On-Chip Asymmetric Microsupercapacitors Combining Reduced Graphene Oxide and Manganese Oxide for High Energy-Power Tradeoff
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Interdigital Gold Micro-Current Collectors
2.2. Active Material Integration
2.3. Material Characterization
2.4. Electrochemical Characterization
3. Results
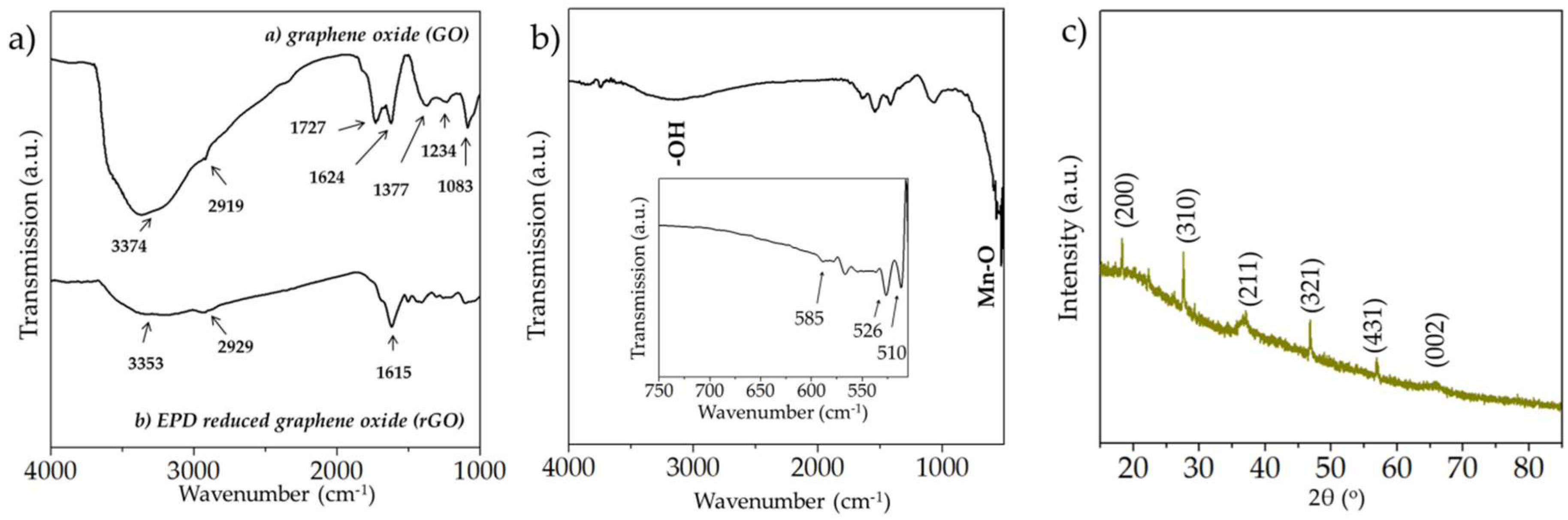
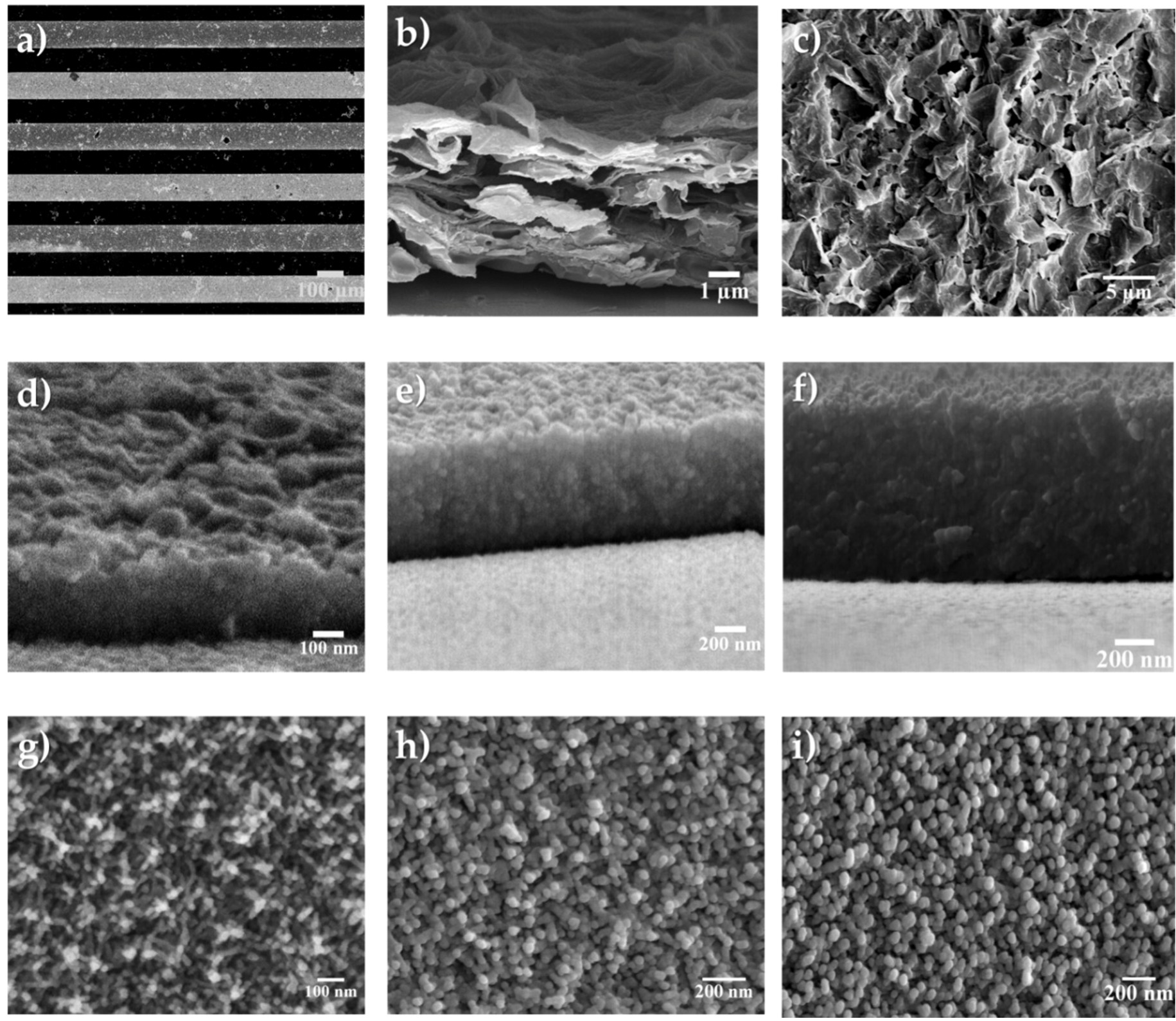
3.1. Spectroscopic, Crystallographic and Microstructural Characterization Performed on the Manganese Oxide and rGO Microelectrodes
3.2. Electrochemical Characterization of the Symmetric rGO//rGO, MnOx//MnOx, and Asymmetric rGO-MnOx Microsupercapacitors
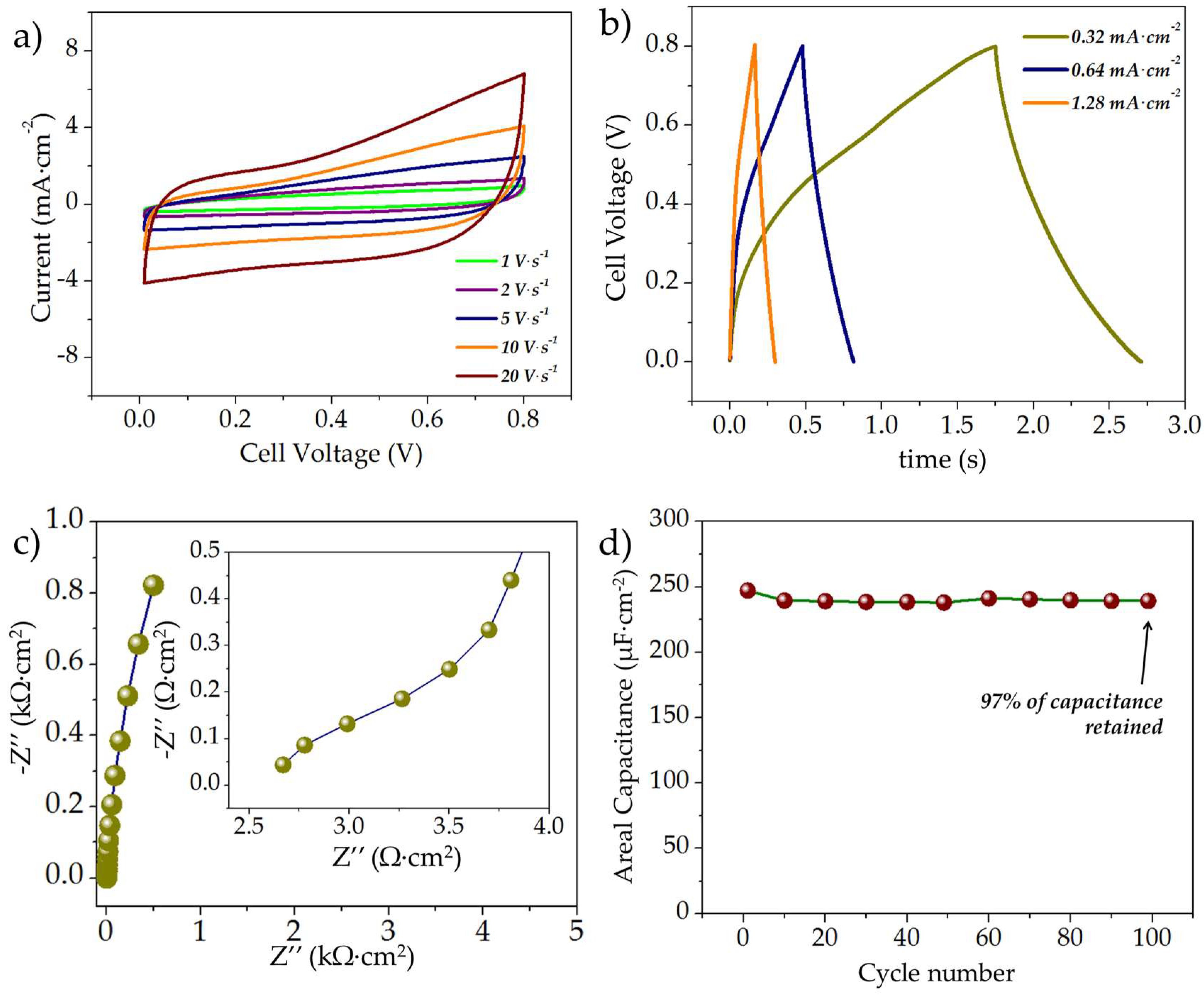
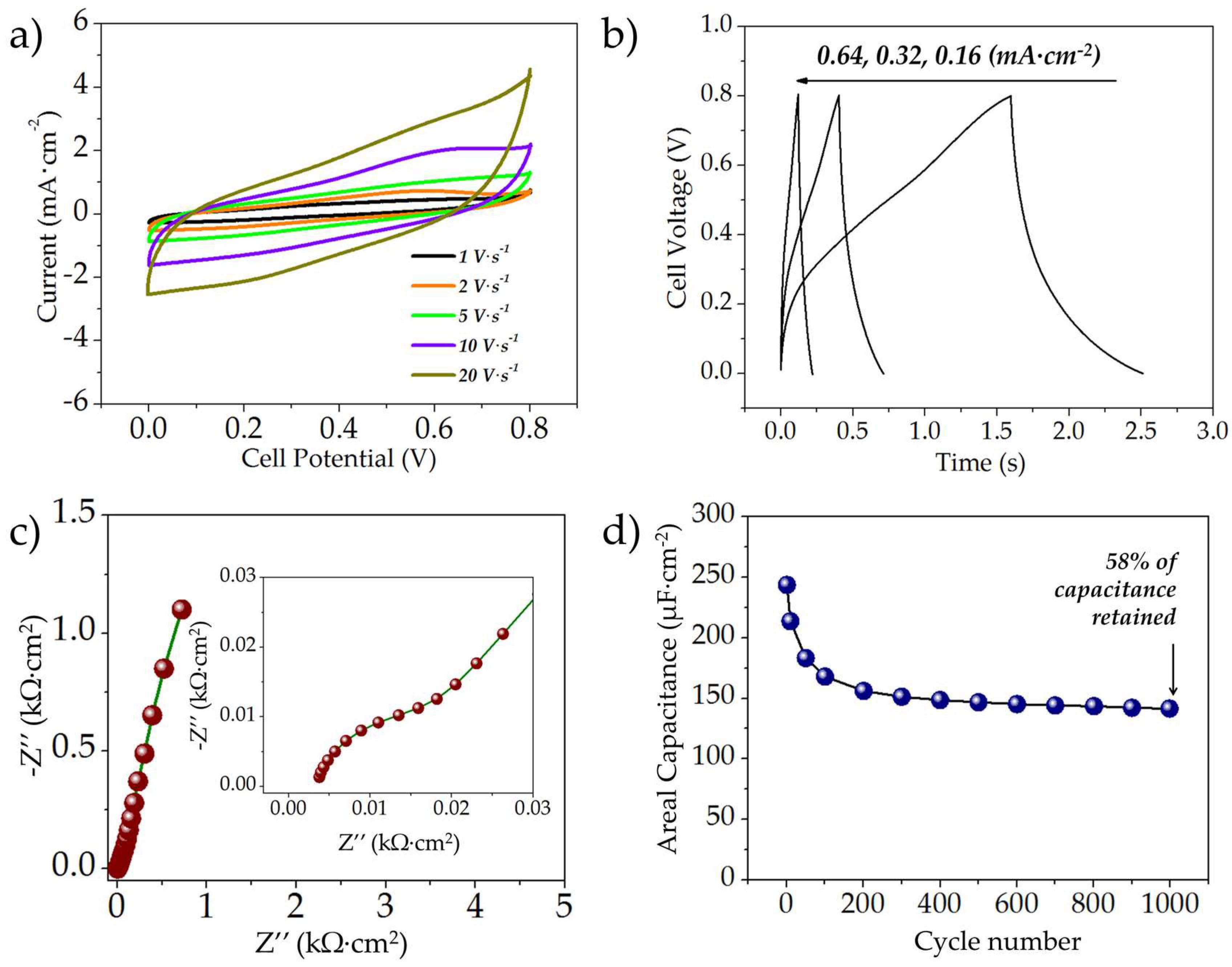
3.2.1. Electrochemical Evaluation of the Symmetric rGO//rGO MSCs
3.2.2. Electrochemical Characterization of the MnOx//MnOx Symmetric MSCs
3.2.3. Electrochemical Evaluation of the Asymmetric rGO//MnOx MSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Agrawal, R.; Chen, C.; Hao, Y.; Song, Y.; Wang, C. Graphene for supercapacitors. In Graphene Based Energy Devices; Rashid bin Mohd Yusoff, A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 171–214. [Google Scholar]
- Algharaibeh, Z.; Liu, X.; Pickup, P.G. An asymmetric anthraquinone-modified carbon/ruthenium oxide supercapacitor. J. Power Sources 2009, 187, 640–643. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Pinero, E.; Béguin, F. Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2 V in aqueous medium. J. Power Sources 2006, 153, 183–190. [Google Scholar] [CrossRef]
