Diagnostic Value of 11C-Methionine (MET) and 18F-Fluorothymidine (FLT) Positron Emission Tomography in Recurrent High-Grade Gliomas; Differentiation from Treatment-Induced Tissue Necrosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Visual Assessment
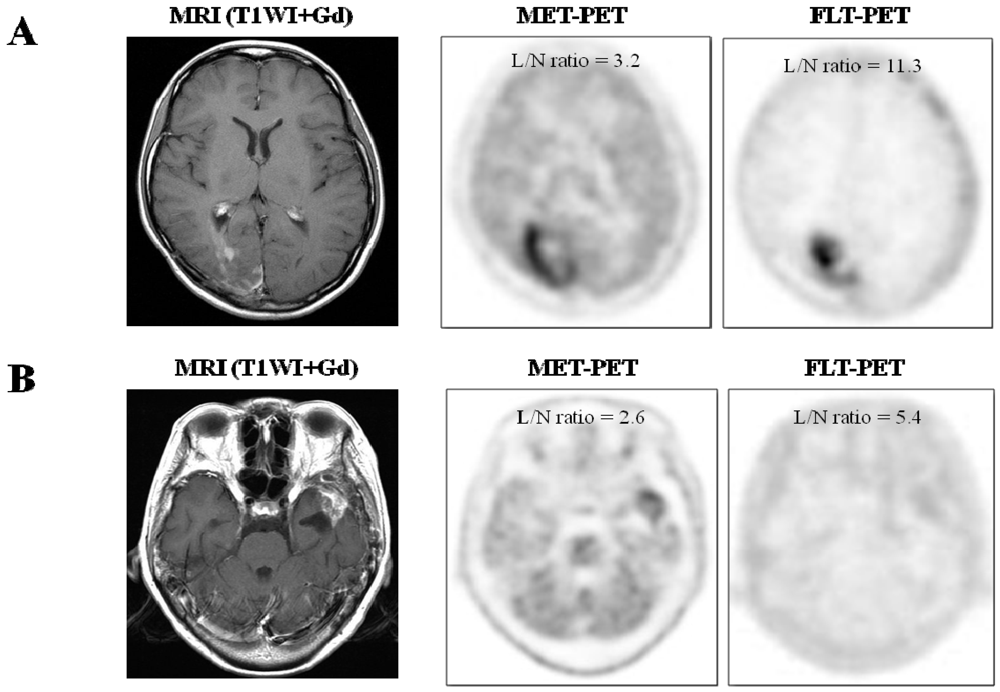
2.1.2. Semi-Quantitative Analysis

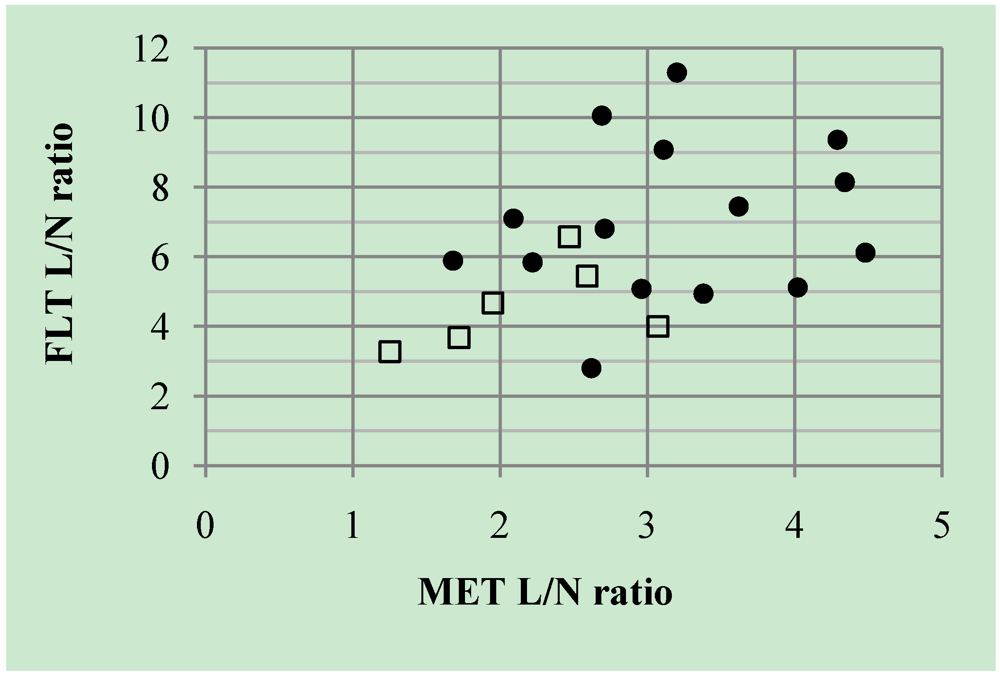
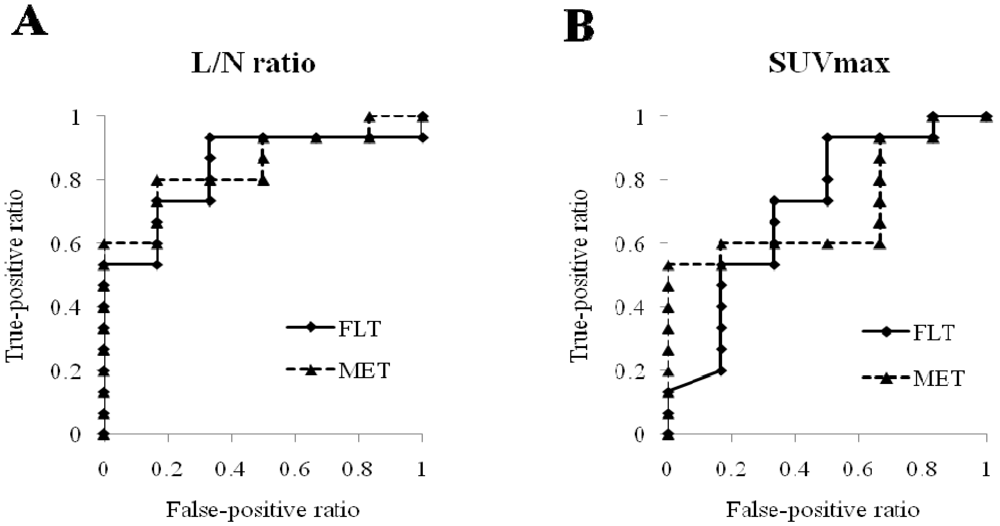
2.2. Discussion
3. Experimental Section
3.1. Patients
| Case | Age | Sex | Primary tumor histopathology | MET | FLT | Method * (months) | Diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|
| SUVmax | L/N ratio | SUVmax | L/N ratio | ||||||
| 1 | 49 | F | Anaplastic astrocytoma | 2.80 | 2.22 | 1.11 | 5.84 | Surgery | Recurrence |
| 2 | 58 | M | Anaplastic astrocytoma | 3.44 | 2.96 | 0.66 | 5.08 | Surgery | Recurrence |
| 3 | 71 | M | Anaplastic oligoastrocytoma | 5.17 | 4.34 | 1.06 | 8.15 | Surgery | Recurrence |
| 4 | 66 | F | Anaplastic oligoastrocytoma | 5.58 | 3.38 | 0.84 | 4.94 | Observation (8) D | Recurrence |
| 5 | 44 | F | Anaplastic oligoastrocytoma | 5.59 | 4.02 | 0.87 | 5.12 | Surgery | Recurrence |
| 6 | 59 | M | Gliomatosis cerebri | 2.69 | 2.09 | 1.49 | 7.10 | Observation (4) D | Recurrence |
| 7 | 59 | M | Glioblastoma | 2.93 | 2.69 | 1.71 | 10.06 | Observation (2) D | Recurrence |
| 8 | 71 | F | Glioblastoma | 2.58 | 1.68 | 1.65 | 5.89 | Observation (2) D | Recurrence |
| 9 | 22 | F | Glioblastoma | 5.21 | 3.20 | 3.05 | 11.30 | Surgery | Recurrence |
| 10 | 30 | F | Glioblastoma | 5.50 | 3.11 | 2.27 | 9.08 | Surgery | Recurrence |
| 11 | 35 | M | Glioblastoma | 8.66 | 5.62 | 0.84 | 2.8 | Surgery | Recurrence |
| 12 | 51 | M | Glioblastoma | 5.56 | 4.48 | 1.04 | 6.12 | Surgery | Recurrence |
| 13 | 60 | M | Glioblastoma | 5.28 | 4.29 | 1.78 | 9.37 | Surgery | Recurrence |
| 14 | 55 | F | Glioblastoma | 3.41 | 2.71 | 1.84 | 6.81 | Observation (5) D | Recurrence |
| 15 | 54 | M | Gliosarcoma | 4.45 | 3.62 | 1.64 | 7.45 | Surgery | Recurrence |
| 16 | 52 | F | Anaplastic astrocytoma | 2.61 | 1.72 | 0.55 | 3.67 | Observation (70) S | Necrosis |
| 17 | 39 | M | Anaplastic astrocytoma | 3.54 | 2.47 | 1.84 | 6.57 | Observation (33) S | Necrosis |
