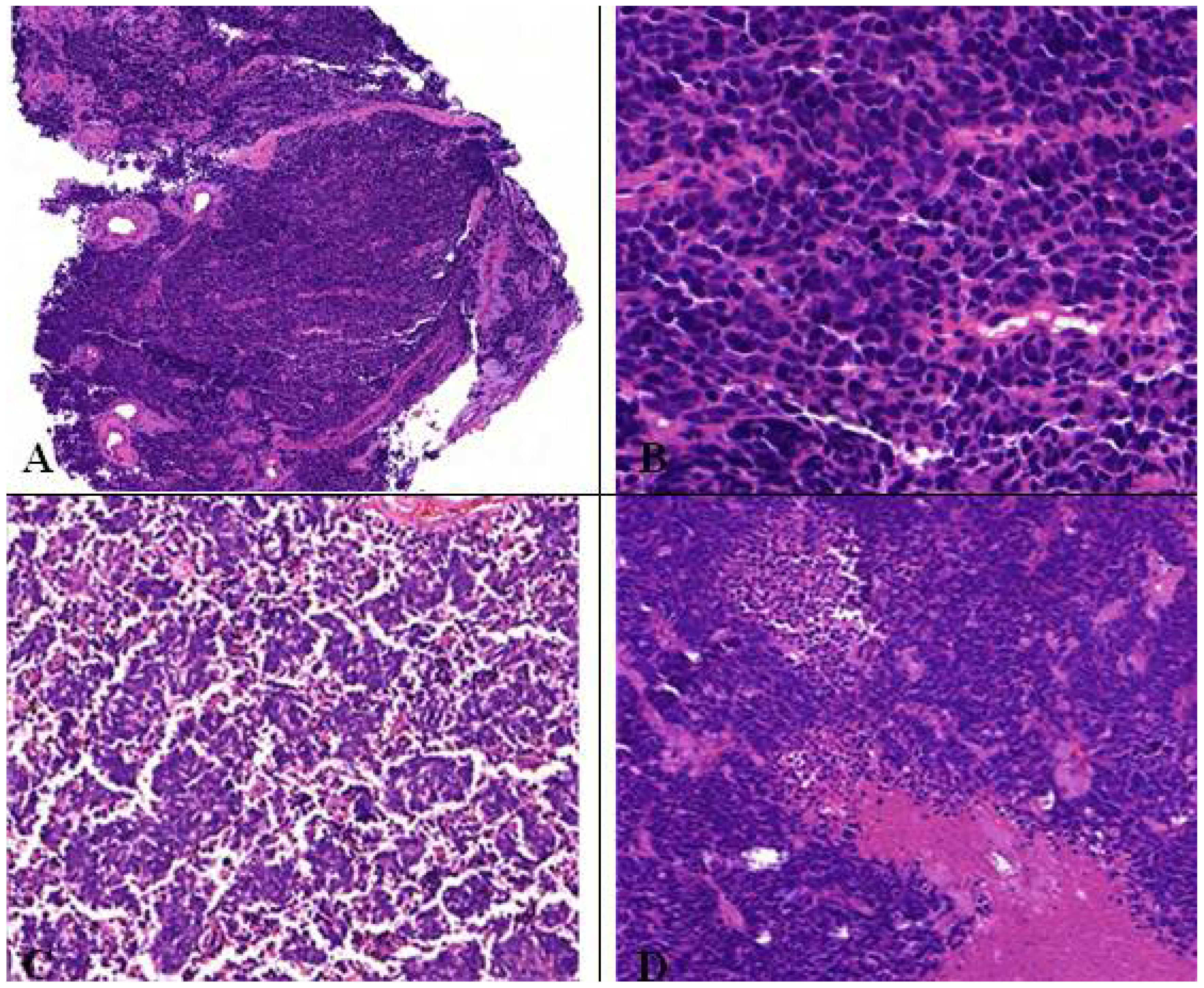
9. SCLC

9.1. Differentiation of SCLC Versus LCNEC—Always Possible?
- classic nuclear morphology, lacking prominent nucleoli and prominent cell;
- membranes.
