Overexpression of Periostin and Lumican in Esophageal Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
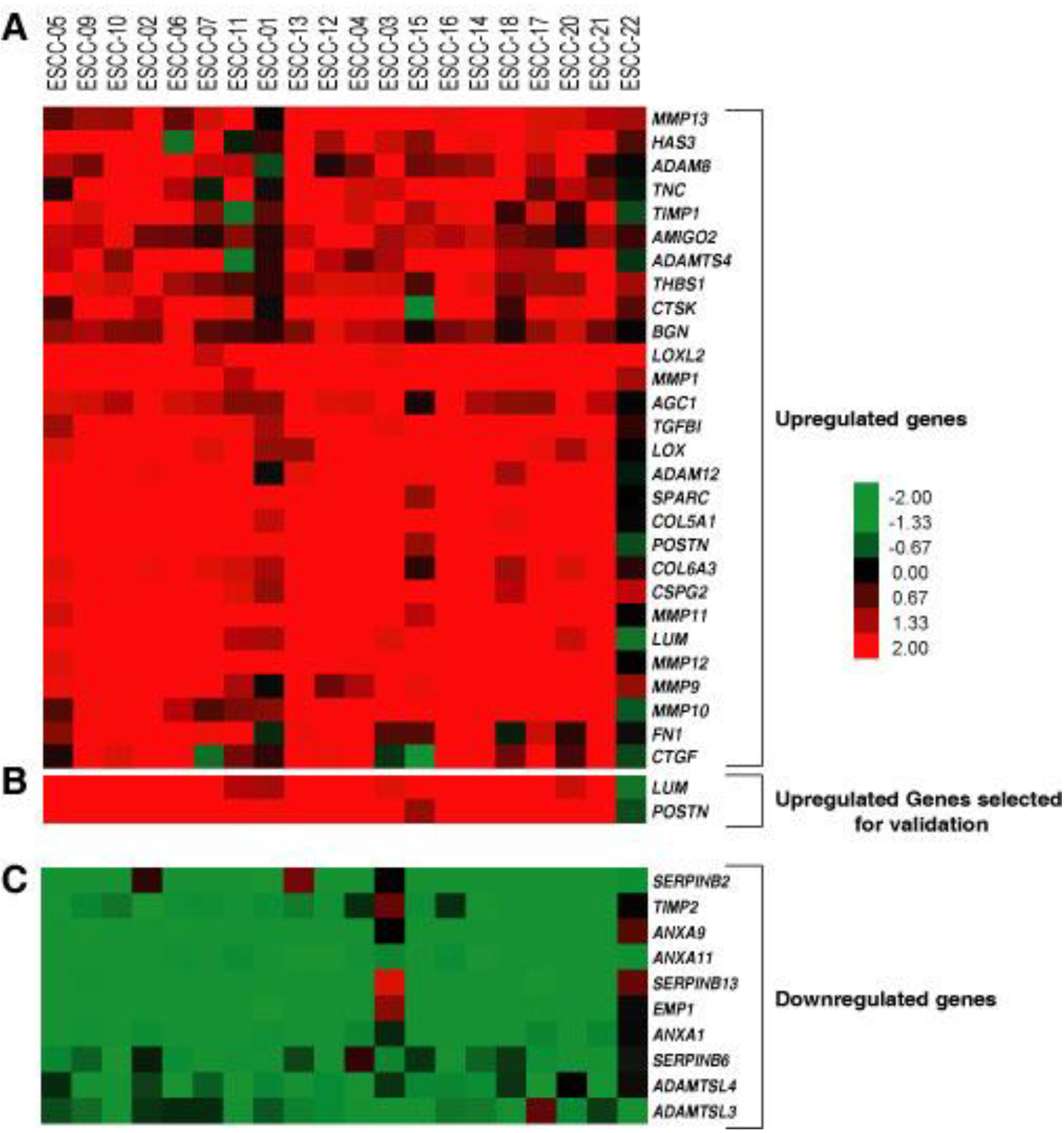
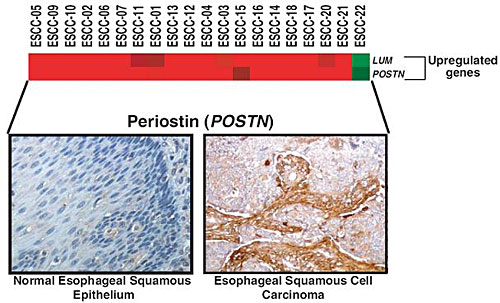
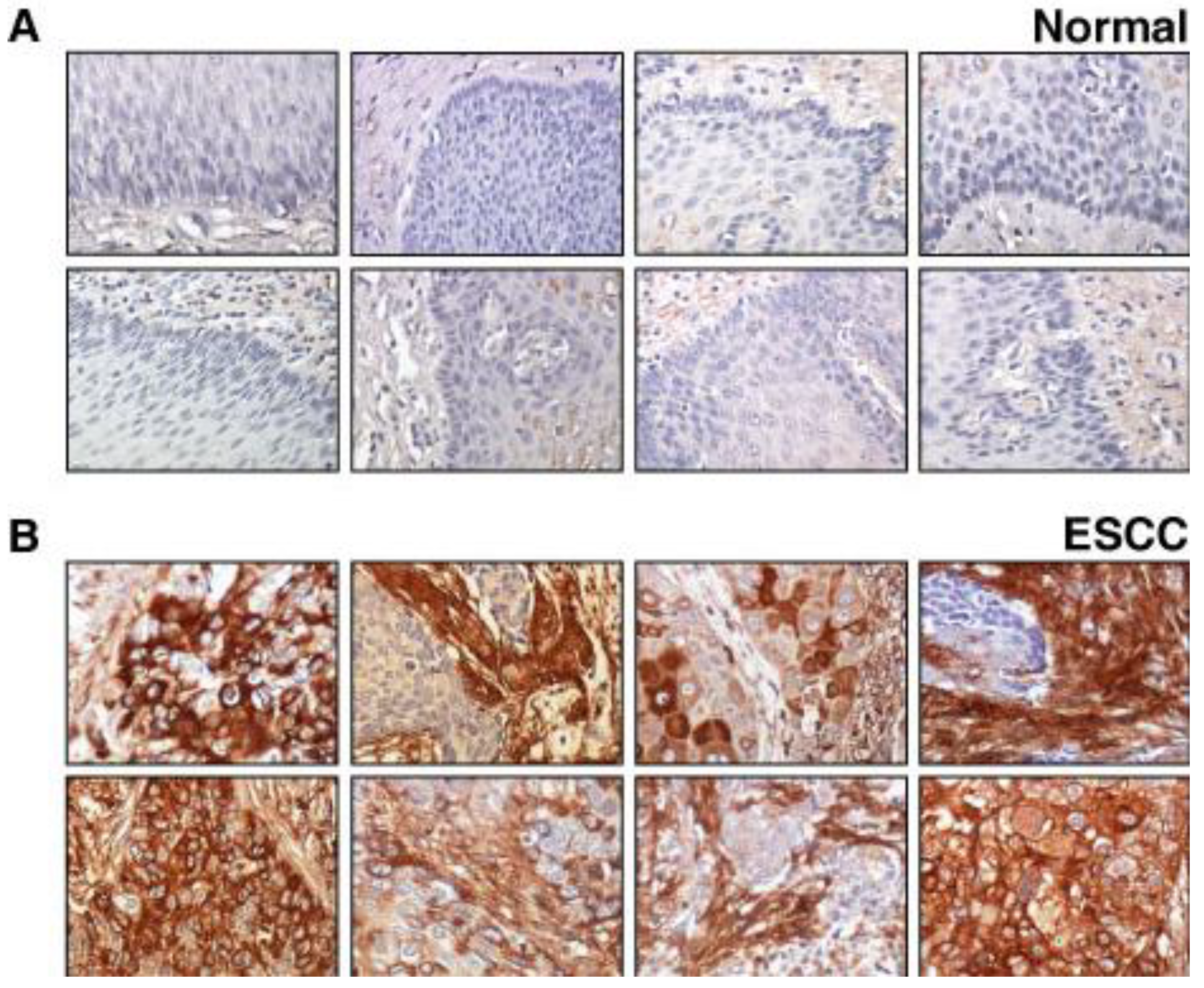
2.1. Validation of Periostin Overexpression in ESCC

2.2. Validation of Lumican Overexpression in ESCC
| Parameters | Periostin | Lumican | |
|---|---|---|---|
| 1 | Number of cases tested | 137 | 137 |
| 2 | Number of positive cases | 137 | 137 |
| 3 | Number of cases with positive staining of carcinoma cells alone | 15 | 19 |
| 4 | Number of cases with positive staining of stroma alone | 23 | 24 |
| 5 | Number of cases with positive staining of both carcinoma and stromal compartments | 99 | 94 |

3. Material and Methods
3.1. DNA Microarray Analysis
3.2. Tissue Samples
3.3. Immunohistochemical Staining
4. Conclusions
Acknowledgements
References
- Stoner, G.D.; Gupta, A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis 2001, 22, 1737–1746. [Google Scholar] [CrossRef]
- Kashyap, M.K.; Marimuthu, A.; Kishore, C.J.; Peri, S.; Keerthikumar, S.; Prasad, T.S.; Mahmood, R.; Rao, S.; Ranganathan, P.; Sanjeeviah, R.C.; Vijayakumar, M.; Kumar, K.V.; Montgomery, E.A.; Kumar, R.V.; Pandey, A. Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer Biol. Ther. 2009, 8, 36–46. [Google Scholar]
- Kandasamy, K.; Keerthikumar, S.; Goel, R.; Mathivanan, S.; Patankar, N.; Shafreen, B.; Renuse, S.; Pawar, H.; Ramachandra, Y.L.; Acharya, P.K.; Ranganathan, P.; Chaerkady, R.; Keshava Prasad, T.S.; Pandey, A. Human Proteinpedia: a unified discovery resource for proteomics research. Nucleic. Acids. Res. 2009, 37, D773–D781. [Google Scholar] [CrossRef]
