Scalable, Modular Three-Dimensional Silicon Microelectrode Assembly via Electroless Plating
Abstract
:1. Introduction
1.1. Overview of 3-D Approaches
1.2. Creating a Scalable 3-D Probe Design
1.3. Scope of the Design
2. Materials and Methods
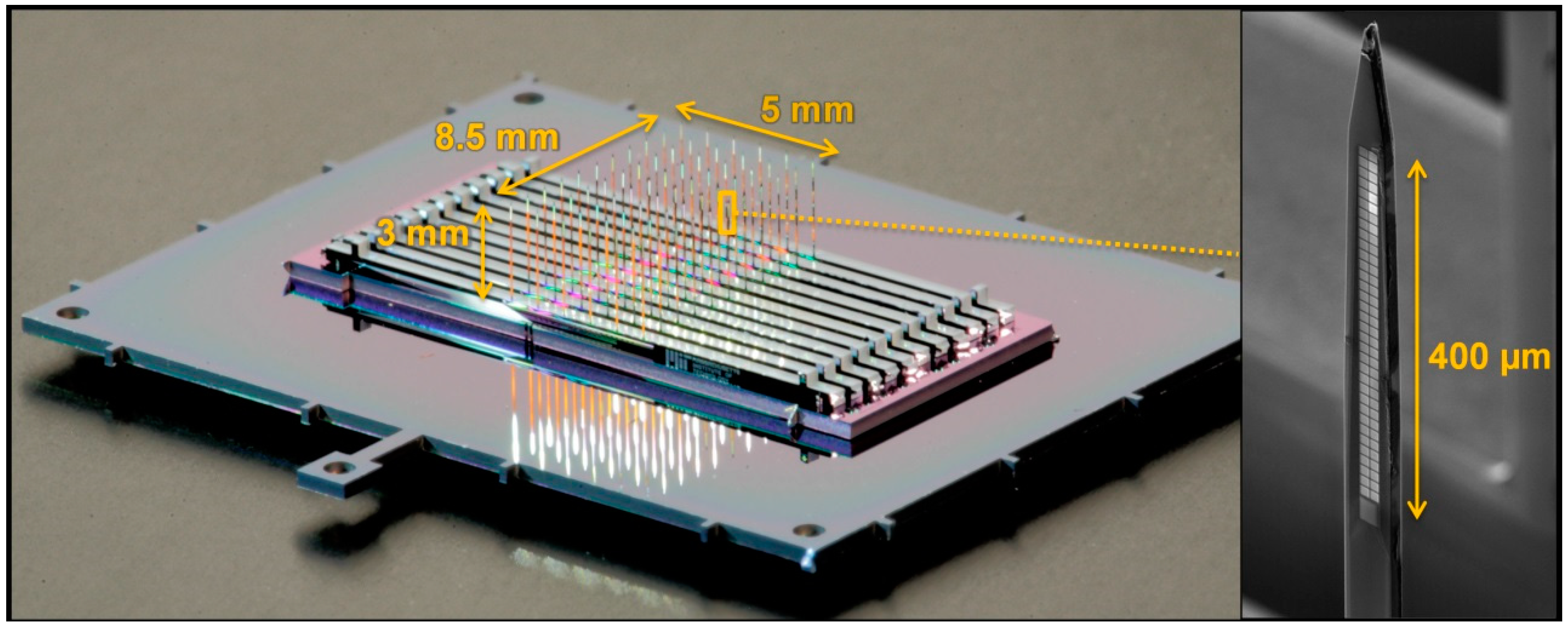
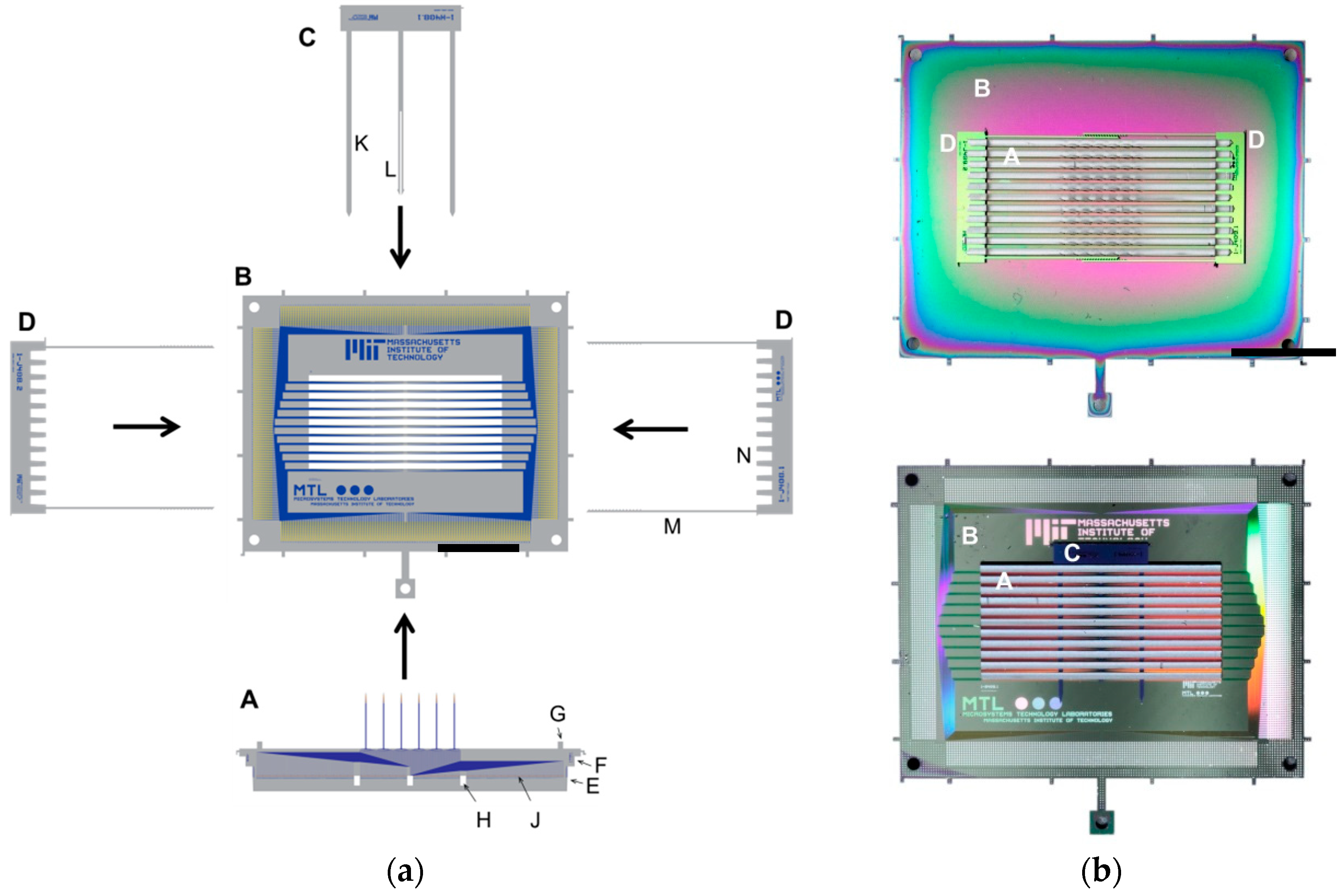
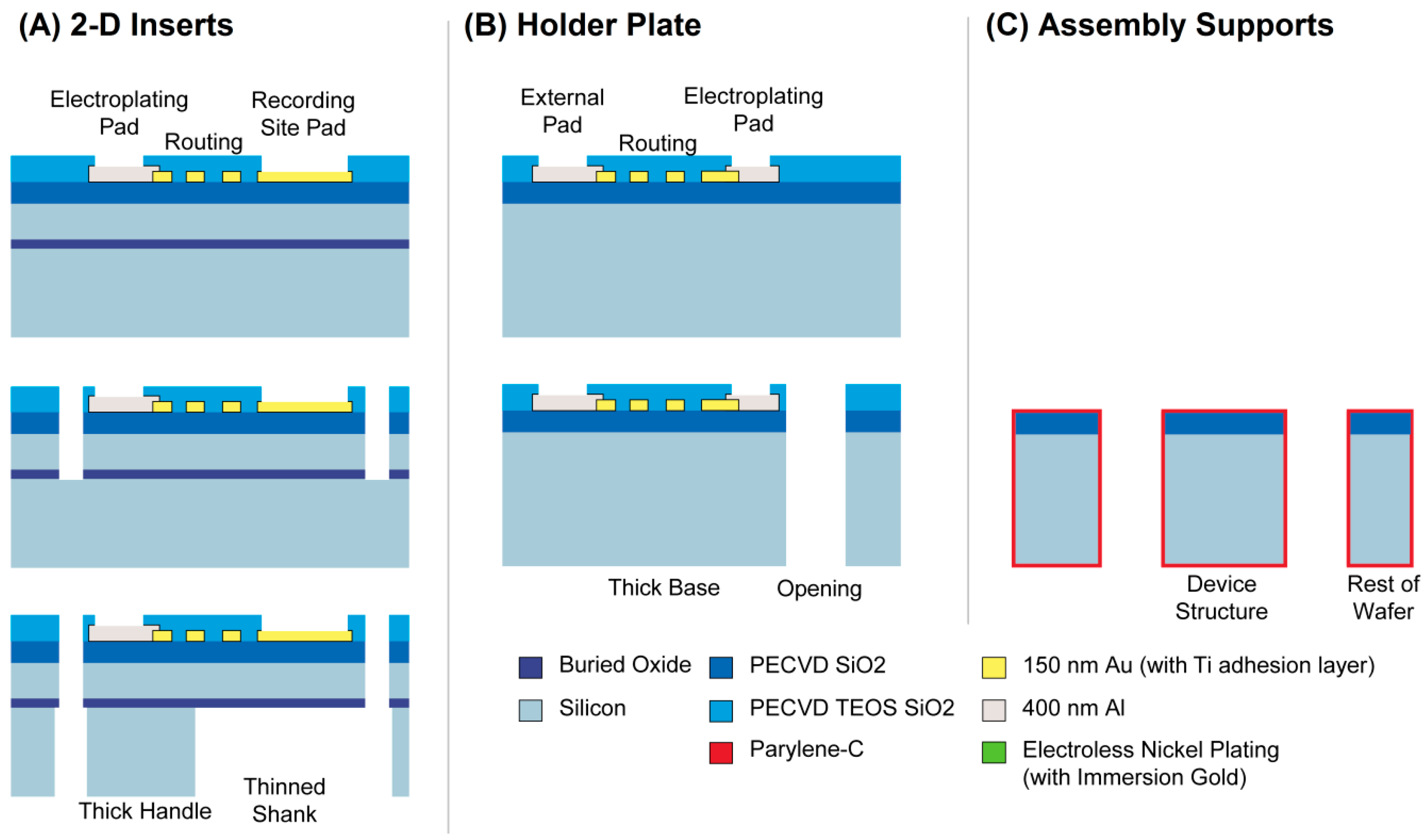
2.1. Fabrication and Assembly Overview
2.2. Mechanical Assembly Procedures
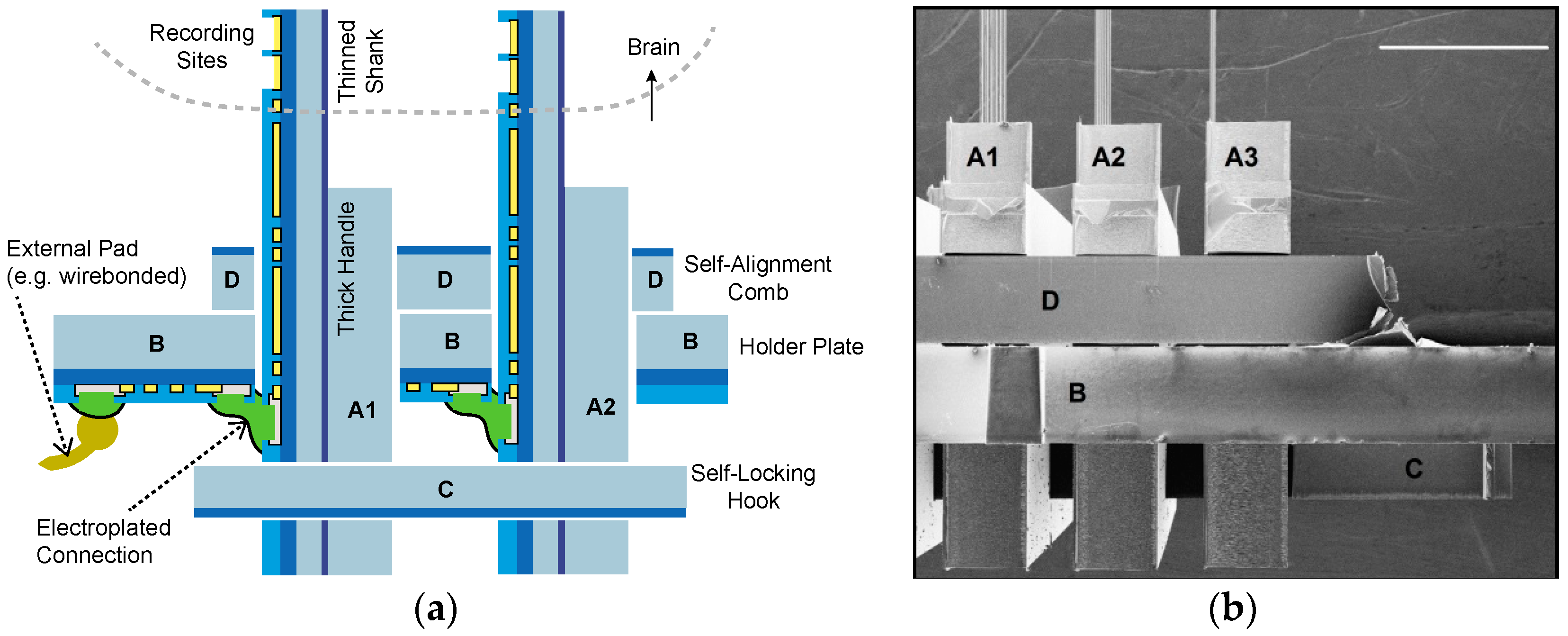
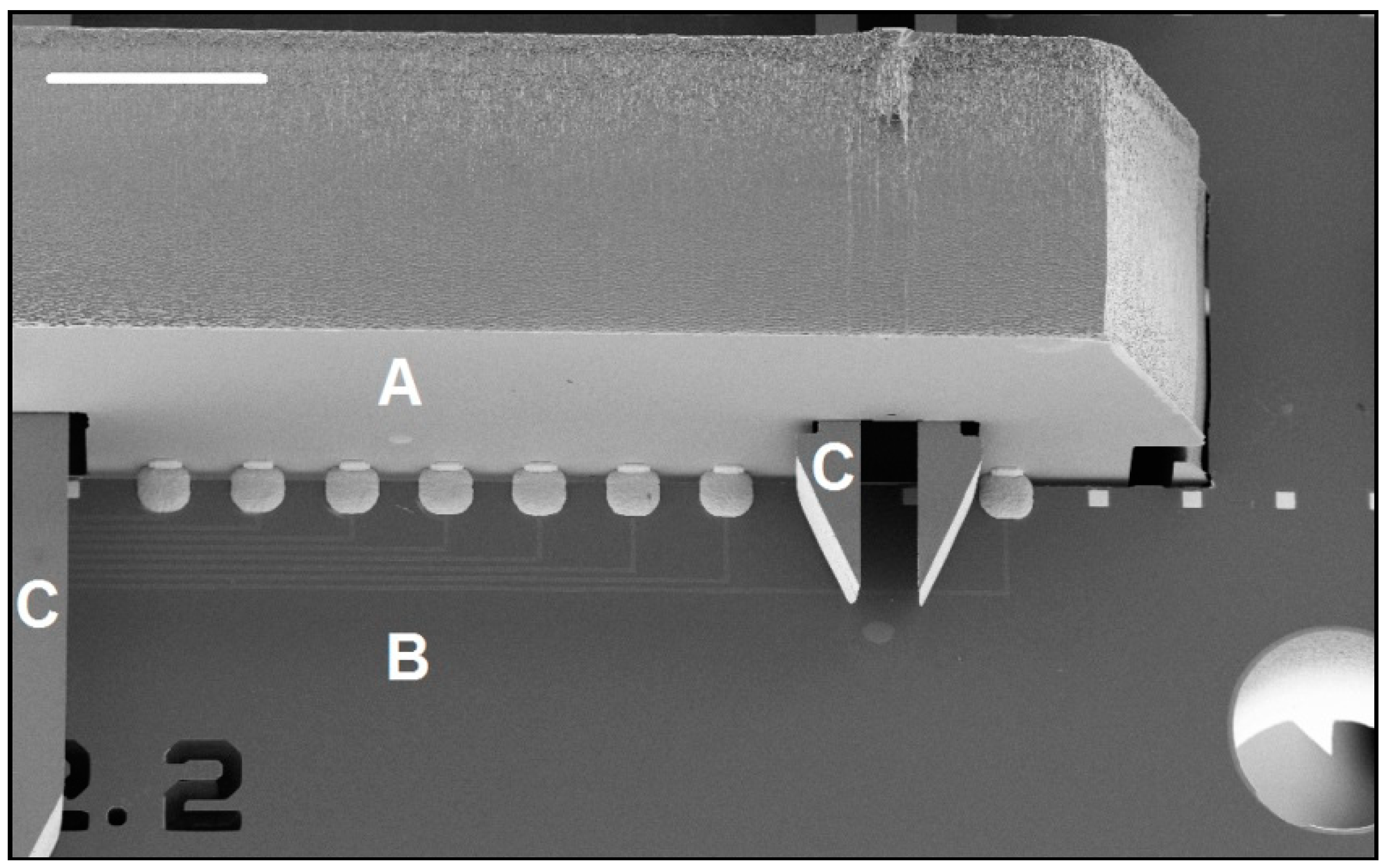
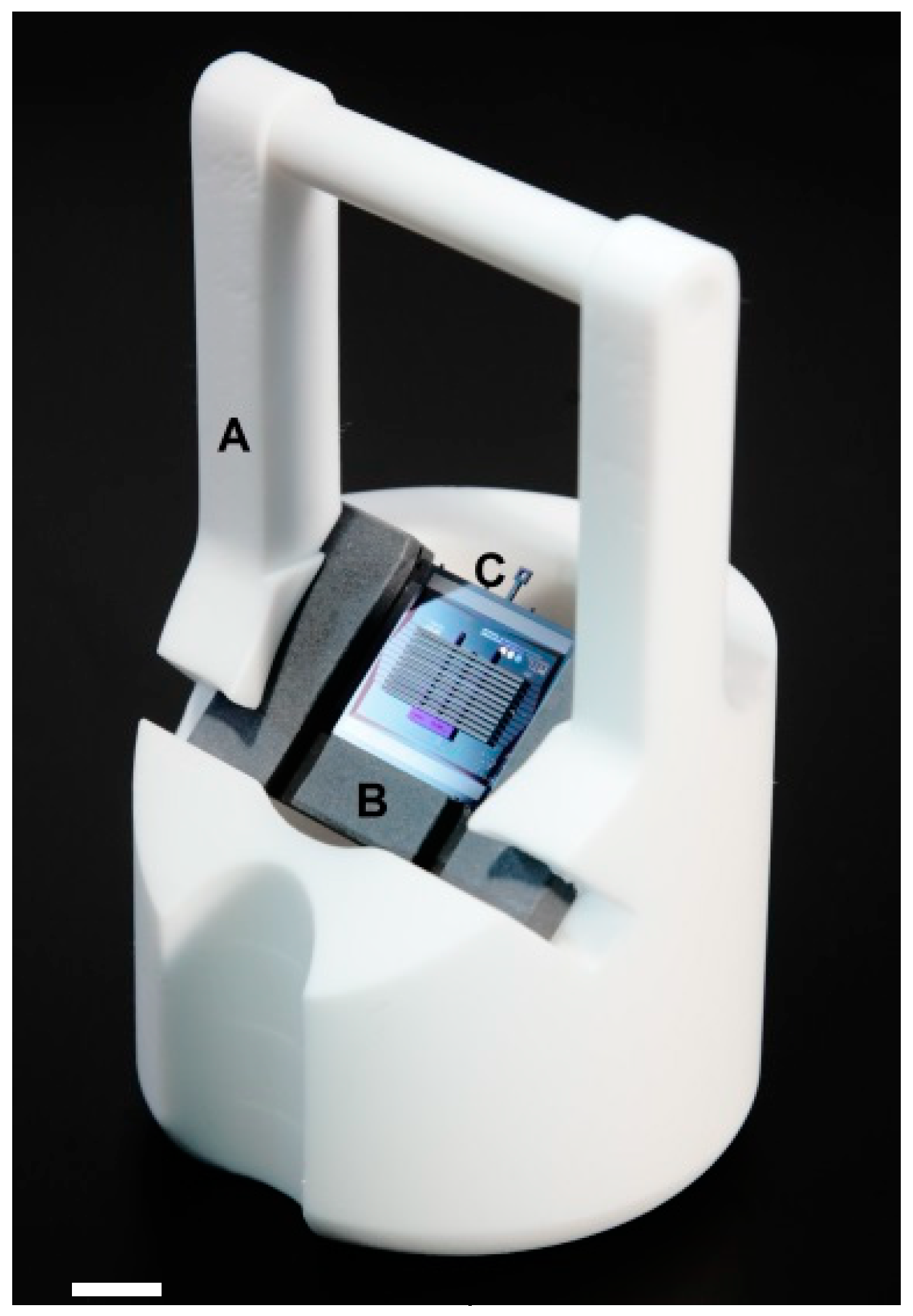
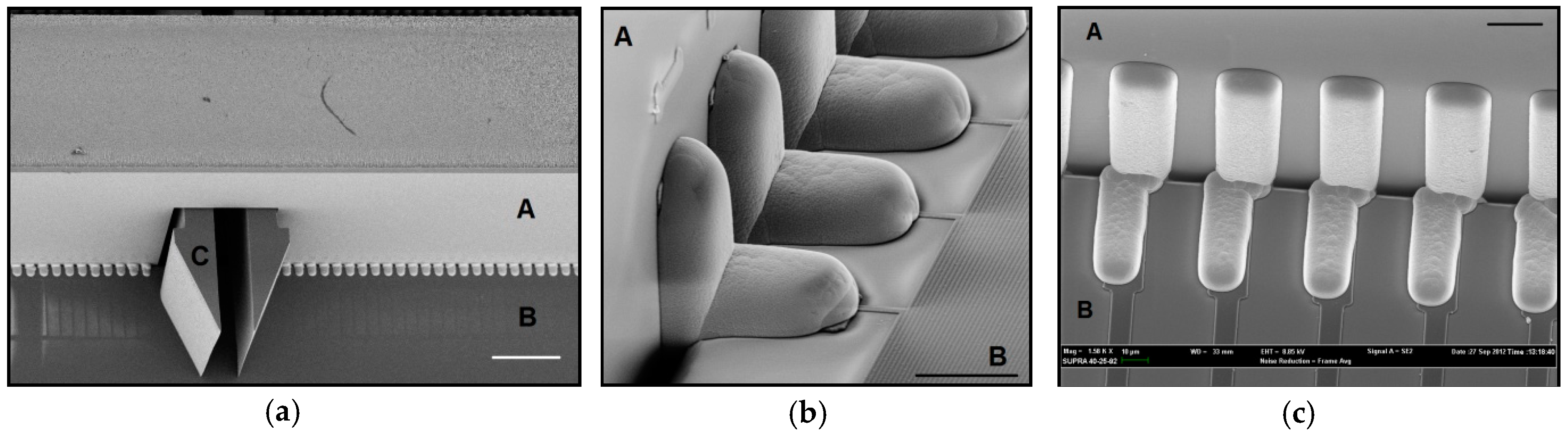
2.2.1. Inserts and Holder Plate
2.2.2. Self-Locking Hook
2.2.3. Self-Alignment Combs
2.2.4. Improvements to the Self-Alignment Combs
2.2.5. Alternative Assembly Methods
2.3. Electrical Assembly Procedures
2.3.1. Post-Package Electrolytic Plating
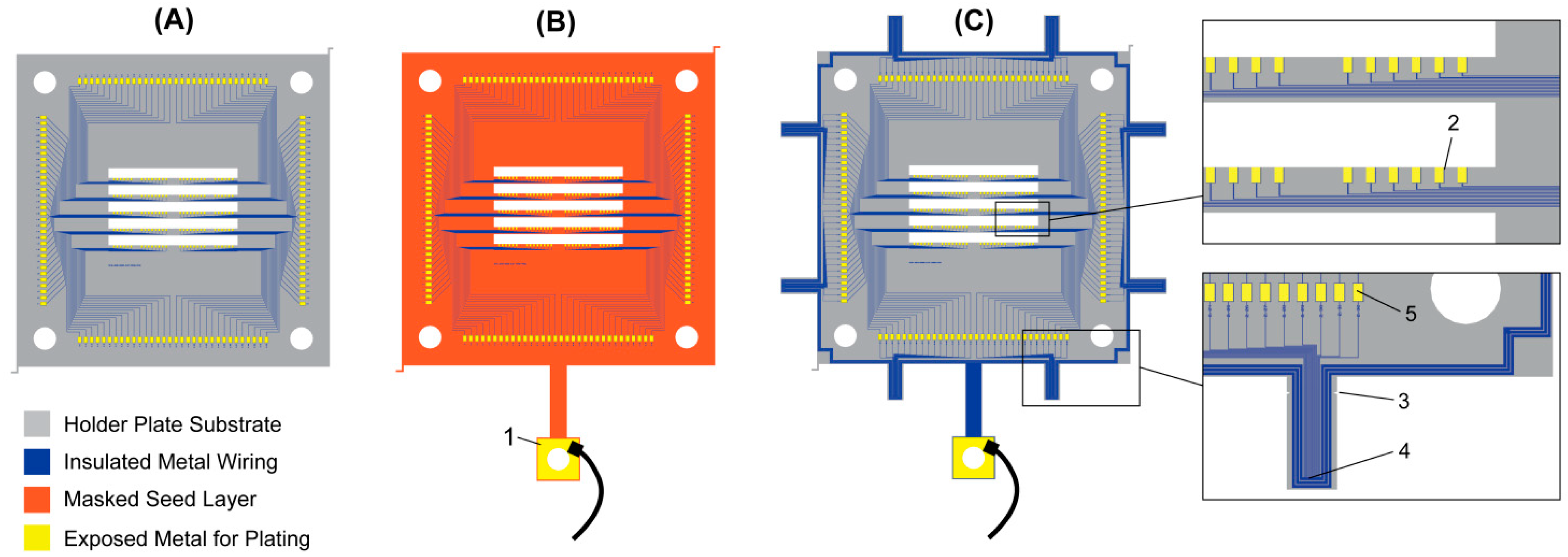
2.3.2. Electrolytic Plating with Seed Layer and Mask
2.3.3. Seedless Plating with Temporary Short Circuits
2.3.4. Electroless Plating
2.3.5. Comparison
3. Results
3.1. Example Designs
3.2. Mechanical Characterization
3.3. Electrical Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wise, K.D.; Angell, J.B.; Starr, A. An integrated-circuit approach to extracellular microelectrodes. IEEE Trans. Biomed. Eng. 1970, 3, 238–247. [Google Scholar] [CrossRef]
- Najafi, K.; Wise, K.D.; Mochizuki, T. A high-yield IC-compatible multichannel recording array. IEEE Trans. Electron Devices 1985, 32, 1206–1211. [Google Scholar] [CrossRef]
- Norlin, P.; Kindlundh, M.; Mouroux, A.; Yoshida, K.; Jensen, W.; Hofmann, U.G. A 32-Site Neural Recording Probe Fabricated by Double-Sided Deep Reactive Ion Etching of Silicon-on-Insulator Substrates. In Proceedings of the 12th Micromechanics Europe Workshop (MME-2001), Cork, Ireland, 16–18 September 2001. [Google Scholar]