9.2. Combined Small Cell Lung Cancer—What Does This Mean?
- squamous cell carcinomas, adenocarcinomas, large cell carcinomas,
- spindle cell or giant cell carcinoma (less common),
9.3. Criteria for Combined Small Cell/Large Cell Carcinoma?
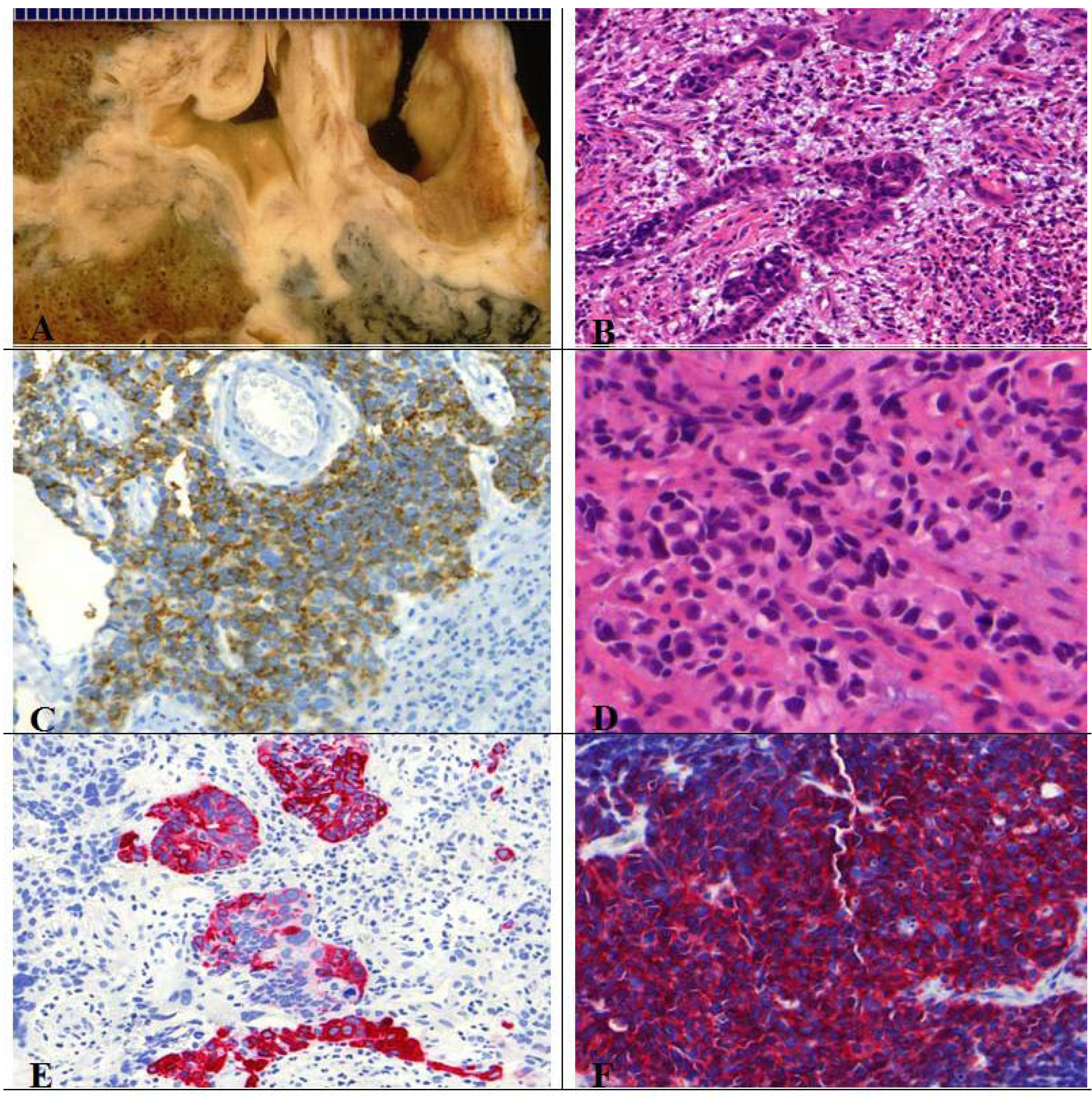
10. Current Treatment and Prognosis of Neuroendocrine Tumors of the Lung
| 5-Year survival rate | Treatment | |
|---|---|---|
| Typical Carcinoid ( G1) | 92–100% | surgical resection/lobectomy |
| Atypical Carcinoid (G2) | 61–88% | surgical resection/lobectomy |
| LCNEC (G3) | 16–57% | cisplatin based chemoth.?/surgery |
| SCLC (G3) | ~2–5% | etoposide/cisplatin or carboplatin combination; early stage patients undergo surgical resection |
- → lung conserving and radical resection including lymph node dissection;
- → anatomic resection (pneumonectomy or lobectomy)—60–70% of cases;
- → sleeve resection (for centrally located carcinoid tumors);
- → lung sparing procedures (wedge resection, segmentectomy, or sleeve resections).