- Hamilton, D.W. Functional role of periostin in development and wound repair: implications for connective tissue disease. J. Cell Commun. Signal. 2008, 2, 9–17. [Google Scholar] [CrossRef]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 1999, 14, 1239–1249. [Google Scholar] [CrossRef]
- Guarino, M.; Rubino, B.; Ballabio, G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007, 39, 305–318. [Google Scholar] [CrossRef]
- Chen, R.; Yi, E.C.; Donohoe, S.; Pan, S.; Eng, J.; Cooke, K.; Crispin, D.A.; Lane, Z.; Goodlett, D.R.; Bronner, M.P.; Aebersold, R.; Brentnall, T.A. Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology 2005, 129, 1187–1197. [Google Scholar] [CrossRef]
- Baril, P.; Gangeswaran, R.; Mahon, P.C.; Caulee, K.; Kocher, H.M.; Harada, T.; Zhu, M.; Kalthoff, H.; Crnogorac-Jurcevic, T.; Lemoine, N.R. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene 2007, 26, 2082–2094. [Google Scholar] [CrossRef]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Hao, Y.; Triadafilopoulos, G.; Sahbaie, P.; Young, H.S.; Omary, M.B.; Lowe, A.W. Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology 2006, 131, 925–933. [Google Scholar] [CrossRef]
- Blanchard, C.; Mingler, M.K.; McBride, M.; Putnam, P.E.; Collins, M.H.; Chang, G.; Stringer, K.; Abonia, J.P.; Molkentin, J.D.; Rothenberg, M.E. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal. Immunol. 2008, 1, 289–296. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009, 137, 1238–1249. [Google Scholar] [CrossRef]
