Immune Response against ALK in Children with ALK-Positive Anaplastic Large Cell Lymphoma
Abstract
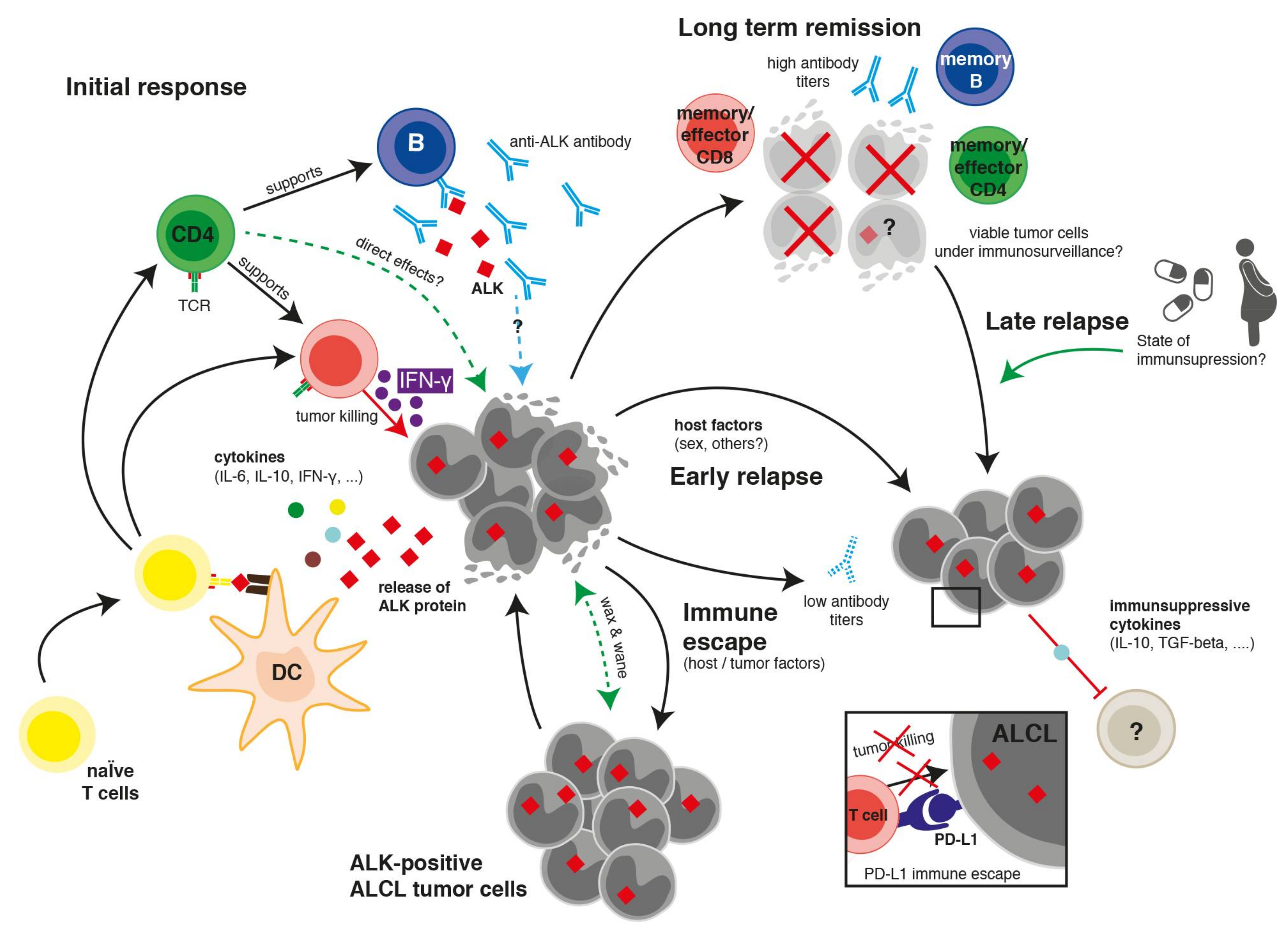
:1. Introduction
2. Clinical, Laboratory, and Pathological Hints towards an Immune Reaction against ALCL
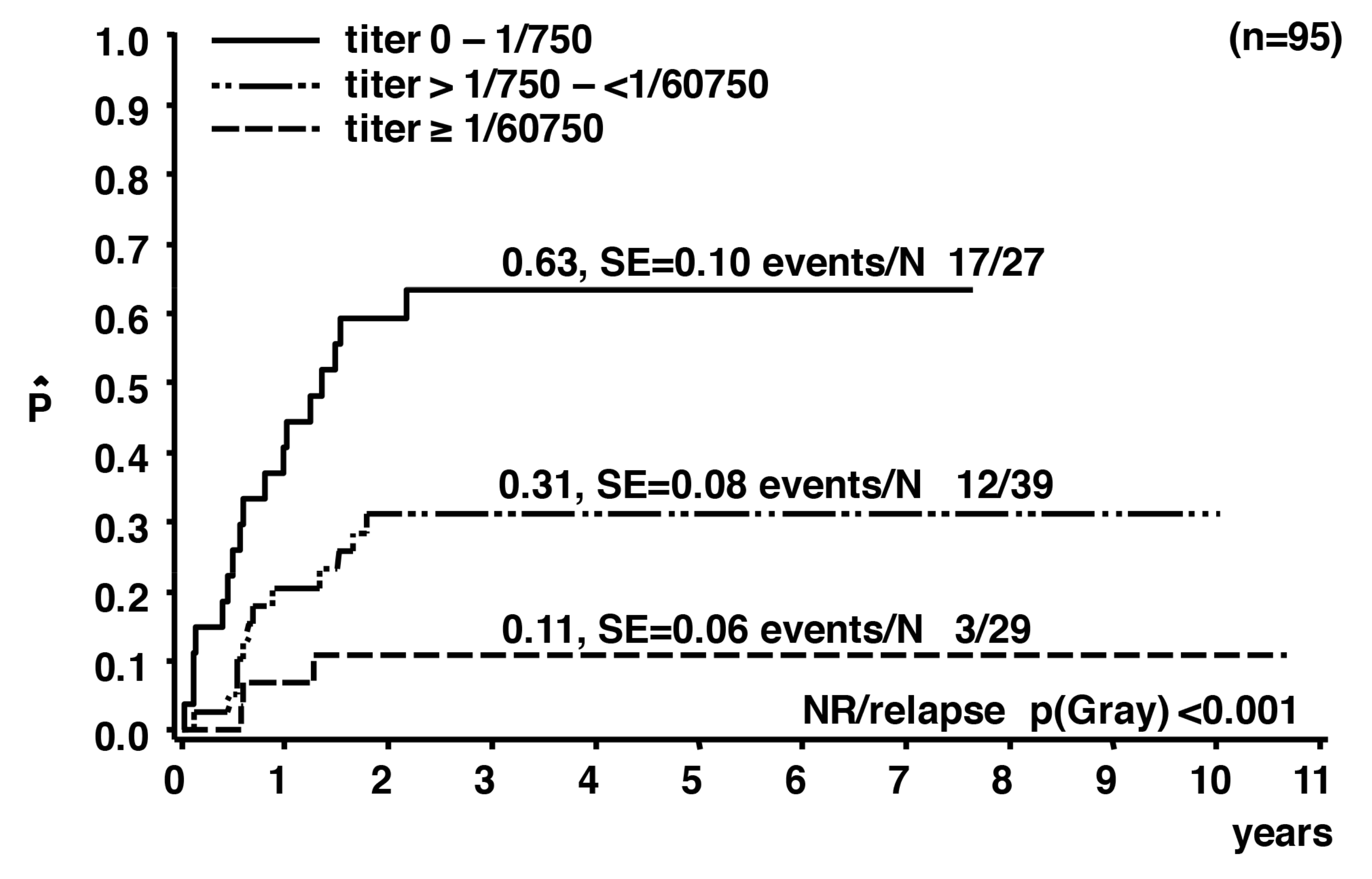
3. Humoral Immune Response against ALK
4. Cellular Immune Response against ALK
4.1. CD8 T Cell Response against ALK
4.2. CD4 T Cell Response against ALK
5. Immune Escape Mechanisms
6. Therapeutic Implications
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Falini, B.; Pileri, S.; Zinzani, P.L.; Carbone, A.; Zagonel, V.; Wolf-Peeters, C.; Verhoef, G.; Menestrina, F.; Todeschini, G.; Paulli, M.; et al. ALK+ lymphoma: Clinico-pathological findings and outcome. Blood 1999, 93, 2697–2706. [Google Scholar] [PubMed]
- Burkhardt, B.; Zimmermann, M.; Oschlies, I.; Niggli, F.; Mann, G.; Parwaresch, R.; Riehm, H.; Schrappe, M.; Reiter, A.; Group, B.F.M. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br. J. Haematol. 2005, 131, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, npm, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.L.; Pickering, D.; Lowe, E.J.; Zwick, D.; Abromowitch, M.; Davenport, G.; Cairo, M.S.; Sanger, W.G. Childhood anaplastic large cell lymphoma has a high incidence of ALK gene rearrangement as determined by immunohistochemical staining and fluorescent in situ hybridisation: A genetic and pathological correlation. Br. J. Haematol. 2005, 131, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Damm-Welk, C.; Klapper, W.; Oschlies, I.; Gesk, S.; Rottgers, S.; Bradtke, J.; Siebert, R.; Reiter, A.; Woessmann, W. Distribution of NPM1-ALK and x-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: A molecular-histological correlation. Br. J. Haematol. 2009, 146, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Morris, S.W.; Turturro, F. Anaplastic lymphoma kinase proteins in growth control and cancer. J. Cell. Physiol. 