11. Molecular Pathology
12. Conclusions
References
- Travis, W.D. Advances in neuroendocrine lung tumors. Ann. Oncol. 2010, 21, ii65–ii71. [Google Scholar] [CrossRef]
- Rekhtmann, N. Neuroendocrine tumors of the lung. Arch. Pathol. Lab. Med. 2010, 134, 1628–1638. [Google Scholar]
- Bertino, E.M. Pulmonary neuroendocrine/carcinoid tumors. Cancer 2009, 115, 4434–4441. [Google Scholar] [CrossRef]
- Taal, B.G.; Visser, O. Epidemiology of neuroendocrine tumours. Neuroendocrin 2004, 80, 3–7. [Google Scholar] [CrossRef]
- Semin Chong, M.D. Teaching points for neuroendocrine tumors of the lung: Clinical, pathologic, and imaging findings. Radiographics 2006, 26, 41–58. [Google Scholar] [CrossRef]
- Moran, C.A. Neuroendocrine carcinomas of the lung. Am. Soc. Clin. Pathol. 2009, 131, 206–221. [Google Scholar] [CrossRef]
- Valente, M.; Catena, L.; Milione, M.; Pusceddu, S.; Formisano, B.; Bajetta, E. Common diagnostic challenges in the histopathologic diagnosis of neuroendocrine lung tumors: A case report. Case Rep. Oncol. 2010, 3, 202–207. [Google Scholar] [CrossRef]
- World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Lung, Pleura, Tymus and Heart; Travis, W.D.; Brambilla, E.; Muller-Hermelink, H.K.; Harris, C.C. (Eds.) IARC Press: Lyon, France, 2004; pp. 19–25.
- Zander, D.S.; Popper, H.H.; Jagirdar, J.; Haque, A.K.; Cagle, P.T.; Barrios, R. Molecular Pathology Of Lung Diseases; Springer: New York, NY, USA, 2008. [Google Scholar]
- Ginsberg, M.S.; Akin, O.; Berger, D.B.; Zakowski, M.F.; Panicek, D.M. Pulmonary tumolets: CT findings. Am. J. Roentgenol. 2004, 183, 293–296. [Google Scholar]
- Churg, A.; Warnock, M.L. Pulmonary tumorlet. A form of peripheral carcinoid. Cancer 1976, 37, 1469–1477. [Google Scholar] [CrossRef]
- Warnock, M.L.; McCowin, M.D. Practical pathology of chest disease-Case studies. Available online: http://pathhsw5m54.ucsf.edu/introduction.html/ (accessed on 28 April 2012).
