A Simple Imaging Device for Fluorescence-Relevant Applications
Abstract
:1. Introduction
2. Materials and Methods
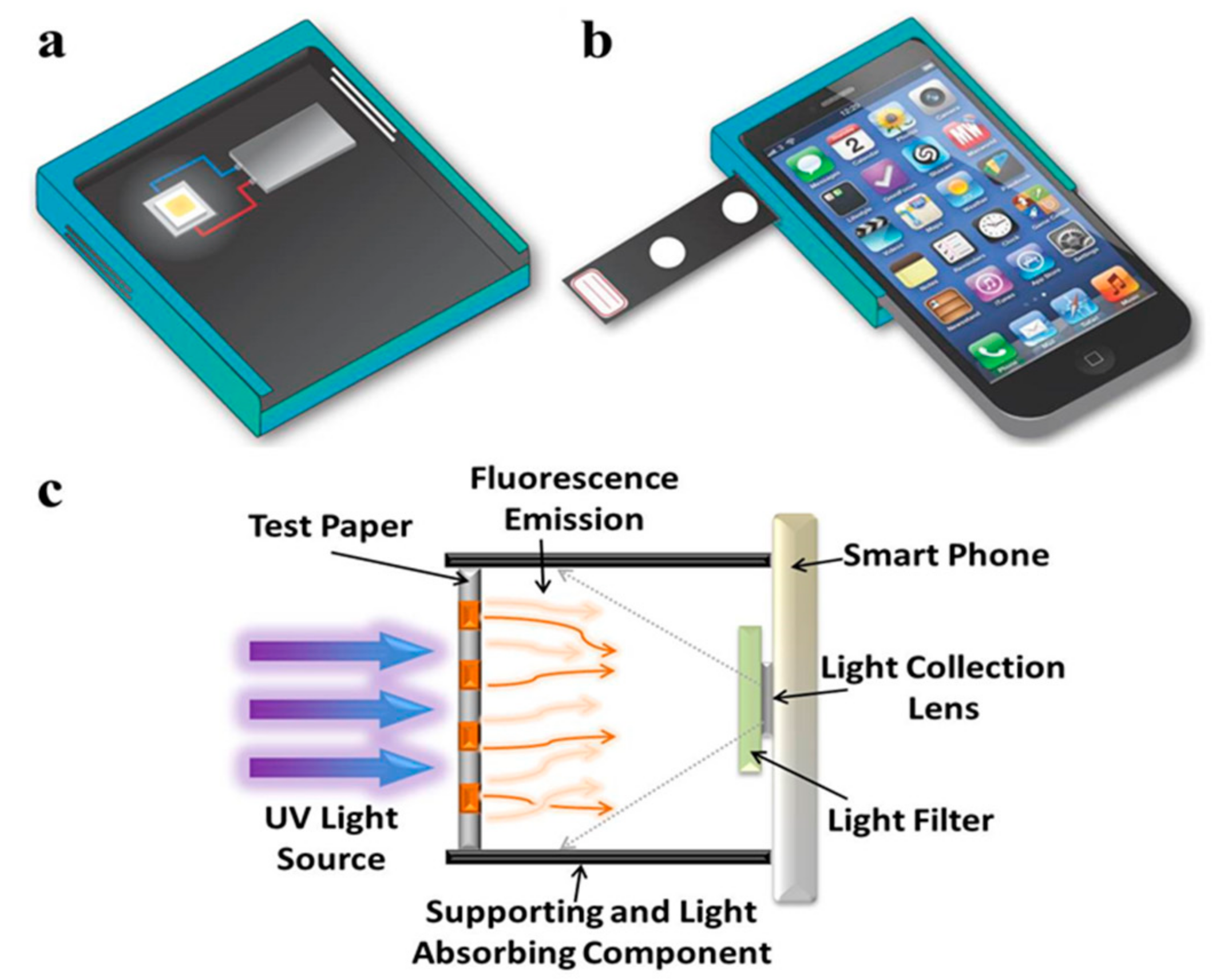
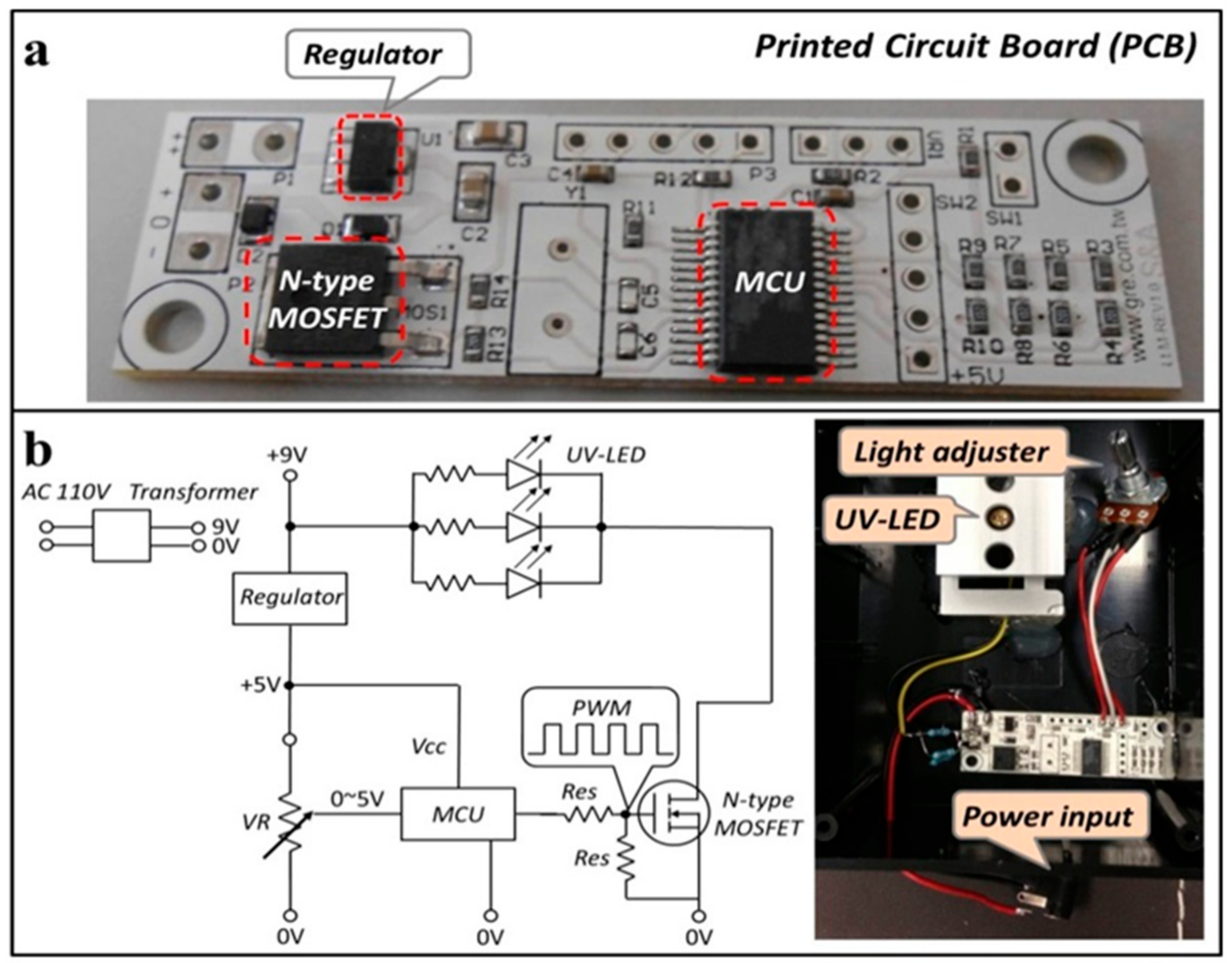
2.1. Portable Fluorescent Image Recording Device
2.2. Chemicals and Materials
2.3. DNA-Stainging Assay and Sperm Analysis
2.4. Image Capture and Intensity Analysis
3. Results and Discussion
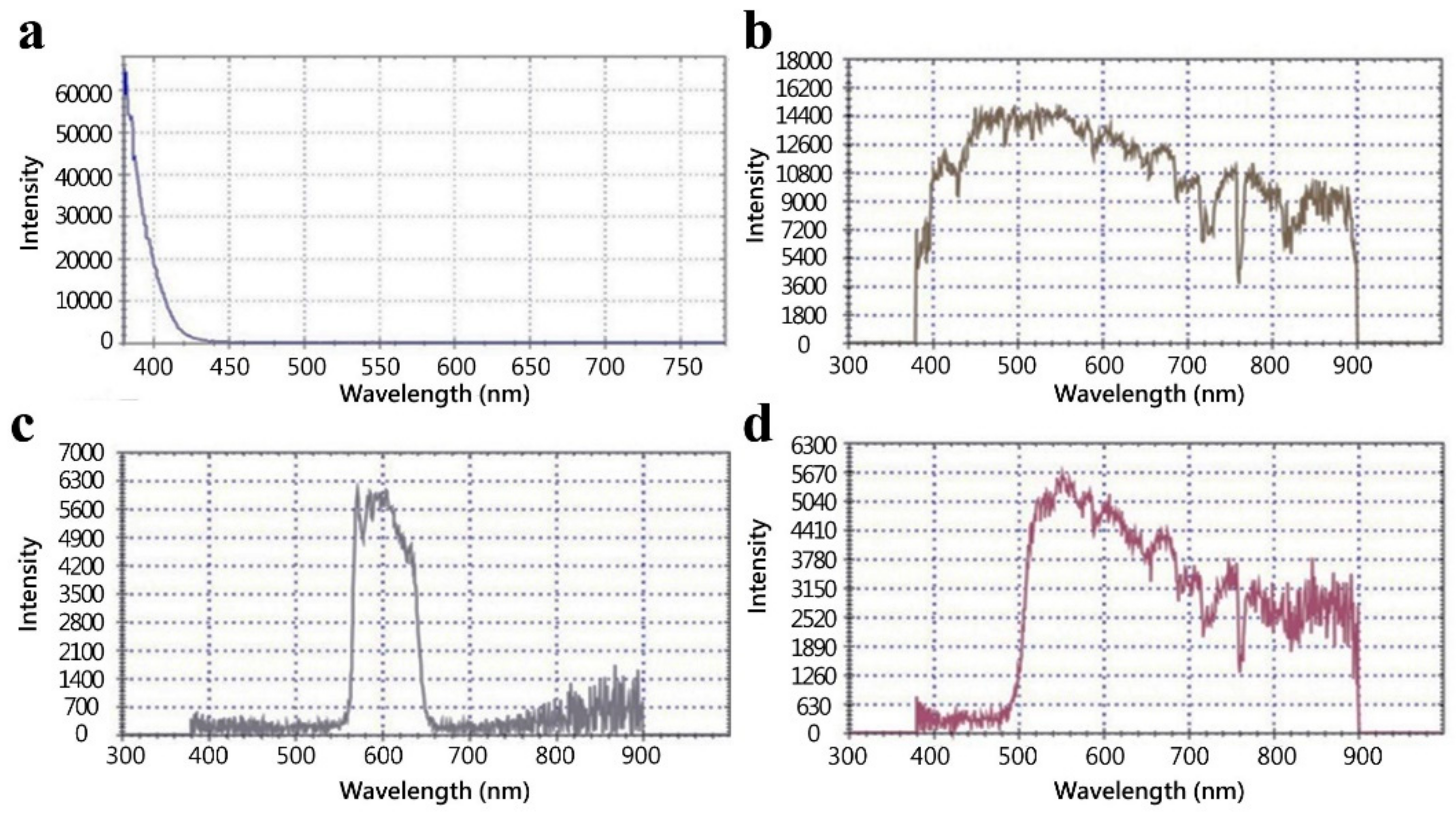
3.1. Functionality of Fluorescence Imaging Device
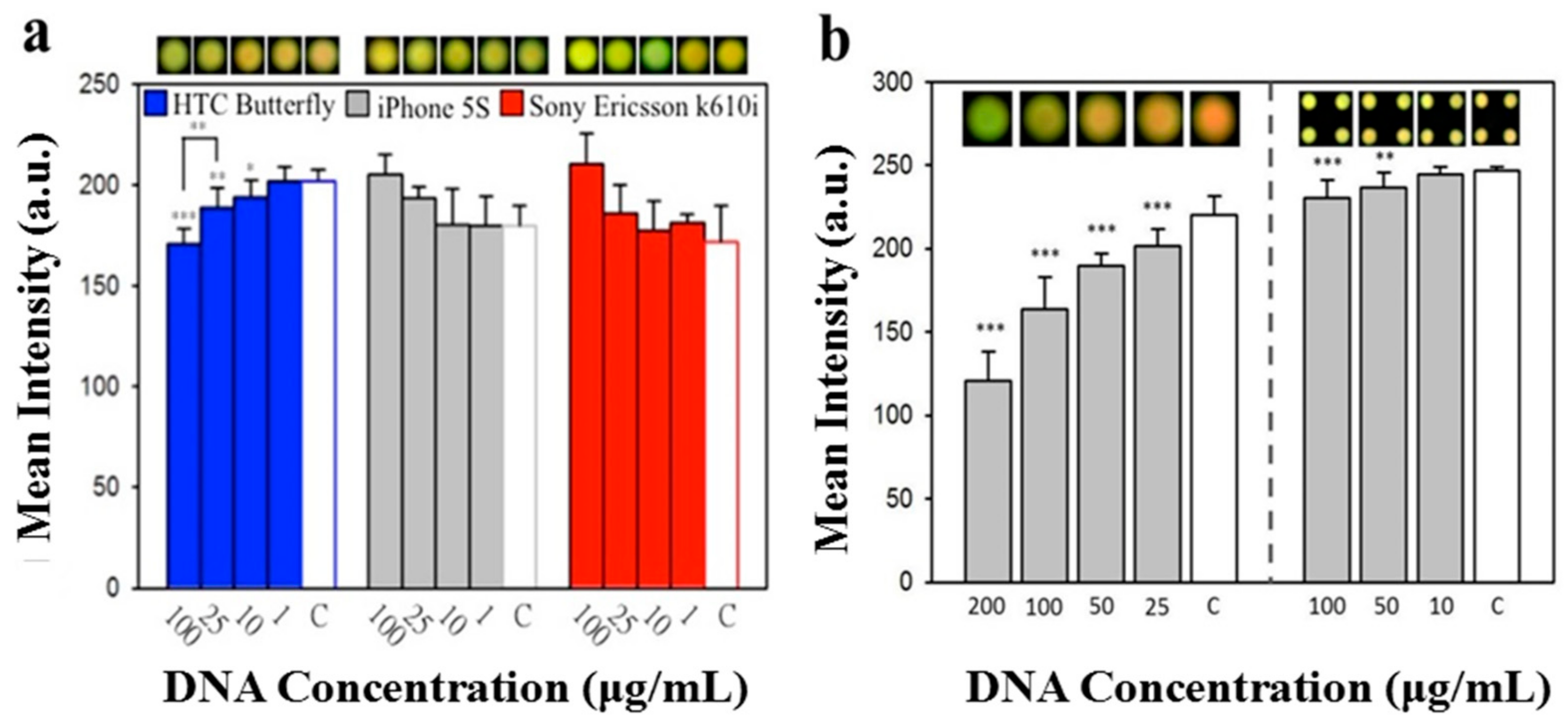
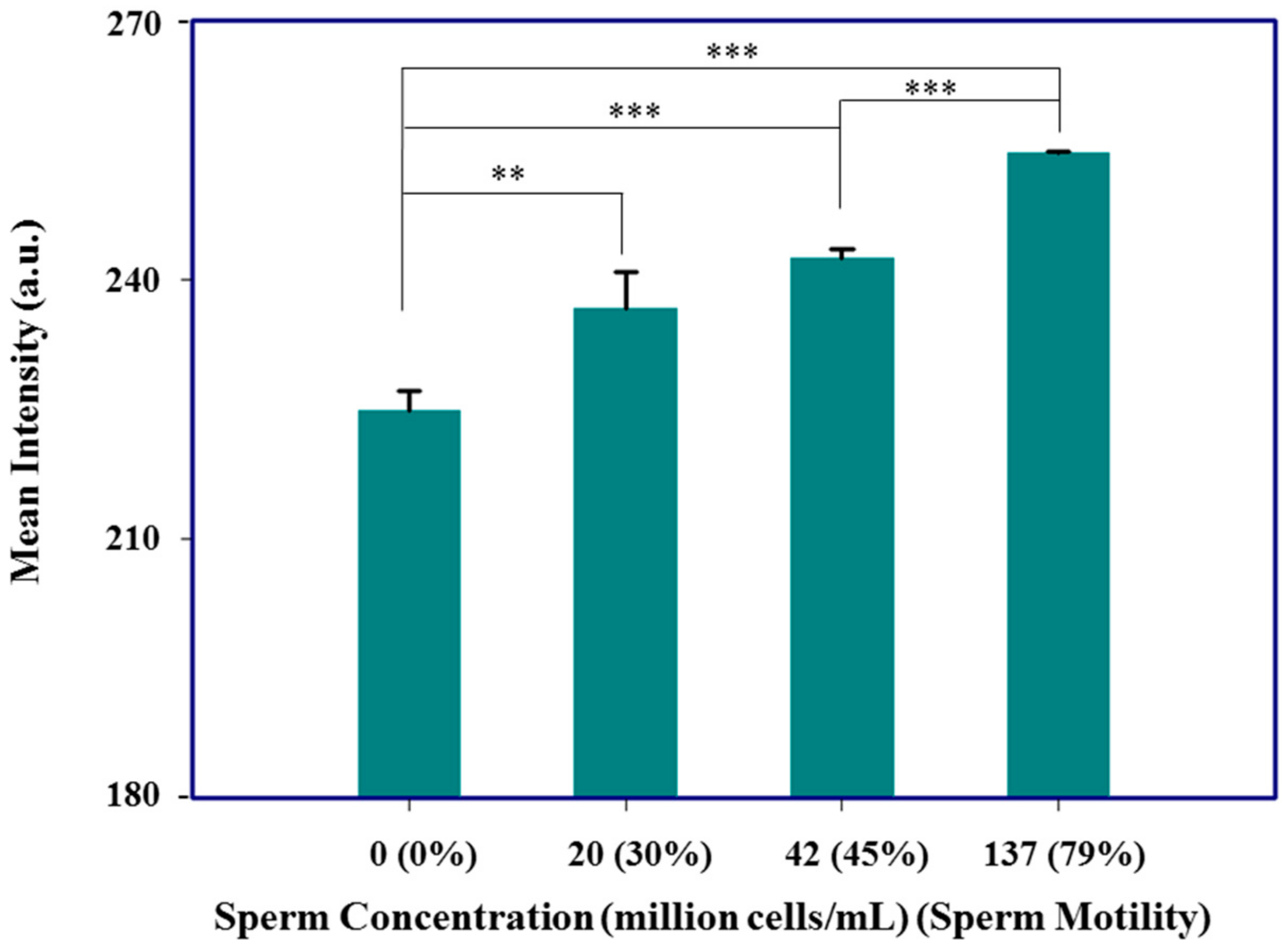
3.2. Fluorescence Detection Device for Nucleotide Analysis and Semen Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mao, X.; Huang, T.J. Microfluidic diagnostics for the developing world. Lab Chip 2012, 12, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Hsu, M.Y.; Kuan, C.M.; Wang, H.K.; Chang, C.L.; Tseng, F.G.; Cheng, C.M. Cotton-based diagnostic devices. Sci. Rep. 2014, 4, 6976. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Oyola-Reynoso, S.; Heim, A.P.; Halbertsma-Black, J.; Zhao, C.; Tevis, I.D.; Cinar, S.; Cademartiri, R.; Liu, X.; Bloch, J.F.; Thuo, M.M. Draw your assay: Fabrication of low-cost paper-based diagnostic and multi-well test zones by drawing on a paper. Talanta 2015, 144, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, R.; Nilghaz, A.; Guan, L.; Zhang, X.; Shen, W. “Periodic-table-style” paper device for monitoring heavy metals in water. Anal. Chem. 2015, 87, 2555–2559. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.; Liang, T.; Fu, E.; Ramachandran, S.; Kauffman, P.; Yager, P. Dissolvable fluidic time delays for programming multi-step assays in instrument-free paper diagnostics. Lab Chip 2013, 13, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-J.; Yang, S.-C.; Yao, D.-J.; Chen, J.-H.; Tu, W.-C.; Cheng, C.-M. Molecular-level dengue fever diagnostic devices made out of paper. Lab Chip 2013, 13, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Ge, L.; Huang, J.; Wang, S.; Ge, S. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011, 11, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.H.; Li, D.; Simon, G.P.; Garnier, G. Gold Nanoparticle–Paper as a Three-Dimensional Surface Enhanced Raman Scattering Substrate. Langmuir 2012, 28, 8782–8790. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Shen, W. Quantitative biomarker assay with microfluidic paper-based analytical devices. Anal. Bioanal. Chem. 2010, 396, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Rolland, J.P.; Kumar, S.; Beattie, P.D.; Jain, S.; Noubary, F.; Wong, V.L.; Pohlmann, R.A.; Ryan, U.S.; Whitesides, G.M. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012, 4, 152ra129. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Gehlot, P.; Sidapra, K.; Edwards, A.D.; Reis, N.M. Portable smartphone quantitation of prostate specific antigen (PSA) in a fluoropolymer microfluidic device. Biosens. Bioelectron. 2015, 70, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.T.; Shen, S.W.; Cheng, C.M.; Chen, C.F. Paper-based tuberculosis diagnostic devices with colorimetric gold nanoparticles. Sci. Technol. Adv. Mater. 2013, 14, 044404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Zhang, L.; Chang, S.; Cui, W.; Zheng, Z. Multiplex microfluidic paper-based immunoassay for the diagnosis of hepatitis C virus infection. Anal. Chem. 2014, 86, 5338–5344. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Green, A.A.; Ferrante, T.; Cameron, D.E.; DaleyKeyser, A.; Yin, P.; Collins, J.J. Paper-based synthetic gene networks. Cell 2014, 159, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.M.; Nosrati, R.; San Gabriel, M.C.; Zini, A.; Sinton, D. Direct DNA Analysis with Paper-Based Ion Concentration Polarization. J. Am. Chem. Soc. 2015, 137, 13913–13919. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Chen, K.-H.; Tsai, C.-H.; Li, W.; Asano, Y.; Naruse, K.; Cheng, C.-M. Paper-based diagnostic devices for evaluating the quality of human sperm. Microfluid. Nanofluid. 2014, 16, 857–867. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, L.; Wang, P.; Yan, M.; Ge, S.; Li, N.; Yu, J.; Huang, J. Photoelectrochemical lab-on-paper device equipped with a porous Au-paper electrode and fluidic delay-switch for sensitive detection of DNA hybridization. Lab Chip 2013, 13, 3945–3955. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, B.-R.; Wang, H.-K.; Fan, S.-T.; Hsu, M.-Y.; Cheng, C.-M. Paper-based immunoaffinity devices for accessible isolation and characterization of extracellular vesicles. Microfluid. Nanofluid. 2014, 16, 849–856. [Google Scholar] [CrossRef]
- Chen, C.; Lin, B.R.; Hsu, M.Y.; Cheng, C.M. Paper-based devices for isolation and characterization of extracellular vesicles. J. Vis. Exp. 2015, 98, 52722. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Li, B.; Ellington, A.D.; Crooks, R.M. DNA Detection Using Origami Paper Analytical Devices. Anal. Chem. 2013, 85, 9713–9720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudanyali, O.; Dimitrov, S.; Sikora, U.; Padmanabhan, S.; Navruz, I.; Ozcan, A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip 2012, 12, 2678–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jani, I.V.; Peter, T.F. How Point-of-Care Testing Could Drive Innovation in Global Health. N. Engl. J. Med. 2013, 368, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed]
- International Telecommunication Union Website. 2015. Available online: http://www.itu.int/pub/D-STR-E_HEALTH.07-2014 (accessed on 20 August 2018).