2004, 199, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Chiarle, R.; Voena, C.; Ambrogio, C.; Piva, R.; Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer 2008, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.T.; Zhao, C.; Zhang, Q.; Wasik, M.A. Nucleophosmin-anaplastic lymphoma kinase: The ultimate oncogene and therapeutic target. Blood 2017, 129, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Schrappe, M.; Tiemann, M.; Parwaresch, R.; Zimmermann, M.; Yakisan, E.; Dopfer, R.; Bucsky, P.; Mann, G.; Gadner, H.; et al. Successful treatment strategy for ki-1 anaplastic large-cell lymphoma of childhood: A prospective analysis of 62 patients enrolled in three consecutive berlin-frankfurt-munster group studies. J. Clin. Oncol. 1994, 12, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Brugieres, L.; Deley, M.C.; Pacquement, H.; Meguerian-Bedoyan, Z.; Terrier-Lacombe, M.J.; Robert, A.; Pondarre, C.; Leverger, G.; Devalck, C.; Rodary, C.; et al. Cd30(+) anaplastic large-cell lymphoma in children: Analysis of 82 patients enrolled in two consecutive studies of the French society of pediatric oncology. Blood 1998, 92, 3591–3598. [Google Scholar] [PubMed]
- Seidemann, K.; Tiemann, M.; Schrappe, M.; Yakisan, E.; Simonitsch, I.; Janka-Schaub, G.; Dorffel, W.; Zimmermann, M.; Mann, G.; Gadner, H.; et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: A report of the Berlin-Frankfurt-Munster group trial NHL-BFM 90. Blood 2001, 97, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Hobson, R.; Imeson, J.; Gerrard, M.; McCarthy, K.; Pinkerton, C.R.; United Kingdom Children’s Cancer Study Group. Anaplastic large cell lymphoma in childhood: Analysis of 72 patients treated on the United Kingdom children’s cancer study group chemotherapy regimens. Br. J. Haematol. 2002, 117, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kiyokawa, N.; Shimada, H.; Miyauchi, J.; Fujimoto, J. Anaplastic large cell lymphoma in Japanese children: Retrospective analysis of 34 patients diagnosed at the national research institute for child health and development. Br. J. Haematol. 