| 18 | 69 | F | Glioblastoma | 3.61 | 1.95 | 1.12 | 4.67 | Surgery | Necrosis |
| 19 | 68 | M | Glioblastoma | 2.52 | 1.25 | 0.82 | 3.28 | Observation (10) S | Necrosis |
| 20 | 62 | M | Glioblastoma | 4.64 | 3.07 | 0.76 | 4.00 | Surgery | Necrosis |
| 21 | 61 | F | Glioblastoma | 4.14 | 2.59 | 0.98 | 5.44 | Surgery | Necrosis |
3.2. 11C-MET and 18F-FLT Synthesis and PET Acquisition
3.3. Data Analysis
3.4. Statistical Analysis
4. Conclusion
Acknowledgements
Conflict of Interest
References
- Wallner, K.F.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma and anaplastic astrocytomas. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 140–149. [Google Scholar]
- Burger, P.C. Malignant astrocytic neoplasm: Classification, pathologic anatomy, and response to treatment. Semin. Oncol. 1986, 13, 16–26. [Google Scholar]
- Dooms, G.C.; Hecht, S.; Brant-Zawadzki, M.; Berthiaume, Y.; Norman, D.; Newton, T.H. Brain radiation lesions: MR imaging. Radiology 1986, 158, 149–155. [Google Scholar]
- Herholz, K.; Hölzer, T.; Bauer, B.; Schröder, R.; Voges, J.; Ernestus, R.-I.; Mendoza, G.; Weber-Luxenburger, G.; Löttgen, J.; Thiel, A.; et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 1998, 50, 1316–1322. [Google Scholar]
- Ceyssens, S.; van Laere, K.; de Groot, T.; Goffin, J.; Bormans, G.; Mortelmans, L. [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am. J. Neuroradiol. 2006, 27, 1432–1437. [Google Scholar]
- Kaschten, B.; Stevenaert, A.; Sadzot, B.; Deprez, M.; Degueldre, C.; Fiore, G.D.; Luxen, A.; Reznik, M. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J. Nucl. Med. 1998, 39, 778–785. [Google Scholar]
- Hatakeyama, T.; Kawai, N.; Nishiyama, Y.; Yamamoto, Y.; Sasakawa, Y.; Ichikawa, T.; Tamiya, T. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur. J. Nucl. Med. Mol. I. 2008, 35, 2009–2017. [Google Scholar] [CrossRef]
- Ribom, D.; Eriksson, A.; Hartman, M.; Engler, H.; Nilsson, A.; Långström, B.; Bolander, H.; Bergström, M.; Smits, A. Positron emission tomography 11C-methionine and survival in patients with low-grade gliomas. Cancer 2001, 92, 2541–1549. [Google Scholar]