- Long, J.W.; Bélanger, D.; Brousse, T.; Sugimoto, W.; Sassin, M.B.; Crosnier, O. Asymmetric electrochemical capacitors—Stretching the limits of aqueous electrolytes. MRS Bull. 2011, 36, 513–522. [Google Scholar] [CrossRef]
- Hong, M.S.; Lee, S.H.; Kim, S.W. Use of KCl aqueous electrolyte for 2 V manganese oxide/activated carbon hybrid capacitor. Electrochem. Solid-State Lett. 2002, 5, A227–A230. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, K. Asymmetric flow electrochemical capacitor with high energy densities based on birnessite-type manganese oxide nanosheets and activated carbon slurries. J. Mater. Sci. 2016, 51, 9306–9313. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Guan, Q.F.; Yu, S.H. Bacterial-cellulose-derived carbon nanofiber@MnO2 and nitrogen-doped carbon nanofiber electrode materials: An asymmetric supercapacitor with high energy and power density. Adv. Mater. 2013, 25, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Li, F.; Cheng, H.M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor. J. Power Sources 2008, 185, 1563–1568. [Google Scholar] [CrossRef]
- Ye, X.-D.; Hu, J.-G.; Yang, Q.; Zheng, Y.-F.; Huang, W.-Z. Preparation and properties of NiO/AC asymmetric capacitor. J. Inorg. Mater. 2014, 29, 250–256. [Google Scholar]
- Sivakkumar, S.R.; Pandolfo, A.G. Evaluation of lithium-ion capacitors assembled with pre-lithiated graphite anode and activated carbon cathode. Electrochim. Acta 2012, 65, 280–287. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, C.; Lv, R.; Bai, Y.; Lin, Y.; Huang, Z.H.; Shen, W.; Qiu, X.; Kang, F. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 15, 43–53. [Google Scholar] [CrossRef]