2003, 121, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Rosolen, A.; Pillon, M.; Garaventa, A.; Burnelli, R.; d’Amore, E.S.; Giuliano, M.; Comis, M.; Cesaro, S.; Tettoni, K.; Moleti, M.L.; et al. Anaplastic large cell lymphoma treated with a leukemia-like therapy: Report of the Italian association of pediatric hematology and oncology (aieop) LNH-92 protocol. Cancer 2005, 104, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.J.; Sposto, R.; Perkins, S.L.; Gross, T.G.; Finlay, J.; Zwick, D.; Abromowitch, M.; Children’s Cancer Group Study. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: Final results of children’s cancer group study 5941. Pediatr. Blood Cancer 2009, 52, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Brugieres, L.; Le Deley, M.C.; Rosolen, A.; Williams, D.; Horibe, K.; Wrobel, G.; Mann, G.; Zsiros, J.; Uyttebroeck, A.; Marky, I.; et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: Results of a randomized trial of the eicnhl group. J. Clin. Oncol. 2009, 27, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Kraveka, J.M.; Weitzman, S.; Lowe, E.; Smith, L.; Lynch, J.C.; Chang, M.; Kinney, M.C.; Perkins, S.L.; Laver, J.; et al. Advanced stage anaplastic large cell lymphoma in children and adolescents: Results of ANHL0131, a randomized phase III trial of apo versus a modified regimen with vinblastine: A report from the children’s oncology group. Pediatr. Blood Cancer 2014, 61, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Le Deley, M.C.; Rosolen, A.; Williams, D.M.; Horibe, K.; Wrobel, G.; Attarbaschi, A.; Zsiros, J.; Uyttebroeck, A.; Marky, I.M.; Lamant, L.; et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: Results of the randomized ALCL99-vinblastine trial. J. Clin. Oncol. 2010, 28, 3987–3993. [Google Scholar] [CrossRef] [PubMed]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase ii study. J. Clin. Oncol. 2012, 30, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Mosse, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A children’s oncology group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Voss, S.D.; Lim, M.S.; Rolland, D.; Minard, C.G.; Fox, E.; Adamson, P.; Wilner, K.; Blaney, S.M.; Weigel, B.J. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A children’s oncology group study. J. Clin. Oncol. 2017, 35, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti Passerini, C.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Brugieres, L.; Quartier, P.; Le Deley, M.C.; Pacquement, H.; Perel, Y.; Bergeron, C.; Schmitt, C.; Landmann, J.; Patte, C.; Terrier-Lacombe, M.J.; et al. Relapses of childhood anaplastic large-cell lymphoma: Treatment results in a series of 41 children—A report from the french society of pediatric oncology. Ann. Oncol. 2000, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Takimoto, T.; Katano, N.; Kikuchi, A.; Tabuchi, K.; Kobayashi, R.; Ayukawa, H.; Kumagai, M.A.; Horibe, K.; Tsurusawa, M. Recurrent childhood anaplastic large cell lymphoma: A retrospective analysis of registered cases in Japan. Br. J. Haematol. 2006, 132, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Zimmermann, M.; Lenhard, M.; Burkhardt, B.; Rossig, C.; Kremens, B.; Lang, P.; Attarbaschi, A.; Mann, G.; Oschlies, I.; et al. Relapsed or refractory anaplastic large-cell lymphoma in children and adolescents after berlin-frankfurt-muenster (BFM)-type first-line therapy: A bfm-group study. J. Clin. Oncol. 2011, 29, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Ruf, S.; Brugieres, L.; Pillon, M.; Zimmermann, M.; Attarbaschi, A.; Mellgren, K.; Williams, D.; Uyttebroeck, A.; Wrobel, G.; Reiter, A.; et al. Risk-adapted therapy for patients with relapsed or refractory ALCL—final report of the prospective ALCL-relapse trial of the eicnhl. Br. J. Haematol. 2015, 171, 45. [Google Scholar]
- Iwahara, T.; Fujimoto, J.; Wen, D.; Cupples, R.; Bucay, N.; Arakawa, T.; Mori, S.; Ratzkin, B.; Yamamoto, T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 1997, 14, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Department of Pediatric Hematology and Oncology, Justus-Liebig University, D-35392 Giessen, Germany. Unpublished work. 2018.