- van Laere, K.; Ceyssens, S.; van Calenbergh, F.; de Groot, T.; Menten, J.; Falmen, P.; Bormans, G.; Mortelmans, L. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: Sensitivity, inter-observer variability and prognostic value. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 39–51. [Google Scholar] [CrossRef]
- Nariai, T.; Tanaka, Y.; Wakimoto, H.; Aoyagi, M.; Tamaki, M.; Ishikawa, K.; Senda, M.; Ishii, K.; Hirakawa, K.; Ohno, K. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J. Neurosurg. 2005, 103, 498–507. [Google Scholar] [CrossRef]
- Ribom, D.; Engler, H.; Blomquist, E.; Smits, A. Potential significance of 11C-methionine PET as a marker for the radiosensitivity of low grade gliomas. Eur. J. Nucl. Med. 2002, 29, 632–640. [Google Scholar] [CrossRef]
- Nuutinen, J.; Sonninen, P.; Lehikoinen, P.; Sutinen, E.; Valavaara, R.; Eronen, E.; Norrgård, S.; Kulmala, J.; Teräs, M.; Minn, H. Radiotherapy treatment planning and long-term follow-up with [11C]methionine PET in patients with low-grade astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 43–52. [Google Scholar] [CrossRef]
- Galldiks, N.; Kracht, L.W.; Burghaus, L.; Thomas, A.; Jacobs, A.H.; Heiss, W.D.; Herholz, K. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 516–524. [Google Scholar] [CrossRef]
- Tsuyuguchi, N.; Takami, T.; Sunada, I.; Iwai, Y.; Yamanaka, K.; Tanaka, K.; Nishikawa, M.; Ohata, K.; Torii, K.; Morino, M.; et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—In malignant glioma. Ann. Nucl. Med. 2004, 18, 291–296. [Google Scholar] [CrossRef]
- Terakawa, Y.; Tsuyuguchi, N.; Iwai, Y.; Yamanaka, K.; Higashiyama, S.; Takami, T.; Ohata, K. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J. Nucl. Med. 2008, 49, 694–699. [Google Scholar] [CrossRef]
- Ishii, K.; Ogawa, T.; Hatazawa, J.; Kanno, I.; Inugami, A.; Fujita, H.; Shimosegawa, E.; Murakami, M.; Okudera, T.; Uemura, K. High L-methyl-[11C]methionine uptake in brain abscess: A PET study. J. Comput. Assist. Tomogr. 1993, 17, 660–661. [Google Scholar]
- Nakagawa, M.; Nuwabara, Y.; Sasaki, M.; Koga, H.; Chen, T.; Kaneko, O.; Hayashi, K.; Morioka, T.; Masuda, K. 11C-methionine uptake in cerebrovascular disease: A comparison with 18F-fDG PET and 99mTc-HMPOA SPECT. Ann. Nucl. Med. 2002, 16, 207–211. [Google Scholar] [CrossRef]