- Ren, J.J.; Su, L.W.; Qin, X.; Yang, M.; Wei, J.P.; Zhou, Z.; Shen, P.W. Pre-lithiated graphene nanosheets as negative electrode materials for Li-ion capacitors with high power and energy density. J. Power Sources 2014, 264, 108–113. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Badway, F.; Du Pasquier, A.; Zheng, T. An asymmetric hybrid nonaqueous energy storage cell. J. Electrochem. Soc. 2001, 148, A930–A939. [Google Scholar] [CrossRef]
- Agrawal, R.; Chen, C.; Dages, S.; Wang, C. A high energy 3V lithium-ion capacitor synthesized via electrostatic spray deposition. Adv. Mater. Lett. 2017, 8, 783–790. [Google Scholar] [CrossRef]
- Agrawal, R.; Hao, Y.; Song, Y.; Chen, C.; Wang, C. Hybridization of lithium-ion batteries and electrochemical capacitors: Fabrication and challenges. In Proceedings of the 2015 SPIE Sensing Technology + Applications, Baltimore, MD, USA, 20–24 April 2015. [Google Scholar]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.I.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Hu, X.; Huai, Y.; Lin, Z.; Suo, J.; Deng, Z. A (LiFePO4–AC)/Li4Ti5O12 hybrid battery capacitor. J. Electrochem. Soc. 2007, 154, A1026–A1030. [Google Scholar] [CrossRef]
- Hu, X.B.; Lin, Z.J.; Liu, L.; Huai, J.Y.; Deng, H.Z. Effects of the LiFePO4 content and the preparation method on the properties of (LiFePO4 + AC)/Li4Ti5O12 hybrid batterycapacitors. J. Serb. Chem. Soc. 2010, 75, 1259–1269. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Flyunt, R.; Knolle, W.; Kahnt, A.; Prager, A.; Lotnyk, A.; Malig, J.; Guldi, D.; Abel, B. Mechanistic aspects of the radiation-chemical reduction of graphene oxide to graphene-like materials. Int. J. Radiat. Biol. 2014, 90, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Fam, D.W.; Boccaccini, A.R.; Shaffer, M.S. Electrophoretic deposition of graphene-related materials: A review of the fundamentals. Prog. Mater. Sci. 2016, 82, 83–117. [Google Scholar] [CrossRef] [Green Version]
- An, S.J.; Zhu, Y.; Lee, S.H.; Stoller, M.D.; Emilsson, T.; Park, S.; Velamakanni, A.; An, J.; Ruoff, R.S. Thin film fabrication and simultaneous anodic reduction of deposited graphene oxide platelets by electrophoretic deposition. J. Phys. Chem. Lett. 2010, 1, 1259–1263. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zhang, D.; Sun, X.; Lin, H.; Wang, C.; Ma, Y. One-step electrophoretic deposition of reduced graphene oxide and Ni(OH)2 composite films for controlled syntheses supercapacitor electrodes. J. Phys. Chem. B 2012, 117, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Adelowo, E.; Baboukani, A.R.; Villegas, M.F.; Henriques, A.; Wang, C. Electrostatic spray deposition-based manganese oxide films—From pseudocapacitive charge storage materials to three-dimensional microelectrode integrands. Nanomaterials 2017, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, X.; Li, S.; Zhang, W.; Kang, F. A high-energy-density micro supercapacitor of asymmetric MnO2–carbon configuration by using micro-fabrication technologies. J. Power Sources 2013, 234, 302–309. [Google Scholar] [CrossRef]