- Du, J.; Blanche, T.J.; Harrison, R.R.; Lester, H.A.; Masmanidis, S.C. Multiplexed, high density electrophysiology with nanofabricated neural probes. PLoS ONE 2011, 6, e26204. [Google Scholar] [CrossRef] [PubMed]
- Blanche, T.J.; Spacek, M.A.; Hetke, J.F.; Swindale, N.V. Polytrodes: High-density silicon electrode arrays for large-scale multiunit recording. J. Neurophysiol. 2005, 93, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Herbawi, A.S.; Larramendy, F.; Galchev, T.; Holzhammer, T.; Mildenberger, B.; Paul, O.; Ruther, P. CMOS-Based Neural Probe with Enhanced Electronic Depth Control. In Proceedings of the 18th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Anchorage, AK, USA, 21–25 June 2015. [Google Scholar]
- Lopez, C.M.; Andrei, A.; Mitra, S.; Welkenhuysen, M.; Eberle, W.; Bartic, C.; Puers, R.; Yazicioglu, R.F.; Gielen, G.G.E. An implantable 455-active-electrode 52-channel CMOS neural probe. IEEE J. Solid-State Circuit. 2014, 49, 248–261. [Google Scholar] [CrossRef]
- Berényi, A.; Somogyvári, Z.; Nagy, A.J.; Roux, L.; Long, J.D.; Fujisawa, S.; Stark, E.; Leonardo, A.; Harris, T.D.; Buzsáki, G. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 2014, 111, 1132–1149. [Google Scholar] [CrossRef] [PubMed]
- Nordhausen, C.T.; Maynard, E.M.; Normann, R.A. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 1996, 726, 129–140. [Google Scholar] [CrossRef]
- Shobe, J.L.; Claar, L.D.; Parhami, S.; Bakhurin, K.I.; Masmanidis, S.C. Brain activity mapping at multiple scales with silicon microprobes containing 1024 electrodes. J. Neurophysiol. 2015, 114, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Drake, K.L.; Wise, K.D.; Farraye, J.; Anderson, D.J.; BeMent, S.L. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity. IEEE Trans. Biomed. Eng. 1988, 35, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Scholvin, J.; Kinney, J.P.; Bernstein, J.G.; Moore-Kochlacs, C.; Kopell, N.; Fonstad, C.G.; Boyden, E.S. Close-packed silicon microelectrodes for scalable spatially