- Chen, W.; Cloughesy, T.; Kamdar, N.; Satyamurthy, N.; Bergsneider, M.; Liau, L.; Mischel, P.; Czernin, J.; Phelps, M.E.; Silverman, D.H.S. Imaging proliferation in brain tumors with 18F-FLT PET: Comparison with 18F-FDG. J. Nucl. Med. 2005, 46, 945–952. [Google Scholar]
- Choi, S.J.; Kim, J.S.; Kim, J.H.; Oh, S.J.; Lee, J.G.; Kim, C.J.; Ra, Y.S.; Yeo, J.S.; Ryu, J.S.; Moon, D.H. [18F]3'-deoxy-3'-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 653–659. [Google Scholar] [CrossRef]
- Saga, T.; Kawashima, H.; Araki, N.; Takahashi, J.A.; Nakashima, Y.; Higashi, T.; Oya, N.; Murai, T.; Hojo, M.; Hashimoto, N.; et al. Evaluation of primary brain tumors with FLT-PET: Usefulness and limitations. Clin. Nucl. Med. 2006, 31, 774–780. [Google Scholar] [CrossRef]
- Shiels, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef]
- Been, L.B.; Suumeijer, A.J.H.; Cobben, D.C.P.; Jager, P.L.; Hoekstra, H.J.; Elsinga, P.H. [18F]FLT-PET in oncology: Current status and opportunities. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1659–1672. [Google Scholar] [CrossRef]
- Jacobs, A.H.; Thomas, A.; Kracht, L.W.; Li, H.; Dittmar, C.; Garlip, G.; Galldiks, N.; Klein, J.C.; Sobesky, J.; Hilker, R.; et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J. Nucl. Med. 2005, 46, 1948–1958. [Google Scholar]
- Yamamoto, Y.; Wong, T.Z.; Turkington, T.G.; Hawk, T.C.; Reardon, D.A.; Coleman, R.E. 3'-deoxy-3'-[F-18]fluorothymidine positron emission tomography in patients with recurrent glioblastoma multiforme: Comparison with Gd-DTPA enhanced magnetic resonance imaging. Mol. Imaging Biol. 2006, 8, 340–347. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, J.H.; Ra, Y.S.; Kwon, D.H.; Oh, S.J.; Kim, J.S. Diagnostic usefulness of 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography in recurrent brain tumor. J. Comput. Assist. Tomogr. 2011, 35, 679–684. [Google Scholar] [CrossRef]
- Ostergaard, L.; Hochberg, F.H.; Rabinov, J.D.; Sorensen, A.G.; Lev, M.; Kim, L.; Weisskoff, R.M.; Gonzales, R.G.; Gyldensted, G.; Rosen, B.R. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J. Neurosurg. 1999, 90, 300–305. [Google Scholar] [CrossRef]