- Pelosi, G.; Zancanaro, C.; Sbabo, L.; Bresaola, E.; Martignoni, G.; Bontempini, L. Development of innumerable neuroendocrine tumorlets in pulmonary lobe scarred by intralobar sequestration. Immunohistochemical and ultrastructural study of an unusual case. Arch. Pathol. Lab. Med. 1992, 116, 1167–1174. [Google Scholar]
- Johney, E.C.; Pfannschmidt, J.; Rieker, R.J.; Schnabel, P.A.; Mechtersheimer, G.; Dienemann, H. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia and a typical carcinoid tumor. J. Thorac. Cardiovasc. Surg. 2006, 132, 1207–1208. [Google Scholar]
- Cagle, P.T. Color Atlas and Text of Pulmonary Pathology; Lippincott William &Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Faggiano, A.; Ferella, P.; Grimaldi, F.; Campana, D.; Manzoni, M.; Davi, M.V.; Bianchi, A.; Valcavi, R.; Papini, E.; Giuffrida, D.; et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian Epidemiological study: The NET management study. J. Endocrinol. Invest. 2011. [Google Scholar]
- Gorshtein, A.; Gross, D.J.; Barak, D.; Strenov, Y.; Refaeli, Y.; Shimon, I.; Grozinsky-Glasberg, S. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia and the associated lung neuroendocrine tumors. Am. Cancer Soc. 2011, 118, 612–619. [Google Scholar]
- Bakhshayesh Karam, M.; Zahirifard, S.; Tahbaz, M.O.; Kaynama, K.; Tolou, F.; Jabari Darjani, H. Bronchial carcinoid tumors: Clinical and radiological findings in 21 patients. J. Thorac. Cardiovasc. Surg. 2005, 2, 111–116. [Google Scholar]
- Sayeg, Y.; Bonnet, R. Neuroendocrine tumors of the lung. Available online: http://www.rhoen-klinikum-ag.com/rka/cms/zbb_2/deu/download/Neuroendocrine_Tumors_of_the_Lung.pdf (accessed on 20 February 2012).
- Grozinsky-Glasberg, S.; Shimon, I.; Korbonits, M.; Grossmann, A.B. Somatostatin analogues in the control of neuroendocrine tumours: Efficacy and mechanisms. Endocr. Relat. Cancer 2008, 15, 701–720. [Google Scholar] [CrossRef]
- Gatto, F.; Hofland, L.J. The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr. Relat. Cancer 2011, 18, R233–R251. [Google Scholar] [CrossRef]
- Reghi, L.; Volante, M.; Tavaglione, V.; Bille, A.; Daniele, L.; Augusti, T.; Inzani, F.; Pelosi, G.; Rindi, G.; Papotti, M. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: A clinicopathologic and immunohistochemical study if 218 clinically aggressive cases. Ann. Oncol. 2010, 21, 548–555. [Google Scholar]
- Terada, T. Pulmonary large cell neuroendocrine carcinoma diagnosed in brain metastasis. Int. J. Clin. Exp. Pathol. 2012, 5, 159–162. [Google Scholar]
- Lowe, K.; Khithani, A.; Lui, E.; Winston, T.; Christian, D.; Saad, J.; Jeyarajah, D.R. Ki-67 labeling: A more sensitive indicator of malignant phenotype than mitotic count or tumor size? J. Surg. Oncol. 2012. [Google Scholar] [CrossRef]
- Erler, B.S.; Presby, M.M.; Finch, M.; Hodges, A.; Horowitz, K.; Topilow, A.A.; Matulewicz, T. CD117, Ki-67, and p53 predict survival in neuroendocrine carcinomas, but not within the subgroup of small cell lung carcinoma. Tumour Biol. 2011, 32, 107–111. [Google Scholar] [CrossRef]
- Skov, B.G.; Holm, B.; Erreboe, A.; Skov, T.; Mellemgaard, A. ERCC1 and Ki-67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: Distribution and impact on survival. J. Thorac. Oncol. 2010, 5, 453–459. [Google Scholar] [CrossRef]
- Asian, D.L.; Gulbahce, H.E.; Pambuccian, S.E.; Manivel, J.C.; Jessurun, J. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am. Clin. Pathol. 2005, 123, 874–878. [Google Scholar] [CrossRef]
- Waser, B.; Rehmann, R.; Sanchez, C.; Fourmy, D.; Reubi, J.C. Glucose-dependent insulinotropic polypeptide receptors in most gastroenteropancreatic and bronchial neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2012, 97, 482–488. [Google Scholar] [CrossRef]
- Junker, K. Tumorregression bösartiger Lungentumoren; Habilitationsschrift., Medizinische Fakultät Bochum: Bochum, Germany, 1998. [Google Scholar]
- Junker, K.; Krapp, D.; Müller, K.-M. Kleinzelliges Bronchialkarzinom nach Chemotherapie-Morphologische Befunde. Pathologe 1995, 16, 217–222. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer Staging Manual, 7th; Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) Springer: New York, NY, USA, 2010.