oversampled neural recording. IEEE Trans. Biomed. Eng. 2016, 63, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Hoogerwerf, A.C.; Wise, K.D. A Three-Dimensional Neural Recording Array. In Proceedings of the International Conference on Solid-State Sensors and Actuators. Digest of Technical Papers, San Francisco, CA, USA, 24–27 June 1991. [Google Scholar]
- Perlin, G.E.; Wise, K.D. A Compact Architecture for Three-Dimensional Neural Microelectrode Arrays. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008. [Google Scholar]
- Bai, Q.; Wise, K.D.; Anderson, D.J. A high-yield microassembly structure for three-dimensional microelectrode arrays. IEEE Trans. Biomed. Eng. 2000, 47, 281–289. [Google Scholar] [PubMed]
- Yao, Y.; Gulari, M.N.; Wiler, J.A.; Wise, K.D. A microassembled low-profile three-dimensional microelectrode array for neural prosthesis applications. J. Microelectromech. Syst. 2007, 16, 977–988. [Google Scholar] [CrossRef]
- Herwik, S.; Kisban, S.; Aarts, A.A.A.; Seidl, K.; Girardeau, G.; Benchenane, K.; Zugaro, M.B.; Wiener, S.I.; Paul, O.; Neves, H.P.; et al. Fabrication technology for silicon-based microprobe arrays used in acute and sub-chronic neural recording. J. Micromech. Microeng. 2009, 19, 074008. [Google Scholar] [CrossRef]
- Aarts, A.A.A.; Srivannavit, O.; Wise, K.D.; Yoon, E.; Puers, R.; Van Hoof, C.; Neves, H.P. Fabrication technique of a compressible biocompatible interconnect using a thin film transfer process. J. Micromech. Microeng. 2011, 21, 074012. [Google Scholar] [CrossRef]
- Kisban, S.; Holzhammer, T.; Herwik, S.; Paul, O.; Ruther, P. Novel method for the assembly and electrical contacting of out-of-plane microstructures. In Proceedings of the 23rd International Conference on Micro Electro Mechanical Systems (MEMS), Hong Kong, China, 24–28 January 2010. [Google Scholar]
