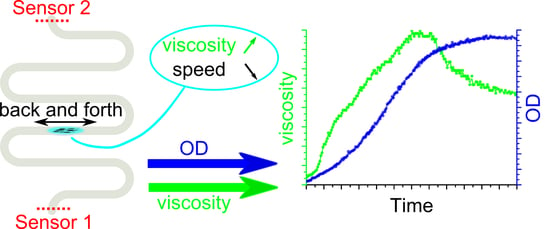
Simultaneous Measurement of Viscosity and Optical Density of Bacterial Growth and Death in a Microdroplet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Requirements for the Microfludic Chip-Method Description
2.3. Construction of the Microfluidic System
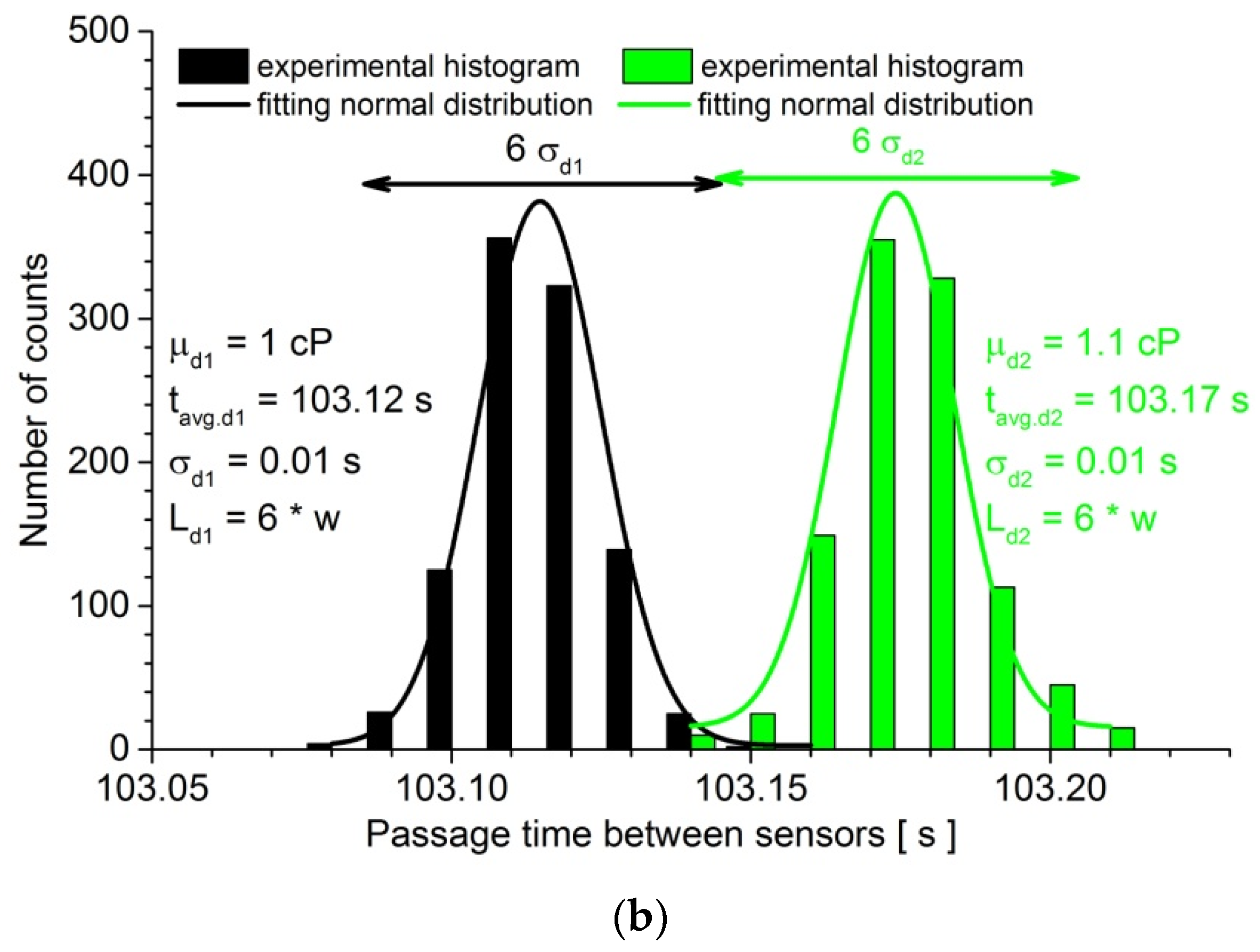
3. Results
3.1. Calibration of the Measurement System
3.2. Viscosity Measurements during Bacterial Culture Growth
3.3. The Verification of Cross-Contamination of the Continious Phase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Einstein, A. Eine neue Bestimmung der der Moleküldimensionen. Annalen Physik 1906, 19, 289–306. [Google Scholar] [CrossRef]
- Karimi, A.; Karig, D.; Kumar, A.; Ardekani, A.M. Interplay of physical mechanisms and biofilm processes: Review of microfluidic methods. Lab Chip 2015, 15, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Cichocki, B.; Ekiel-Jeżewska, M.L.; Słowicka, A.; Wajnryb, E.; Wasilewska, M. Fibrinogen conformations and charge in electrolyte solutions derived from DLS and dynamic viscosity measurements. J. Colloid Interface Sci. 2012, 385, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, J.-K. Microfluidic Rheometer for Characterization of Protein Unfolding and Aggregation in Microflows. Small 2010, 6, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.X. Measurements of fluid viscosity using a miniature ball drop device. Rev. Sci. Instrum. 2016, 87, 054301. [Google Scholar] [CrossRef] [PubMed]
- McKennell, R. Cone-Plate Viscometer. Anal. Chem. 1956, 28, 1710–1714. [Google Scholar] [CrossRef]
- Dennington, S.; Mekkhunthod, P.; Rides, M.; Gibbs, D.; Salta, M.; Stoodley, V.; Wharton, J.; Stoodley, P. Miniaturized rotating disc rheometer test for rapid screening of drag reducing marine coatings. Surf. Topogr. Metrol. Prop. 2015, 3, 034004. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Cho, Y.I.; Lee, D.-H.; Park, C.-M.; Moon, H.-W.; Hur, M.; Kim, J.Q.; Yun, Y.-M. Analytical performance evaluation of the scanning capillary tube viscometer for measurement of whole blood viscosity. Clin. Biochem. 2013, 46, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Rhoden, F. A modified Ubbelohde viscometer for measurement of the viscosity of polymer solutions. J. Phys. E Sci. Instrum. 1969, 2, 1134. [Google Scholar] [CrossRef]
- Anderson, A.M.; Bruno, B.A.; Smith, L.S. Viscosity Measurement. In Handbook of Measurement in Science and Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA; American Cancer Society: Atlanta, GA, USA, 2013; pp. 947–979. ISBN 978-1-118-43670-7. [Google Scholar]