- Junker, K.; Thomas, M.; Schulmann, K.; Klinke, F.; Bosse, U.; Müller, K.-M. Tumor regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J. Cancer Res. Clin. Oncol. 1997, 123, 469–477. [Google Scholar] [CrossRef]
- Fisseler-Eckhoff, A.; Laberke, H.G.; Fischer, M.; Müller, K.-M. Karzinoidtumoren der Lunge und Asbest. Versicherungsmedizinische Aspekte. Pathologe 1998, 19, 425–429. [Google Scholar] [CrossRef]
- Nicholson, S.A.; Beasley, M.B.; Brambilla, E. Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am. J. Surg. Pathol. 2002, 26, 1184–1197. [Google Scholar] [CrossRef]
- Müller, K.M.; Fisseler-Eckhoff, A. Small cell bronchial cancer-Pathologic anatomy. Langenbecks Arch. Chir. Suppl. Kongressbd. 1991, 1991, 534–543. [Google Scholar]
- Elliott, J.A.; Osterlind, K.; Hirsch, F.R.; Hansen, H.H. Metastatic patterns in small-cell lung cancer: Correlation of autopsy findings with clinical parameters in 537 patients. J. Clin. Oncol. 1987, 5, 246–254. [Google Scholar]
- Abeloff, M.D.; Eggleston, J.C.; Mendelsohn, G.; Ettinger, D.S.; Baylin, S.B. Changes in morphologic and biochemical characteristics of small cell carcinoma of the lung: A clinicopathologic study. Am. J. Med. 1979, 66, 757–764. [Google Scholar] [CrossRef]
- Waddell, T.K.; Shepherd, F.A. Should aggressive surgery ever be part of the management of small cell lung cancer? Thorac. Surg. Clin. 2004, 14, 271–281. [Google Scholar]
- Skuladottin, H.; Hirsch, F.H.; Hansen, H.H.; Olsen, J.H. Pulmonary neuroendocrine tumors: Incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung Cancer 2002, 37, 127–135. [Google Scholar] [CrossRef]
- Isaka, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Watanabe, R.; Ito, I.; Nakajima, T.; Kondo, H. A clinicopathological study of peripheral, small-sized high-grade neuroendocrine tumours of the lung: Differences between small-cell lung carcinoma and large-cell neuroendocrine carcinoma. Eur. J. Cardiothorac. Surg. 2012, 41, 841–846. [Google Scholar] [CrossRef]
- Johnson, R.; Trocha, S.; McLawhorn, M.; Worley, M.; Wheeler, G.; Thompson, L.; Schisler, N.; Schammel, D.; Schammel, C.; Stephenson, J.; Bolton, W. Histology, not lymph node involvement, predicts long-term survival in bronchopulmonary carcinods. Am. Surg. 2011, 77, 1669–1674. [Google Scholar]
- Martini, N.; Zaman, M.B.; Bains, M.S.; Burt, M.E.; McCormack, P.M.; Rusch, V.W.; Ginsberg, R.J. Treatment and prognosis in bronchial carcinoids involving regional lymph nodes. J. Thorac. Cardiovasc. Surg. 1994, 107, 1–6. [Google Scholar]
- Sun, J.M.; Ahn, M.J.; Um, S.W.; Kim, H.; Kim, H.K.; Choi, Y.S.; Han, J.; Kim, J.; Kwon, O.J.; Shim, Y.M.; Park, K. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: Similar to that for small cell lung cancer of non-small cell lung cancer? Lung Cancer 2012, 77, 365–370. [Google Scholar] [CrossRef]
- Kenmotsu, Y.; Oshita, F.; Sugiura, M.; Murakami, S.; Kondo, T.; Saito, H.; Yamada, K. Anticancer Res. 2012, 32, 1453–1456.