- Vashist, S.K.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sikora, U.; Ozcan, A. Quantum dot enabled detection of Escherichia coli using a cell-phone. Analyst 2012, 137, 2541–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, T.S.; Li, W.; McCracken, K.E.; Yoon, J.-Y. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip 2013, 13, 4832–4840. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, P.B.; Huang, M.-C.; Truong, N.; Ho, C.-M. Rapid electrochemical detection on a mobile phone. Lab Chip 2013, 13, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Preechaburana, P.; Gonzalez, M.C.; Suska, A.; Filippini, D. Surface plasmon resonance chemical sensing on cell phones. Angew. Chem. Int. Ed. Engl. 2012, 51, 11585–11588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yaglidere, O.; Su, T.W.; Tseng, D.; Ozcan, A. Wide-field fluorescent microscopy on a cell-phone. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 6801–6804. [Google Scholar]
- Zhu, H.; Mavandadi, S.; Coskun, A.F.; Yaglidere, O.; Ozcan, A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal. Chem. 2011, 83, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Qi, H.; Luo, W.; Tseng, D.; Ki, S.J.; Wan, Z.; Göröcs, Z.; Bentolila, L.A.; Wu, T.-T.; Sun, R.; et al. Fluorescent Imaging of Single Nanoparticles and Viruses on a Smart Phone. ACS Nano 2013, 7, 9147–9155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Yaglidere, O.; Su, T.-W.; Tseng, D.; Ozcan, A. Cost-effective and compact wide-field fluorescent imaging on a cell-phone. Lab Chip 2011, 11, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Luo, W.; Chiang, S.; Kappel, T.; Mejia, C.; Tseng, D.; Chan, R.Y.L.; Yan, E.; Qi, H.; Shabbir, F.; et al. Imaging and Sizing of Single DNA Molecules on a Mobile Phone. ACS Nano 2014, 8, 12725–12733. [Google Scholar] [CrossRef] [PubMed]
- Nwajiaku, L.; Mbachu, I.; Ikeako, L. Prevalence, Clinical Pattern and Major Causes of Male Infertility in Nnewi, South East Nigeria: A Five Year Review. Afrimedic J. 2012, 3, 16–19. [Google Scholar]
- Centers for Disease Control and Prevention. 2016. Available online: http://www.cdc.gov/reproductivehealth/infertility/ (accessed on 20 August 2018).

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, S.-J.; Kuan, C.-M.; Hung, M.-W.; Fu, Y.; Yeh, J.A.; Yao, D.-J.; Cheng, C.-M. A Simple Imaging Device for Fluorescence-Relevant Applications. Micromachines 2018, 9, 418. https://doi.org/10.3390/mi9080418
Lo S-J, Kuan C-M, Hung M-W, Fu Y, Yeh JA, Yao D-J, Cheng C-M. A Simple Imaging Device for Fluorescence-Relevant Applications. Micromachines. 2018; 9(8):418. https://doi.org/10.3390/mi9080418
Chicago/Turabian StyleLo, Shih-Jie, Chen-Meng Kuan, Min-Wei Hung, Yun Fu, J. Andrew Yeh, Da-Jeng Yao, and Chao-Min Cheng. 2018. "A Simple Imaging Device for Fluorescence-Relevant Applications" Micromachines 9, no. 8: 418. https://doi.org/10.3390/mi9080418