- Dinh, T.M.; Mesnilgrente, F.; Conédéra, V.; Kyeremateng, N.A.; Pech, D. Realization of an asymmetric interdigitated electrochemical micro-capacitor based on carbon nanotubes and manganese oxide. J. Electrochem. Soc. 2015, 162, A2016–A2020. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.W.; Feng, Y.Q.; Yan, X.B.; Chen, J.T.; Xue, Q.J. Superior micro-supercapacitors based on graphene quantum dots. Adv. Funct. Mater. 2013, 23, 4111–4122. [Google Scholar] [CrossRef]
- Beidaghi, M.; Wang, C. Micro-supercapacitors based on interdigital electrodes of reduced graphene oxide and carbon nanotube composites with ultrahigh power handling performance. Adv. Funct. Mater. 2012, 22, 4501–4510. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Witit-Anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on epoxy resins-identification, monitoring the curing process, phase separation and water uptake. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophanides Theo., Ed.; InTech: London, UK, 2012. [Google Scholar]
- Sim, L.C.; Leong, K.H.; Ibrahim, S.; Saravanan, P. Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J. Mater. Chem. A 2014, 2, 5315–5322. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Qian, D.; Li, Y.; Zhang, W. Single-crystal α-MnO2 nanorods: Synthesis and electrochemical properties. Nanotechnology 2007, 18, 115616. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Z.; Dai, L.; Chen, L. Synthesis and characterization of single-crystalline nanorods of α-MnO2 and γ-MnOOH. Mater. Chem. Phys. 2005, 93, 149–153. [Google Scholar] [CrossRef]