- Zhang, Y.; Wu, X.; Wang, Y.; Zhu, S.; Gao, B.Z.; Yuan, X.-C. Measurement of the microscopic viscosities of microfluids with a dynamic optical tweezers system. Laser Phys. 2014, 24, 065601. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Xing, D. Photoacoustic measurement of liquid viscosity. Appl. Phys. Lett. 2010, 96, 211102. [Google Scholar] [CrossRef]
- Parker, W.C.; Chakraborty, N.; Vrikkis, R.; Elliott, G.; Smith, S.; Moyer, P.J. High-resolution intracellular viscosity measurement using time-dependent fluorescence anisotropy. Opt. Express 2010, 18, 16607–16617. [Google Scholar] [CrossRef] [PubMed]
- Pipe, C.J.; McKinley, G.H. Microfluidic rheometry. Mech. Res. Commun. 2009, 36, 110–120. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, W.S.; Vanapalli, S.A. Microfluidic viscometers for shear rheology of complex fluids and biofluids. Biomicrofluidics 2016, 10, 043402. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Burns, M.A. Analysis of Non-Newtonian Liquids Using a Microfluidic Capillary Viscometer. Anal. Chem. 2006, 78, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, M.G.; Park, J.-K. Microfluidic parallel circuit for measurement of hydraulic resistance. Biomicrofluidics 2010, 4, 034110. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.M.; Furst, E.M. High-throughput rheology in a microfluidic device. Lab Chip 2011, 11, 3802–3809. [Google Scholar] [CrossRef] [PubMed]
- Livak-Dahl, E.; Lee, J.; Burns, M.A. Nanoliter droplet viscometer with additive-free operation. Lab Chip 2013, 13, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ward, K.R.; Burns, M.A. Viscosity Measurements Using Microfluidic Droplet Length. Anal. Chem. 2017, 89, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Bronfenbrenner, J. Changes in viscosity during lysis of bacteria by bacteriophage. Proc. Soc. Exp. Biol. Med. 1926, 23, 635–636. [Google Scholar] [CrossRef]
- Patrício, P.; Almeida, P.L.; Portela, R.; Sobral, R.G.; Grilo, I.R.; Cidade, T.; Leal, C.R. Living bacteria rheology: Population growth, aggregation patterns, and collective behavior under different shear flows. Phys. Rev. E 2014, 90, 022720. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Patrício, P.; Almeida, P.L.; Sobral, R.G.; Franco, J.M.; Leal, C.R. Rotational tumbling of Escherichia coli aggregates under shear. Phys. Rev. E 2016, 94, 062402. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.R.; Doetsch, R.N. Effect of viscosity on bacterial motility. J. Bacteriol. 1974, 117, 696–701. [Google Scholar] [PubMed]