- Borbély, K.; Nyáry, I.; Tóth, M.; Ericson, K.; Gulyás, B. Optimization of semi-quantification in metabolic PET studies with 18F-fluorodeoxyglucose and 11C-methionine in the determination of malignancy of gliomas. J. Neurol. Sci. 2006, 246, 85–94. [Google Scholar] [CrossRef]
- Ogawa, T.; Hatazawa, J.; Inugami, A.; Murakami, M.; Fujita, H.; Shimosegawa, E.; Noguchi, K.; Okudera, T.; Kanno, I.; Uemura, K.; et al. Carbon-11-methionine PET evaluation of intracerebral hematoma: Distinguishing neoplastic from non-neoplastic hematoma. J. Nucl. Med. 1995, 36, 2175–2179. [Google Scholar]
- Kim, S.; Chung, J.-K.; Im, S.-H.; Jeong, J.M.; Lee, D.S.; Kim, D.G.; Jung, H.W.; Lee, M.C. 11C-methionine PET as a prognostic marker in patients with glioma: Comparison with 18F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 52–59. [Google Scholar] [CrossRef]
- Okubo, S.; Zhen, H.N.; Kawai, N.; Nishiyama, Y.; Haba, R.; Tamiya, T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J. Neurooncol. 2010, 99, 217–225. [Google Scholar] [CrossRef]
- Kracht, L.W.; Friese, M.; Herholz, K.; Schroeder, R.; Bauer, B.; Jacobs, A.; Heiss, W.-D. Methyl-[11C]-L-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 868–873. [Google Scholar] [CrossRef]
- Tsuyuguchi, N.; Takami, T.; Sunada, I.; Iwai, Y.; Yamanaka, K.; Tanaka, K.; NIshikawa, M.; Ohata, K.; Torii, T.; Morino, M.; et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery: In malignant glioma. Ann. Nucl. Med. 2004, 18, 291–296. [Google Scholar] [CrossRef]
- Toyohara, J.; Waki, A.; Takamatsu, S.; Yonekura, Y.; Magata, Y.; Fujibayashi, Y. Basis of FLT as a cell proliferation marker: Comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell-analysis in 22 asynchronously growing tumor cell lines. Nucl. Med. Biol. 2002, 29, 281–287. [Google Scholar] [CrossRef]
- de Reuck, J.; Santens, P.; Goethals, P.; Strijckmans, K.; Lemahieu, I.; Boon, P.; Achten, E.; Lemmerling, M.; Vandekerckhove, T.; Caemaert, J. [Methyl-11C]thymidine positron emission tomography in tumoral and non-tumoral cerebral lesions. Acta Neurol. Belg. 1999, 99, 118–125. [Google Scholar]