- Cheng, M.Y.; Yao, L.; Tan, K.L.; Lim, R.; Li, P.; Chen, W. 3D probe array integrated with a front-end 100-channel neural recording ASIC. J. Micromech. Microeng. 2014, 24, 125010. [Google Scholar] [CrossRef]
- Malhi, S.D.S.; Davis, H.E.; Stierman, R.J.; Bean, K.E.; Driscoll, C.C.; Chatterjee, P.K. Orthogonal Chip Mount—A 3D Hybrid Wafer Scale Integration Technology. In Proceedings of the International Electron Devices Meeting, Washington, DC, USA, 6–9 December 1987. [Google Scholar]
- Lee, Y.T.; Lin, C.W.; Lin, C.M.; Yeh, S.R.; Chang, Y.C.; Fu, C.C.; Fang, W. A 3D Glass Microprobe Array with Embedded Silicon for Alignment and Electrical Connection. In Proceedings of the International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009. [Google Scholar]
- Hoogerwerf, A.C.; Wise, K.D. A three-dimensional microelectrode array for chronic neural recording. IEEE Trans. Biomed. Eng. 1994, 41, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Barz, F.; Holzhammer, T.; Paul, O.; Ruther, P. Novel Technology for the In-plane to Out-of-plane Transfor of Multiple Interconnection Lines in 3D Neural Probes. In Proceedings of the 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013. [Google Scholar]
- John, J.; Li, Y.; Zhang, J.; Loeb, J.A.; Xu, Y. Microfabrication of 3D neural probes with combined electrical and chemical interfaces. J. Micromech. Microeng. 2011, 21, 105011. [Google Scholar] [CrossRef]
- Merriam, S.M.E.; Srivannavit, O.; Gulari, M.N.; Wise, K.D. A three-dimensional 64-site folded electrode array using planar fabrication. J. Microelectromech. Syst. 2011, 20, 594–600. [Google Scholar] [CrossRef]
- Wang, M.F.; Maleki, T.; Ziaie, B. A self-assembled 3D microelectrode array. J. Micromech. Microeng. 2010, 20, 035013. [Google Scholar] [CrossRef]
- Chen, C.H.; Chuang, S.C.; Su, H.C.; Hsu, W.L.; Yew, T.R.; Chang, Y.C.; Yeh, S.R.; Yao, D.J. A three-dimensional flexible microprobe array for neural recording assembled through electrostatic actuation. Lab Chip 2011, 11, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Suzuki, T.; Mabuchi, K.; Fujita, H. 3D flexible multichannel probe array. J. Micromech. Microeng. 2004, 14, 104–107. [Google Scholar] [CrossRef]