- Harman, M.; Vig, D.K.; Radolf, J.D.; Wolgemuth, C.W. Viscous Dynamics of Lyme Disease and Syphilis Spirochetes Reveal Flagellar Torque and Drag. Biophys. J. 2013, 105, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, V.; Ceri, H.; Martinuzzi, R.J. Increased tolerance of Staphylococcus aureus to vancomycin in viscous media. FEMS Immunol. Med. Microbiol. 2007, 51, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Kummerli, R.; Griffin, A.S.; West, S.A.; Buckling, A.; Harrison, F. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. R. Soc. B Biol. Sci. 2009, 276, 3531–3538. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.H.; Kretschmer, L.; Will, S.; Leipertz, A.; Fröba, A.P. Density, Surface Tension, and Kinematic Viscosity of Hydrofluoroethers HFE-7000, HFE-7100, HFE-7200, HFE-7300, and HFE-7500. J. Chem. Eng. Data 2015, 60, 3759–3765. [Google Scholar] [CrossRef]
- Battino, R.; Rettich, T.R.; Tominaga, T. The Solubility of Oxygen and Ozone in Liquids. J. Phys. Chem. Ref. Data 1983, 12, 163–178. [Google Scholar] [CrossRef]
- Jakiela, S.; Korczyk, P.M.; Makulska, S.; Cybulski, O.; Garstecki, P. Discontinuous Transition in a Laminar Fluid Flow: A Change of Flow Topology inside a Droplet Moving in a Micron-Size Channel. Phys. Rev. Lett. 2012, 108, 134501. [Google Scholar] [CrossRef] [PubMed]
- Makulska, S.; Jakiela, S.; Garstecki, P. A micro-rheological method for determination of blood type. Lab Chip 2013, 13, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, S. Measurement of the hydrodynamic resistance of microdroplets. Lab Chip 2016, 16, 3695–3699. [Google Scholar] [CrossRef] [PubMed]
- Vanapalli, S.A.; Banpurkar, A.G.; van den Ende, D.; Duits, M.H.G.; Mugele, F. Hydrodynamic resistance of single confined moving drops in rectangular microchannels. Lab Chip 2009, 9, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Puel, F.; Chevalier, Y.; Galvan, J.-M.; Rivoire, A.; Klein, J.-P. Study of droplet size distribution during an emulsification process using in situ video probe coupled with an automatic image analysis. Chem. Eng. J. 2010, 165, 946–957. [Google Scholar] [CrossRef]
- Coulliette, C.; Pozrikidis, C. Motion of an array of drops through a cylindrical tube. J. Fluid Mech. 1998, 358, 1–28. [Google Scholar] [CrossRef]
- Erb, R.M.; Obrist, D.; Chen, P.W.; Studer, J.; Studart, A.R. Predicting sizes of droplets made by microfluidic flow-induced dripping. Soft Matter 2011, 7, 8757–8761. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Mahler, L.; Tovar, M.; Weber, T.; Brandes, S.; Michael Rudolph, M.; Ehgartner, J.; Mayr, T.; Thilo Figge, M.; Roth, M.; Zang, E. Enhanced and homogeneous oxygen availability during incubation of microfluidic droplets. RSC Adv. 2015, 5, 101871–101878. [Google Scholar] [CrossRef]