- Woessmann, W.; Peters, C.; Lenhard, M.; Burkhardt, B.; Sykora, K.W.; Dilloo, D.; Kremens, B.; Lang, P.; Fuhrer, M.; Kuhne, T.; et al. Allogeneic haematopoietic stem cell transplantation in relapsed or refractory anaplastic large cell lymphoma of children and adolescents—A Berlin-Frankfurt-Munster group report. Br. J. Haematol. 2006, 133, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.G.; Hale, G.A.; He, W.; Camitta, B.M.; Sanders, J.E.; Cairo, M.S.; Hayashi, R.J.; Termuhlen, A.M.; Zhang, M.J.; Davies, S.M.; et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol. Blood Marrow Transplant. 2010, 16, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Strullu, M.; Thomas, C.; Le Deley, M.C.; Chevance, A.; Kanold, J.; Bertrand, Y.; Jubert, C.; Dalle, J.H.; Paillard, C.; Baruchel, A.; et al. Hematopoietic stem cell transplantation in relapsed ALK+ anaplastic large cell lymphoma in children and adolescents: A study on behalf of the sfce and SFGM-TC. Bone Marrow Transplant. 2015, 50, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Fukano, R.; Mori, T.; Kobayashi, R.; Mitsui, T.; Fujita, N.; Iwasaki, F.; Suzumiya, J.; Chin, M.; Goto, H.; Takahashi, Y.; et al. Haematopoietic stem cell transplantation for relapsed or refractory anaplastic large cell lymphoma: A study of children and adolescents in Japan. Br. J. Haematol. 2015, 168, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Lamant, L.; McCarthy, K.; d’Amore, E.; Klapper, W.; Nakagawa, A.; Fraga, M.; Maldyk, J.; Simonitsch-Klupp, I.; Oschlies, I.; Delsol, G.; et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: Results of the ALCL99 study. J. Clin. Oncol. 2011, 29, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Abramov, D.; Oschlies, I.; Zimmermann, M.; Konovalov, D.; Damm-Welk, C.; Wossmann, W.; Klapper, W. Expression of CD8 is associated with non-common type morphology and outcome in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Haematologica 2013, 98, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Damm-Welk, C.; Busch, K.; Burkhardt, B.; Schieferstein, J.; Viehmann, S.; Oschlies, I.; Klapper, W.; Zimmermann, M.; Harbott, J.; Reiter, A.; et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative pcr in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood 2007, 110, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Savan, R.; McFarland, A.P.; Reynolds, D.A.; Feigenbaum, L.; Ramakrishnan, K.; Karwan, M.; Shirota, H.; Klinman, D.M.; Dunleavy, K.; Pittaluga, S.; et al. A novel role for IL-22R1 as a driver of inflammation. Blood 2011, 117, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, K.; Hedegaard, C.J.; Schmiegelow, K.; Muller, K. Plasma cytokine profiles at diagnosis in pediatric patients with non-Hodgkin lymphoma. J. Pediatr. Hematol. Oncol. 2012, 34, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Knorr, F.; Damm-Welk, C.; Ruf, S.; Singh, V.K.; Zimmermann, M.; Reiter, A.; Woessmann, W. Blood cytokine concentrations of pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma patients. Haematologica 2017. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, C.; Minard-Colin, V.; Saada, V.; Lamant, L.; Delsol, G.; Patte, C.; Le Deley, M.C.; Valteau-Couanet, D.; Brugieres, L. Clinical analysis and prognostic significance of haemophagocytic lymphohistiocytosis-associated anaplastic large cell lymphoma in children. Br. J. Haematol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X.; Song, W.; Fang, Y.; Yu, L.; Liu, S.; Churilov, L.P.; Zhang, F. The roles and applications of autoantibodies in progression, diagnosis, treatment and prognosis of human malignant tumours. Autoimmun. Rev. 2017, 16, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Zaenker, P.; Gray, E.S.; Ziman, M.R. Autoantibody production in cancer—The humoral immune response toward autologous antigens in cancer patients. Autoimmun. Rev. 2016, 15, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The emerging role of b cells in tumor immunity. Cancer Res. 2016, 76, 5597–5601. [Google Scholar] [CrossRef] [PubMed]
- Pulford, K.; Falini, B.; Banham, A.H.; Codrington, D.; Roberton, H.; Hatton, C.; Mason, D.Y. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood 2000, 96, 1605–1607. [Google Scholar] [PubMed]