- Chiou, J.C.; Chang, C.W. Development of Three Dimensional Neural Sensing Device by Stacking Method. In Proceedings of the IEEE Sensors 2010 Conference, Kona, HI, USA, 1–4 November 2010. [Google Scholar]
- Merriam, M.E.; Dehmel, S.; Srivannavit, O.; Shore, S.E.; Wise, K.D. A 3-D 160-Site Microelectrode Array for Cochlear Nucleus Mapping. IEEE Trans. Biomed. Eng. 2011, 58, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Riedel-Kruse, I.H.; Nawroth, J.C.; Roukes, M.L.; Laurent, G.; Masmanidis, S.C. High-Resolution three-dimensional extracellular recording of neuronal activity with microfabricated electrode arrays. J. Neurophysiol. 2009, 101, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Barz, F.; Paul, O.; Ruther, P. Modular Assembly Concept for 3D Neural Probe Prototypes Offering High Freedom of Design and Alignment Precision. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014. [Google Scholar]
- Langhals, N.B.; Kipke, D.R. Validation of a Novel Three-Dimensional Electrode Array Within Auditory Cortex. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009. [Google Scholar]
- Scholvin, J.; Kinney, J.P.; Bernstein, J.G.; Moore-Kochlacs, C.; Kopell, N.J.; Fonstad, C.G.; Boyden, E.S. Heterogeneous neural amplifier integration for scalable extracellular microelectrodes. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Rios, G.; Lubenov, E.V.; Chi, D.; Roukes, M.L.; Siapas, A.G. Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett. 2016, 16, 6857–6862. [Google Scholar] [CrossRef] [PubMed]