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of Phosphorescence from Nanoparticles Yields Accurate Measurement of Concentration of Oxygen in Microdroplets, Allowing One to Monitor the Metabolism of Bacteria. Anal. Chem. 2016, 88, 12006–12012. [Google Scholar] [CrossRef] [PubMed]
- Sklodowska, K.; Jakiela, S. Enhancement of bacterial growth with the help of immiscible oxygenated oils. RSC Adv. 2017, 7, 40990–40995. [Google Scholar] [CrossRef]
- Gachelin, J.; Miño, G.; Berthet, H.; Lindner, A.; Rousselet, A.; Clément, É. Non-Newtonian viscosity of E-coli suspensions. Phys. Rev. Lett. 2013, 110, 268103. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, Y.; Wang, J.; Zhu, P.; Tian, Y.; Xu, M.; Wang, L.; Huang, X. Passive Mixing inside Microdroplets. Micromachines 2018, 9, 160. [Google Scholar] [CrossRef]
- Wioland, H.; Lushi, E.; Goldstein, R.E. Directed collective motion of bacteria under channel confinement. New J. Phys. 2016, 18, 075002. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Be’er, A.; Florin, E.-L.; Swinney, H.L. Collective motion and density fluctuations in bacterial colonies. Proc. Natl. Acad. Sci. USA 2010, 107, 13626–13630. [Google Scholar] [CrossRef] [PubMed]
- Saintillan, D.; Shelley, M.J. Emergence of coherent structures and large-scale flows in motile suspensions. J. R. Soc. Interface 2012, 9, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Collective motion of bacteria in two dimensions. Quant. Biol. 2015, 3, 199–205. [Google Scholar] [CrossRef]
- Douarche, C.; Buguin, A.; Salman, H.; Libchaber, A.E. Coli and Oxygen: A Motility Transition. Phys. Rev. Lett. 2009, 102, 198101. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sklodowska, K.; Debski, P.R.; Michalski, J.A.; Korczyk, P.M.; Dolata, M.; Zajac, M.; Jakiela, S. Simultaneous Measurement of Viscosity and Optical Density of Bacterial Growth and Death in a Microdroplet. Micromachines 2018, 9, 251. https://doi.org/10.3390/mi9050251
Sklodowska K, Debski PR, Michalski JA, Korczyk PM, Dolata M, Zajac M, Jakiela S. Simultaneous Measurement of Viscosity and Optical Density of Bacterial Growth and Death in a Microdroplet. Micromachines. 2018; 9(5):251. https://doi.org/10.3390/mi9050251
Chicago/Turabian StyleSklodowska, Karolina, Pawel R. Debski, Jacek A. Michalski, Piotr M. Korczyk, Miroslaw Dolata, Miroslaw Zajac, and Slawomir Jakiela. 2018. "Simultaneous Measurement of Viscosity and Optical Density of Bacterial Growth and Death in a Microdroplet" Micromachines 9, no. 5: 251. https://doi.org/10.3390/mi9050251