- Muzi, M.; Spence, A.M.; O’Sullivan, F.; Mankoff, D.A.; Wells, J.M.; Grierson, J.R.; Link, J.M.; Krohn, K.A. Kinetic analysis of 3'-deoxy-3'-18F-fluorothymidine in patients with gliomas. J. Nucl. Med. 2006, 47, 1612–1621. [Google Scholar]
- Schiepers, C.; Chen, W.; Dahlbom, M.; Cloughesy, T.; Hoh, C.K.; Huang, S.C. 18F-fluorothymidine kinetics of malignant brain tumors. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 4714–4721. [Google Scholar]
- Dandois, V.; Rommel, D.; Renard, L.; Jamart, J.; Cosnard, G. Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas: Preliminary results in clinical practice. J. Neuroradiol. 2010, 34, 89–97. [Google Scholar]
- Machulla, H.J.; Blocher, A.; Kuntzsch, M.; Grierson, J.R. Simplified labeling approach for synthesizing 3'-deoxy-3'-[18F]fluorothymidine ([18F]FLT). J. Radioanal. Nucl. Chem. 2000, 24, 843–846. [Google Scholar]
- Ishiwata, K.; Ido, T.; Vaalburg, W. Increased amounts of D-enantiomer dependent on alkaline concentration in the synthesis of L-[methyl-11C]methionine. Appl. Radiat. Isot. 1998, 39, 311–314. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shishido, H.; Kawai, N.; Miyake, K.; Yamamoto, Y.; Nishiyama, Y.; Tamiya, T. Diagnostic Value of 11C-Methionine (MET) and 18F-Fluorothymidine (FLT) Positron Emission Tomography in Recurrent High-Grade Gliomas; Differentiation from Treatment-Induced Tissue Necrosis. Cancers 2012, 4, 244-256. https://doi.org/10.3390/cancers4010244
Shishido H, Kawai N, Miyake K, Yamamoto Y, Nishiyama Y, Tamiya T. Diagnostic Value of 11C-Methionine (MET) and 18F-Fluorothymidine (FLT) Positron Emission Tomography in Recurrent High-Grade Gliomas; Differentiation from Treatment-Induced Tissue Necrosis. Cancers. 2012; 4(1):244-256. https://doi.org/10.3390/cancers4010244
Chicago/Turabian StyleShishido, Hajime, Nobuyuki Kawai, Keisuke Miyake, Yuka Yamamoto, Yoshihiro Nishiyama, and Takashi Tamiya. 2012. "Diagnostic Value of 11C-Methionine (MET) and 18F-Fluorothymidine (FLT) Positron Emission Tomography in Recurrent High-Grade Gliomas; Differentiation from Treatment-Induced Tissue Necrosis" Cancers 4, no. 1: 244-256. https://doi.org/10.3390/cancers4010244