- Wang, X.; Myers, B.D.; Yan, J.; Shekhawat, G.; Dravid, V.; Lee, P.S. Manganese oxide micro-supercapacitors with ultra-high areal capacitance. Nanoscale 2013, 5, 4119–4122. [Google Scholar] [CrossRef] [PubMed]
- Bakardjieva, S.; Bezdička, P.; Grygar, T.; Vorm, P. Reductive dissolution of microparticulate manganese oxides. J. Solid-State Electrochem. 2000, 4, 306–313. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zou, W.; Quan, B.; Yu, A.; Wu, H.; Jiang, P.; Wei, Z. An all-solid-state flexible micro-supercapacitor on a chip. Adv. Energy Mater. 2011, 1, 1068–1072. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-dimensional graphene carbon nanotube carpet-based microsupercapacitors with high electrochemical performance. Nano Lett. 2012, 13, 72–78. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nanotechnology 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Long, X.; Zhou, H.; Guo, E.; Wang, X.; Hu, Z. On-chip interdigitated supercapacitor based on nano-porous gold/manganese oxide nanowires hybrid electrode. Electrochim. Acta 2015, 163, 107–115. [Google Scholar] [CrossRef]



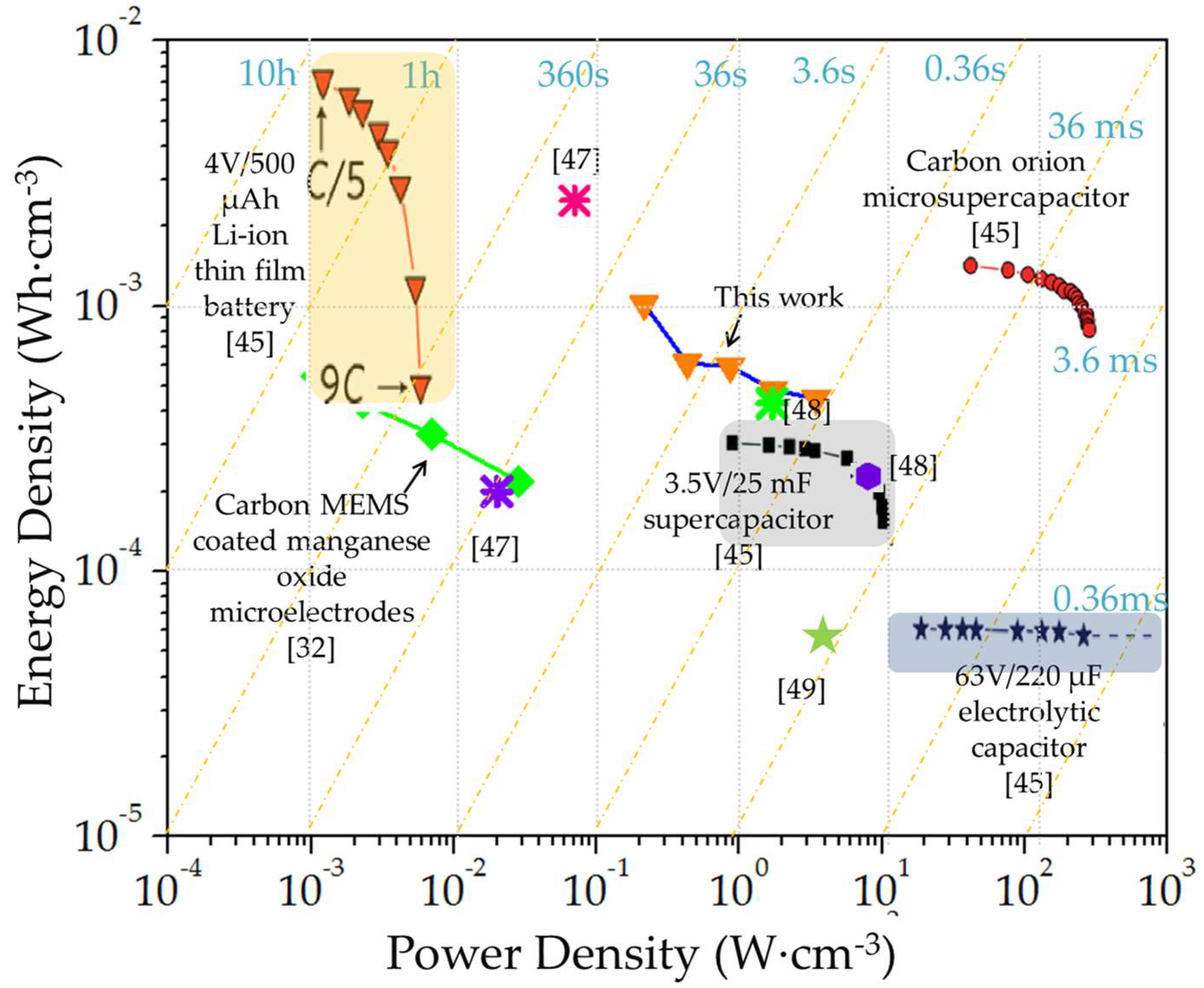
| Device Design | Electro-Active Materials | Electrolyte | Specific Capacitance | Energy-Power Characteristics | Ref |
|---|---|---|---|---|---|
| Sandwich | Carbon Microelectromechanical systems (C-MEMS) coated manganese oxide | Aqueous 1 M Na2SO4 | Maximal areal capacitance of 0.055 F·cm−2 and stack capacitance of 7.4 F·cm−3 | Stack energy and power densities of 0.51 mWh·cm−3 and 28.3 mW·cm−3, respectively | [32] |