- Fournel, L.; Falcoz, P.E.; Alifano, M.; Charpentier, M.C.; Boudaya, M.S.; Magdeleinat, P.; Damotte, D.; Regnard, J.F. Surgical management of pulmonary large cell neuroendocrine carcinomas: A 10-year experience. Eur. J. Cardiothorac Surg. 2012, 42, 2. [Google Scholar]
- Abedalla, N.; Tremblay, L.; Baey, C.; Fabre, D.; Planchard, D.; Pignon, J.P.; Guigay, J.; Pechoux, C.L.; Soria, J.C.; de Montpreville, V.T.; et al. Effect of chemotherapy in patients with resected small-cell or large-cell neuroendocrine carcinoma. J. Thorac. Oncol. 2012, 7, 1179–1183. [Google Scholar]
- de Pas, T.M.; Giovannini, M.; Manzotti, M.; Trifiro, G.; Toffalorio, F.; Catania, C.; Spaggiari, L.; Labianca, R.; Barberis, M. Large-cell neuroendocrine carcinoma of the lung harboring EGFR mutation and responding to gifitinib. J. Clin. Oncol. 2011, 29, 819–822. [Google Scholar] [CrossRef]
- Pelosi, G.; Rodrigues, J.; Viale, G.; Rosai, J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens. Am. J. Surg. Pathol. 2005, 29, 179–187. [Google Scholar] [CrossRef]
- Bertina, E.M.; Confer, P.D.; Colonna, J.E.; Ross, P.; Otterson, G.A. Pulmonary neuroendocrine/carcinoid tumors. Cancer 2009, 115, 4434–4441. [Google Scholar]
- Johnson, B.E.; Russel, E.; Simmons, A.M.; Phelbs, R.; Steinberg, S.M.; Ihde, D.C.; Gazdar, A.F. Myc family DNA amplification in 126 tumor cell lines from patients with small cell lung cancer. J. Cell Biochem. 1996, 24, 210–217. [Google Scholar]
- Walch, A.K.; Zitzelberger, H.F.; Aubele, M.M.; Mattis, A.E.; Bauchinger, M.; Candidus, S.; Prauer, H.W.; Werner, M.; Hofler, H. Typical and atypical carcinoid tumors of the lung are characterized by 11q deletions as detected by comparative genomic hybridization. Am. J. Pathol. 1998, 153, 1089–1098. [Google Scholar]
- Swarts, D.R.; Ramaekers, F.C.; Speel, E.J. Molecular and cellular biology of neuroendocrine lung tumors: Evidence for separate biological entities. Biochim. Biophys. Acta 1826, 255–271. [Google Scholar]
- Sartelet, H.; Decaussin, M.; Devouassoux, G.; Nawrocki-Raby, B.; Brichon, P.-Y.; Brambilla, C.; Brambilla, E. Expression of vascular endothelial growth factor (VEGF) and its receptors (VEGF-R1 [Flt-1] and VEGF-R2 [KDR/Flk-1]) in tumorlets and in neuroendocrine cell hyperplasia of the lung. Hum. Pathol. 2004, 35, 1210–1217. [Google Scholar] [CrossRef]
- Edmonston, T.B.; Kushnir, M.; Aharonov, R.; Lithwick Yanai, G.; Benjamin, H.; Bibbo, M.; Thurm, C.; Horowitz, L.; Huang, Y.; Gilad, S.; et al. MicroRNAs as clinical biomarkers for lung cancer classification (Meeting abstract). AACR 2010. No. 145.. [Google Scholar]
- Meiri, M.; Spector, Y.; Cohen, L.; Rosenwald, S.; Bentwich, Z.; Perelman, M.; Aharonov, R.; Barshack, I. MicroRNAs as powerful diagnostic tools for the differential diagnosis of lung tumors (Meeting abstract). J. Clin. Oncol. 2008, 26. No. 15_suppl 11112.. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fisseler-Eckhoff, A.; Demes, M. Neuroendocrine Tumors of the Lung. Cancers 2012, 4, 777-798. https://doi.org/10.3390/cancers4030777
Fisseler-Eckhoff A, Demes M. Neuroendocrine Tumors of the Lung. Cancers. 2012; 4(3):777-798. https://doi.org/10.3390/cancers4030777
Chicago/Turabian StyleFisseler-Eckhoff, Annette, and Melanie Demes. 2012. "Neuroendocrine Tumors of the Lung" Cancers 4, no. 3: 777-798. https://doi.org/10.3390/cancers4030777