- Michon, F.; Aarts, A.; Borghs, G.; Bruce, M.; Fabian, K. Integration of silicon-based probes and micro-drive array for chronic recordings of large populations of neurons in behaving animals. J. Neural Eng. 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dombeck, D.A.; Khabbaz, A.N.; Collman, F.; Adelman, T.L.; Tank, D.W. Imaging Large-Scale Neural Activity with Cellular Resolution in Awake, Mobile Mice. Neuron 2007, 56, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.D.; Collman, F.; Dombeck, D.A.; Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 2009, 461, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Scholvin, J.; Fonstad, C.G.; Boyden, E.S. Scaling models for microfabricated in vivo neural recording technologies. In Proceedings of the 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017. [Google Scholar]
- Hetke, J.F.; Lund, J.L.; Najafi, K.; Wise, K.D.; Anderson, D.J. Silicon ribbon cables for chronically implantable microelectrode arrays. IEEE Trans. Biomed. Eng. 1994, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ledochowitsch, P.; Tiefenauer, R.F.; Pepin, B.; Maharbiz, M.M.; Blanche, T.J. Nanoflex for Neural Nanoprobes. In Proceedings of the The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013. [Google Scholar]
- Sun, T.; Park, W.T.; Cheng, M.Y.; An, J.Z.; Xue, R.F.; Tan, K.L.; Je, M. Implantable polyimide cable for multichannel high-data-rate neural recording microsystems. IEEE Trans. Biomed. Eng. 2012, 59, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Hetke, J.F.; Najafi, K.; Wise, K.D. Flexible miniature ribbon cables for long-term connection to implantable sensors. Sensor Actuators A-Phys. 1990, 23, 999–1002. [Google Scholar] [CrossRef]
- Thacker, H.D.; Bottoms, W.R. Wafer-Level testing of gigascale integrated circuits. In Integrated Interconnect Technologies for 3D Nanoelectronic Systems; Artech House: Norwood, MA, USA, 2009. [Google Scholar]
- International Technology Roadmap for Semiconductors. Available online: https://www.semiconductors.org/clientuploads/Research_Technology/ITRS/2015/0_2015%20ITRS%202.0%20Executive%20Report%20(1).pdf (accessed on 24 August 2018).