| Interdigital | Graphene quantum dots//manganese oxide | Aqueous 0.5 M Na2SO4 | 1.1 mF·cm−2 | 0.154 μWh·cm−2 at a specific power of 7.51 μW·cm−2 | [36] |
| Interdigital | Onion-like carbon | 1 M Et4NBF4 in PC | 1.35 F·cm−3 at 1 V·s−1 | Stack energy density of ~1.7 mWh·cm−3 and power density of 200–250 W·cm−3 | [45] |
| Interdigital | Nano-porous gold/MnO2 | (PVA)/H2SO4 | - | Energy density of 55 μWh·cm−3 Power density of 3.4 W·cm−3 | [49] |
| Interdigital | Manganese oxide Reduced graphene oxide | 1 M Na2SO4 | Maximal areal capacitance of 1.63 mF·cm−2, equivalent to a stack/volumetric capacitance of 3.6 F·cm−3 | Maximal energy density of 1.02 mWh·cm−3 Maximal power density of 3.44 W·cm−3 Areal energy density of 0.274 μWh·cm−2 at a power density of 193.6 μW·cm−2 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, R.; Wang, C. On-Chip Asymmetric Microsupercapacitors Combining Reduced Graphene Oxide and Manganese Oxide for High Energy-Power Tradeoff. Micromachines 2018, 9, 399. https://doi.org/10.3390/mi9080399
Agrawal R, Wang C. On-Chip Asymmetric Microsupercapacitors Combining Reduced Graphene Oxide and Manganese Oxide for High Energy-Power Tradeoff. Micromachines. 2018; 9(8):399. https://doi.org/10.3390/mi9080399
Chicago/Turabian StyleAgrawal, Richa, and Chunlei Wang. 2018. "On-Chip Asymmetric Microsupercapacitors Combining Reduced Graphene Oxide and Manganese Oxide for High Energy-Power Tradeoff" Micromachines 9, no. 8: 399. https://doi.org/10.3390/mi9080399