- Ait-Tahar, K.; Cerundolo, V.; Banham, A.H.; Hatton, C.; Blanchard, T.; Kusec, R.; Becker, M.; Smith, G.L.; Pulford, K. B and CTL responses to the ALK protein in patients with ALK-positive ALCL. Int. J. Cancer 2006, 118, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Bonvini, P.; Ait-Tahar, K.; Pillon, M.; Tridello, G.; Buffardi, S.; Lombardi, A.; Pulford, K.; Rosolen, A. Kinetics of humoral response to ALK and its relationship with minimal residual disease in pediatric ALCL. Leukemia 2009, 23, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Ait-Tahar, K.; Damm-Welk, C.; Burkhardt, B.; Zimmermann, M.; Klapper, W.; Reiter, A.; Pulford, K.; Woessmann, W. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood 2010, 115, 3314–3319. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Pillon, M.; Zimmermann, M.; Carraro, E.; Basso, G.; Knoerr, F.; Woessmann, W.; Damm-Welk, C. Course of anti-ALK antibody titres during chemotherapy in children with anaplastic large cell lymphoma. Br. J. Haematol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Damm-Welk, C.; Pillon, M.; Zimmermann, M.; Franceschetto, G.; Pulford, K.; Reiter, A.; Rosolen, A.; Woessmann, W. Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia 2013, 27, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Damm-Welk, C.; Siddiqi, F.; Fischer, M.; Hero, B.; Narayanan, V.; Camidge, D.R.; Harris, M.; Burke, A.; Lehrnbecher, T.; Pulford, K.; et al. Anti-ALK antibodies in patients with ALK-positive malignancies not expressing NPM-ALK. J. Cancer 2016, 7, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Mastini, C.; Blasco, R.B.; Mologni, L.; Voena, C.; Mussolin, L.; Mach, S.L.; Adeni, A.E.; Lydon, C.A.; Sholl, L.M.; et al. Epitope mapping of spontaneous autoantibodies to anaplastic lymphoma kinase (ALK) in non-small cell lung cancer. Oncotarget 2017, 8, 92265–92274. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.B.; Makary, E.; Schiffman, K.; Goodell, V.; Disis, M.L. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005, 65, 650–656. [Google Scholar] [PubMed]
- Chiarle, R.; Martinengo, C.; Mastini, C.; Ambrogio, C.; D’Escamard, V.; Forni, G.; Inghirami, G. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat. Med. 2008, 14, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Passoni, L.; Scardino, A.; Bertazzoli, C.; Gallo, B.; Coluccia, A.M.; Lemonnier, F.A.; Kosmatopoulos, K.; Gambacorti-Passerini, C. ALK as a novel lymphoma-associated tumor antigen: Identification of 2 HLA-A2.1-restricted CD8+ T-cell epitopes. Blood 2002, 99, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Passoni, L.; Gallo, B.; Biganzoli, E.; Stefanoni, R.; Massimino, M.; Di Nicola, M.; Gianni, A.M.; Gambacorti-Passerini, C. In vivo t-cell immune response against anaplastic lymphoma kinase in patients with anaplastic large cell lymphomas. Haematologica 2006, 91, 48–55. [Google Scholar] [PubMed]
- Beckhove, P.; Feuerer, M.; Dolenc, M.; Schuetz, F.; Choi, C.; Sommerfeldt, N.; Schwendemann, J.; Ehlert, K.; Altevogt, P.; Bastert, G.; et al. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J. Clin. Investig. 2004, 114, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martinez-Cano, S.; Mejias-Perez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) t cells. Nat. Commun. 2017, 8, 16073. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Roussel, H.; Diniz, M.O.; Karaki, S.; Tran, T.; Voron, T.; Dransart, E.; Sandoval, F.; Riquet, M.; Rance, B.; et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 2017, 8, 15221. [Google Scholar] [CrossRef] [PubMed]
- Voena, C.; Menotti, M.; Mastini, C.; Di Giacomo, F.; Longo, D.L.; Castella, B.; Merlo, M.E.B.; Ambrogio, C.; Wang, Q.; Minero, V.G.; et al. Efficacy of a cancer vaccine against ALK-rearranged lung tumors. Cancer Immunol. Res. 2015, 3, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Werner, S.; Hackstein, H.; Lennerz, V.; Reiter, A.; Wolfel, T.; Damm-Welk, C.; Woessmann, W. Analysis of nucleophosmin-anaplastic lymphoma kinase (NPM-ALK)-reactive CD8(+) t cell responses in children with NPM-ALK(+) anaplastic large cell lymphoma. Clin. Exp. Immunol. 2016, 186, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.; Lemmens, E.E.; Wolfe, T.; Christen, U.; von Herrath, M.G.; Schoenberger, S.P. Cd4+ t cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003, 421, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. A brief history of t cell help to b cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ait-Tahar, K.; Barnardo, M.C.; Pulford, K. CD4 T-helper responses to the anaplastic lymphoma kinase (ALK) protein in patients with ALK-positive anaplastic large-cell lymphoma. Cancer Res. 2007, 67, 1898–1901. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Coukos, G. Deciphering and reversing tumor immune suppression. Immunity 2013, 39, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar] [CrossRef] [PubMed]