- Zorzos, A.N.; Scholvin, J.; Boyden, E.S.; Fonstad, C.G. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt. Lett. 2012, 37, 4841–4843. [Google Scholar] [CrossRef] [PubMed]
- Gaither, M.S.; Gates, R.S.; Kirkpatrick, R.; Cook, R.F.; DelRio, F.W. Etching process effects on surface structure, fracture strength and reliability of single-crystal silicon theta-like specimens. J. Microelectromech. Syst. 2013, 22, 589–602. [Google Scholar] [CrossRef]
- Najafi, K.; Ji, J.; Wise, K.D. Scaling limitations of silicon multichannel recording probes. IEEE Trans. Biomed. Eng. 1990, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Aiple, F. Multimicroelectrode investigation of monkey striate cortex: Spike train correlations in the infragranular layers. J. Neurophysiol. 1988, 60, 798–828. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J.; Normann, R.A. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 1992, 20, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Edell, D.J.; Toi, V.V.; McNeil, V.M.; Clark, L.D. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans. Biomed. Eng. 1992, 39, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Pak, N.; Siegle, J.H.; Kinney, J.P.; Denman, D.J.; Blanche, T.J.; Boyden, E.S. Closed-loop, ultraprecise, automated craniotomies. J. Neurophysiol. 2015, 113, 3943–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Reference | Method a | Design b | Total Sites | Connection k Count Pitch | |
|---|---|---|---|---|---|
| Nordhausen 1996 [9] | Monolithic | 10 × 10 × 1 | 100 | n/a | n/a |
| Hoogerwerf 1991 [14] | Electrolytic | 4 × 4 × 16 | 256 | 16 g | - |
| Hoogerwerf 1994 [24] | Electrolytic | 4 × 4 × 8 | 128 | 16 g | - |
| Barz 2013 [25] | Electrolytic | 4 × 4 × 4 | 64 | 64 | 70 µm |
| Herwik 2009 [18] | Pressed | 4 × 4 × 5 | 80 | 80 | 70 µm |
| Kisban 2010 [20] | Pressed | 2 × 4 × 5 | 80 | 80 | 35 µm d |
| Aarts 2011 [19] | Pressed | 4 × 4 × 5 | 80 | 80 | 70 µm e |
| Bai 2000 [16] | Ultrasonic | 4 × 4 × 4 | 32 | 32 g | - |
| Yao 2007 [17] | Ultrasonic | 4 × 8 × 32 | 1024 | 32 g | - |
| Perlin 2008 [15] | Ultrasonic | 4 × 4 × 4 | 64 | 64 | 40 µm |
| Malhi 1987 c [22] | Solder | 9 × 1 × 22 | 198 | 198 | - |
| Cheng 2014 [21] | Solder | 5 × 4 × 5 | 100 | 100 | 150 µm |
| Lee 2009 [23] | Silver Paste | 4 × 4 × 1 | 16 | 16 | 800 µm e |
| Takeuchi 2004 [30] | Folding | 2 × 3 × 3 | 18 | n/a | n/a |
| Wang 2010 [28] | Folding | 2 × 2 × 4 | 32 | n/a | n/a |
| John 2011 [26] | Folding | 3 × 3 × 2 | 18 | n/a | n/a |
| Chen 2011 [29] | Folding | 2 × 2 × 2 | 8 | n/a | n/a |
| Merriam 2011 a [27] | Folding | 4 × 4 × 4 | 64 | n/a | n/a |
| Chiou 2010 [31] | Die Stacking | 4 × 4 × 4 | 64 | n/a | n/a |
| Rios 2016 [37] | Die Stacking | 4 × 4 × 64 | 1024 | 256 | 200 µm |
| Du 2009 [33] | Package | 4 × 2 × 8 h | 64 | n/a | n/a |
| Langhals 2009 [35] | Package | 4 × 4 × 4 | 64 | n/a | n/a |
| Merriam 2011 b [32] | Package | 5 × 4 × 8 | 160 | n/a | n/a |
| Barz 2014 [34] | Package | 2 × 2 × 8 | 32 | n/a | n/a |
| Barz 2017 [34] | Package | 2 × 2 × 8 | 32 | n/a | n/a |
| Shobe 2015 [10] | Package | 4 × 4 × 64 f | 1024 | n/a | n/a |
| Michon 2016 [38] | Micro-Drive | 16 × 2 × 8 | 256 | n/a | n/a |
| Step | 2-D Inserts (A) | Holder Plate (B) | Mechanical Supports (C,D) |
|---|---|---|---|
| Starting material | 150 mm SOI wafer, thicknesses: 15 µm device layer, 0.8 µm buried oxide, 510 µm handle | 150 mm wafer, 525 µm thick, double-sided polished | 150 mm wafer, 525 µm thick, double-sided polished |
| Clean wafers and insulation | Piranha clean 1 µm of PECVD SiO2 | Piranha clean 1 µm of PECVD SiO2 | Omitted |
| Electron beam lithography metallization (liftoff) | 10 nm Ti/150 nm Au/5 nm Ti, mask is 400 nm of PMMA 495A8 | 10 nm Ti/150 nm Au/5 nm Ti mask is 400 nm of PMMA 495A8 | Omitted |
| Optical lithography metallization (liftoff) | 50 nm Ti/400 nm Al, mask is 1.5 µm of AZ5214E | 50 nm Ti/400 nm Al mask is 1.5 µm of AZ5214E | Omitted |
| Upper insulation | 1 µm of PECVD TEOS | 1 µm of PECVD TEOS | Omitted |
| Electron beam lithography small recording site etch | CF4/CHF3 based SiO2 etch, mask is 800 nm of PMMA 495A11 | CF4/CHF3 based SiO2 etch, mask is 800 nm of PMMA 495A11 | Omitted |
| Optical lithography large pad etch | CF4/CHF3 based SiO2 etch, mask is 1 µm of SPR-700 | CF4/CHF3 based SiO2 etch, mask is 1 µm of SPR-700 | Omitted |