- Blanchard, C.; Wang, N.; Stringer, K.F.; Mishra, A.; Fulkerson, P.C.; Abonia, J.P.; Jameson, S.C.; Kirby, C.; Konikoff, M.R.; Collins, M.H.; Cohen, M.B.; Akers, R.; Hogan, S.P.; Assa'ad, A.H.; Putnam, P.E.; Aronow, B.J.; Rothenberg, M.E. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Invest. 2006, 116, 536–547. [Google Scholar] [CrossRef]
- Puglisi, F.; Puppin, C.; Pegolo, E.; Andreetta, C.; Pascoletti, G.; D'Aurizio, F.; Pandolfi, M.; Fasola, G.; Piga, A.; Damante, G.; Di Loreto, C. Expression of periostin in human breast cancer. J. Clin. Pathol. 2008, 61, 494–498. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kashyap, M.K.; Marimuthu, A.; Peri, S.; Kumar, G.S.S.; Jacob, H.K.C.; Prasad, T.S.K.; Mahmood, R.; Kumar, K.V.V.; Kumar, M.V.; Meltzer, S.J.; et al. Overexpression of Periostin and Lumican in Esophageal Squamous Cell Carcinoma. Cancers 2010, 2, 133-142. https://doi.org/10.3390/cancers2010133
Kashyap MK, Marimuthu A, Peri S, Kumar GSS, Jacob HKC, Prasad TSK, Mahmood R, Kumar KVV, Kumar MV, Meltzer SJ, et al. Overexpression of Periostin and Lumican in Esophageal Squamous Cell Carcinoma. Cancers. 2010; 2(1):133-142. https://doi.org/10.3390/cancers2010133
Chicago/Turabian StyleKashyap, Manoj Kumar, Arivusudar Marimuthu, Suraj Peri, Ghantasala S. Sameer Kumar, Harrys K.C. Jacob, Thottethodi Subrahmanya Keshava Prasad, Riaz Mahmood, K. V. Veerendra Kumar, M. Vijaya Kumar, Stephen J. Meltzer, and et al. 2010. "Overexpression of Periostin and Lumican in Esophageal Squamous Cell Carcinoma" Cancers 2, no. 1: 133-142. https://doi.org/10.3390/cancers2010133