- Andorsky, D.J.; Yamada, R.E.; Said, J.; Pinkus, G.S.; Betting, D.J.; Timmerman, J.M. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. 2011, 17, 4232–4244. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycka, M.; Marzec, M.; Liu, X.; Zhang, Q.; Wasik, M.A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the t regulatory cell phenotype by activating stat3. Proc. Natl. Acad. Sci. USA 2006, 103, 9964–9969. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, J.; Bourgeois-Daigneault, M.C.; Huppe, G.; Tremblay, J.; Aumont, A.; Houde, M.; Bartee, E.; Brunet, A.; Gauvreau, M.E.; de Gassart, A.; et al. Interleukin-10-induced march1 mediates intracellular sequestration of mhc class ii in monocytes. Eur. J. Immunol. 2008, 38, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, G.; Shevach, E.M. Antigen-specific induced t regulatory cells impair dendritic cell function via an IL-10/march1-dependent mechanism. J. Immunol. 2013, 191, 5875–5884. [Google Scholar] [CrossRef] [PubMed]
- Tze, L.E.; Horikawa, K.; Domaschenz, H.; Howard, D.R.; Roots, C.M.; Rigby, R.J.; Way, D.A.; Ohmura-Hoshino, M.; Ishido, S.; Andoniou, C.E.; et al. CD83 increases mhc ii and CD86 on dendritic cells by opposing IL-10-driven march1-mediated ubiquitination and degradation. J. Exp. Med. 2011, 208, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Department of Pediatric Hematology and Oncology, Justus-Liebig University, D-35392 Giessen, Germany. Unpublished work. 2018.
- Zitvogel, L.; Kepp, O.; Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 2011, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsushima, H.; Mizumoto, N.; Takashima, A. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009, 69, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsushima, H.; Nishibu, A.; Clausen, B.E.; Takashima, A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009, 69, 6987–6994. [Google Scholar] [CrossRef] [PubMed]
- Brugieres, L.; Pacquement, H.; Le Deley, M.C.; Leverger, G.; Lutz, P.; Paillard, C.; Baruchel, A.; Frappaz, D.; Nelken, B.; Lamant, L.; et al. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: A report from the french society of pediatric oncology. J. Clin. Oncol. 2009, 27, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Hebart, H.; Lang, P.; Woessmann, W. Nivolumab for refractory anaplastic large cell lymphoma: A case report. Ann. Intern Med. 2016, 165, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, C.; Abbou, S.; Minard-Colin, V.; Geoerger, B.; Scoazec, J.Y.; Vassal, G.; Jaff, N.; Heuberger, L.; Valteau-Couanet, D.; Brugieres, L. Efficacy of nivolumab in a patient with systemic refractory ALK+ anaplastic large cell lymphoma. Pediatr. Blood Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.A.; Gorgens, A.; Chmielewski, M.; Murke, F.; Kimpel, J.; Giebel, B.; Abken, H. Superior therapeutic index in lymphoma therapy: CD30(+) CD34(+) hematopoietic stem cells resist a chimeric antigen receptor T-cell attack. Mol. Ther. 2016, 24, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Majzner, R.G.; Zhang, L.; Wanhainen, K.; Long, A.H.; Nguyen, S.M.; Lopomo, P.; Vigny, M.; Fry, T.J.; Orentas, R.J.; et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 2017, 25, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V.; Fraietta, J.A.; Levine, B.L.; Kalos, M.; Zhao, Y.; June, C.H. Adoptive immunotherapy for cancer or viruses. Annu. Rev. Immunol. 2014, 32, 189–225. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadler, S.; Singh, V.K.; Knörr, F.; Damm-Welk, C.; Woessmann, W. Immune Response against ALK in Children with ALK-Positive Anaplastic Large Cell Lymphoma. Cancers 2018, 10, 114. https://doi.org/10.3390/cancers10040114
Stadler S, Singh VK, Knörr F, Damm-Welk C, Woessmann W. Immune Response against ALK in Children with ALK-Positive Anaplastic Large Cell Lymphoma. Cancers. 2018; 10(4):114. https://doi.org/10.3390/cancers10040114
Chicago/Turabian StyleStadler, Serena, Vijay Kumar Singh, Fabian Knörr, Christine Damm-Welk, and Wilhelm Woessmann. 2018. "Immune Response against ALK in Children with ALK-Positive Anaplastic Large Cell Lymphoma" Cancers 10, no. 4: 114. https://doi.org/10.3390/cancers10040114