| Frontside DRIE etch a | CF4/CHF3 based etch of frontside SiO2, then 15 µm etch of Si device layer to buried oxide. Mask is 8 µm of AZ4620. | CF4/CHF3 based etch of frontside SiO2, then 15 µm etch of Si device layer to buried oxide. Mask is 8 µm of AZ4620. | Etch 525 µm through wafer, mask is 8 µm of AZ4620 |
| SOI wafer buried oxide etch | CF4/CHF3 based etch of 0.8 µm of buried oxide. | Omitted | Omitted |
| Backside DRIE etch | Etch 510 µm through wafer from the backside, mask is 8 µm of AZ4620 | Omitted | Omitted |
| Clean wafers | Barrel ash in oxygen plasma | Barrel ash in oxygen plasma | Barrel ash in oxygen plasma |
| Insulation on full wafer | Omitted | Omitted | 0.1 µm c of PECVD SiO2 1 µm of Parylene-C |
| Remove parts | Break out devices | Break out devices | Break out devices |
| Insulation on individual parts | (optional) Place dies facing down onto a Si wafer and deposit 100 nm PECVD Si3N4 to insulate backside b | Place dies facing down onto a Si wafer and deposit 100 nm PECVD Si3N4 to insulate backside b | Omitted |
| Detail | Packaged Plating | Short-Circuited Breakout Beams | Seed and Mask | Electroless |
|---|---|---|---|---|
| Method | Electrolytic | Electrolytic | Electrolytic | Electroless |
| Common metal choices | Au, Ni, Cu | Au, Ni, Cu | Au, Ni, Cu | Ni, Cu |
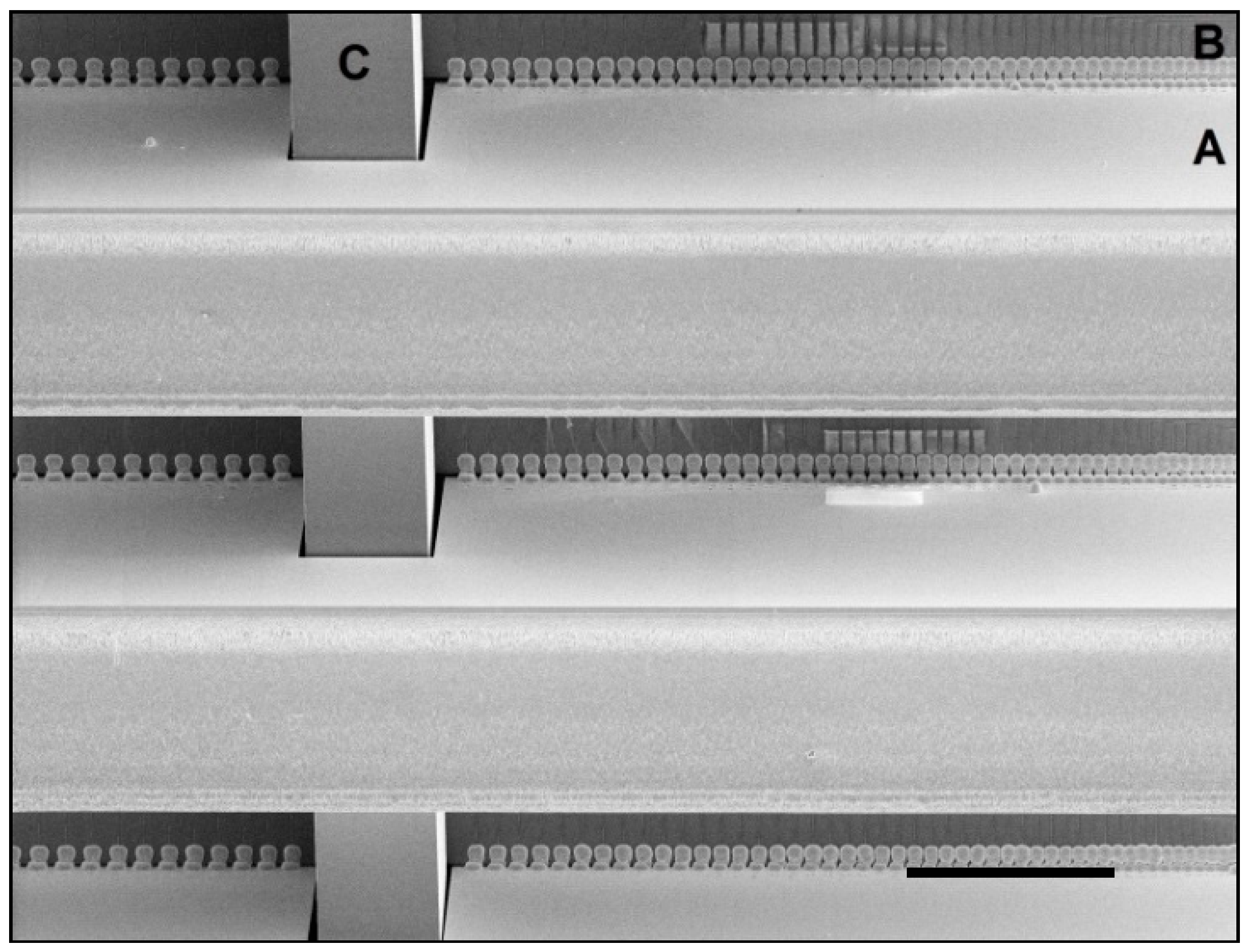
| Holder plate design type (see Figure 7) | “A” | “C” | “B” | “A” |
| Can be plated before packaging | No | Yes | Yes | Yes |
| Requires further processing after plating | No | Yes | Yes | No |
| Minimum pitch a | Wpad + 2 Wgap | Wpad + 2 Wgap | Wpad + 2 Wgap | Wpad + Wgap |
| Requires direct wiring access to plating pads | Yes | Yes | No | No |
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
| ID | Design (Part Names) | Recording Site Configuration a | 3-D Array Configuration b | Connections per 2-D Insertc | Total Connections | Connection Pitch | Device Purpose | |
|---|---|---|---|---|---|---|---|---|
| 1a | B160–F160 | 2 × 20 @ 13.0 µm | 9 × 4 | 160 | 1440 | 60 µm | Conservative design | |
| 1b | B160–F20 | 2 × 2 @ 9.5/14 µm | 9 × 40 | 160 | 1440 | 60 µm | Large shank count, tetrode tips | |
| 2 | B160–F30 | 1 × 9 @ 250.0 µm | 9 × 17 | 160 | 1440 | 60 µm | Optical-only lithography | |
| 3 | 3a | B408–F408 | 2 × 34 @ 13.0 µm | 11 × 6 | 408 | 4488 | 40 µm | Standard design |
| 3b | B409–F408 | 2 × 34 @ 13.0 µm | 11 × 6 | 408 | 4488 | 40 µm | Compact holder plate | |
| 4 | B1000–F1006 | 4 × 42 @ 13.0 µm | 10 × 6 | 1008 | 10,080 | 26 µm | Aggressive design | |
| 5 | B1000–F1010 | 2 × 50 @ 13.0 µm | 10 × 10 | 1008 | 10,080 | 26 µm | Aggressive design | |
| 6 | B10–F10 | 1 × 1 | 8 × 80 | 149 | 1192 | 16.5 to 113.7 µm | Pitch and DRIE etch testing | |
| Design | Wire Length (mm) | Wire Aspect Ratio (nsq = L/W) | Resistance (kΩ) | Capacitance (pF) |
|---|---|---|---|---|
| Holder B10 | 5.6 | 1900 | 1.02 | 0.56 |
| Insert F10 a | 1.9 | 1050 | n/a | 0.08 |
| Holder B160 | 11.3 | 7200 | 8.53 | 1.39 |
| Insert F30 | 2.1 | 950 | 0.29 | 0.09 |
| Insert F160 | 4.0 | 7700 | 9.30 | 0.28 |
| Holder B408 | 13.0 | 14,200 | 21.7 | 1.82 |
| Holder B409 | 13.9 | 34,750 | 55.1 | 1.28 |
| Insert F408 | 5.3 | 8700 | 9.45 | 0.48 |
| Holder B1000 | 15.8 | 39,400 | 66.1 | 1.88 |
| Insert F1006 | 8.2 | 14,100 | 18.1 | 0.71 |
| Insert F1010 | 5.4 | 11,300 | 12.8 | 0.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholvin, J.; Zorzos, A.; Kinney, J.; Bernstein, J.; Moore-Kochlacs, C.; Kopell, N.; Fonstad, C.; Boyden, E.S. Scalable, Modular Three-Dimensional Silicon Microelectrode Assembly via Electroless Plating. Micromachines 2018, 9, 436. https://doi.org/10.3390/mi9090436
Scholvin J, Zorzos A, Kinney J, Bernstein J, Moore-Kochlacs C, Kopell N, Fonstad C, Boyden ES. Scalable, Modular Three-Dimensional Silicon Microelectrode Assembly via Electroless Plating. Micromachines. 2018; 9(9):436. https://doi.org/10.3390/mi9090436
Chicago/Turabian StyleScholvin, Jörg, Anthony Zorzos, Justin Kinney, Jacob Bernstein, Caroline Moore-Kochlacs, Nancy Kopell, Clifton Fonstad, and Edward S. Boyden. 2018. "Scalable, Modular Three-Dimensional Silicon Microelectrode Assembly via Electroless Plating" Micromachines 9, no. 9: 436. https://doi.org/10.3390/mi9090436




