A Visualization Technique of a Unique pH Distribution around an Ion Depletion Zone in a Microchannel by Using a Dual-Excitation Ratiometric Method
Abstract
:1. Introduction
2. Materials and Methods
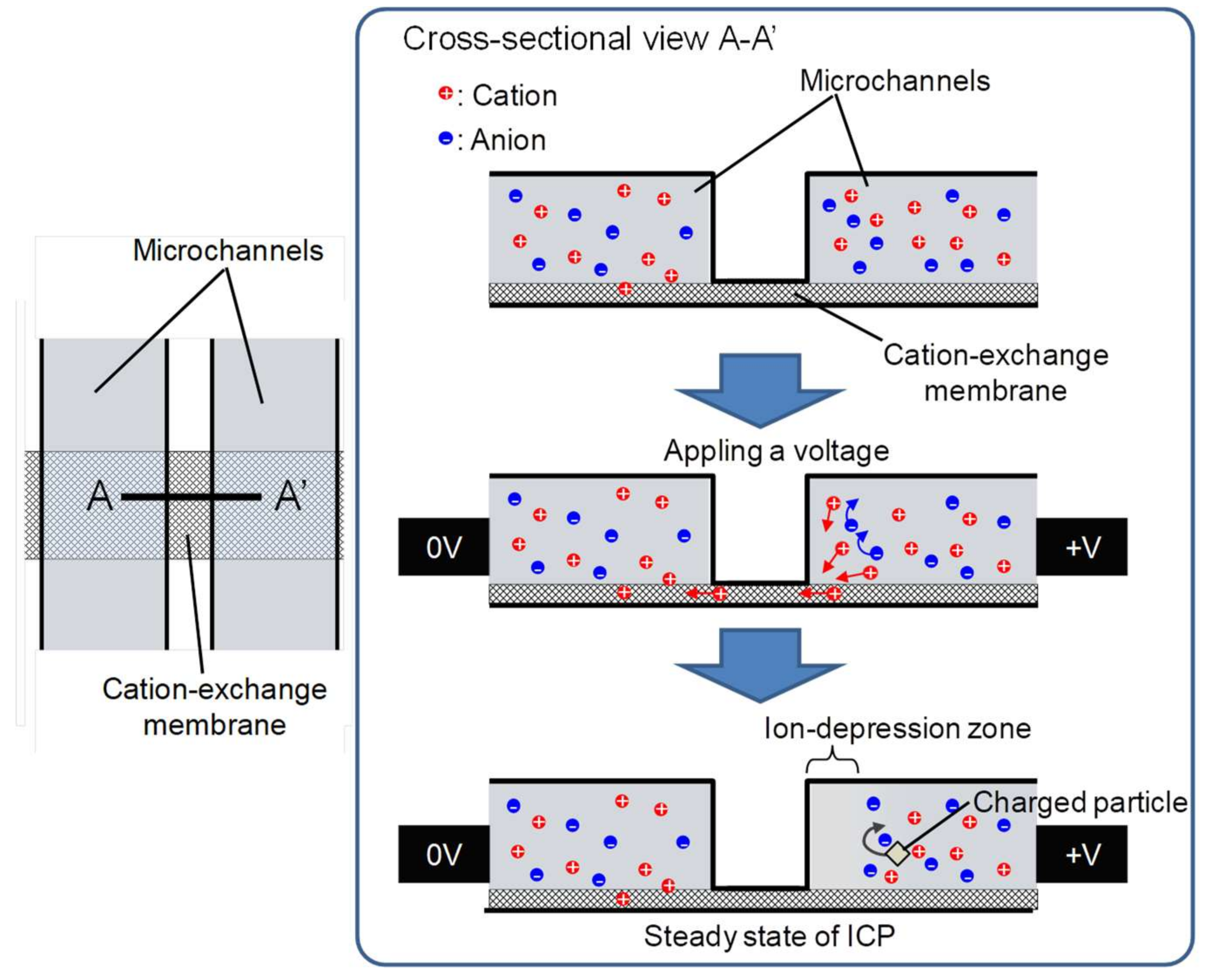
2.1. Ion Concentration Polarization (ICP) in Microchannels
2.2. Measurement Principles
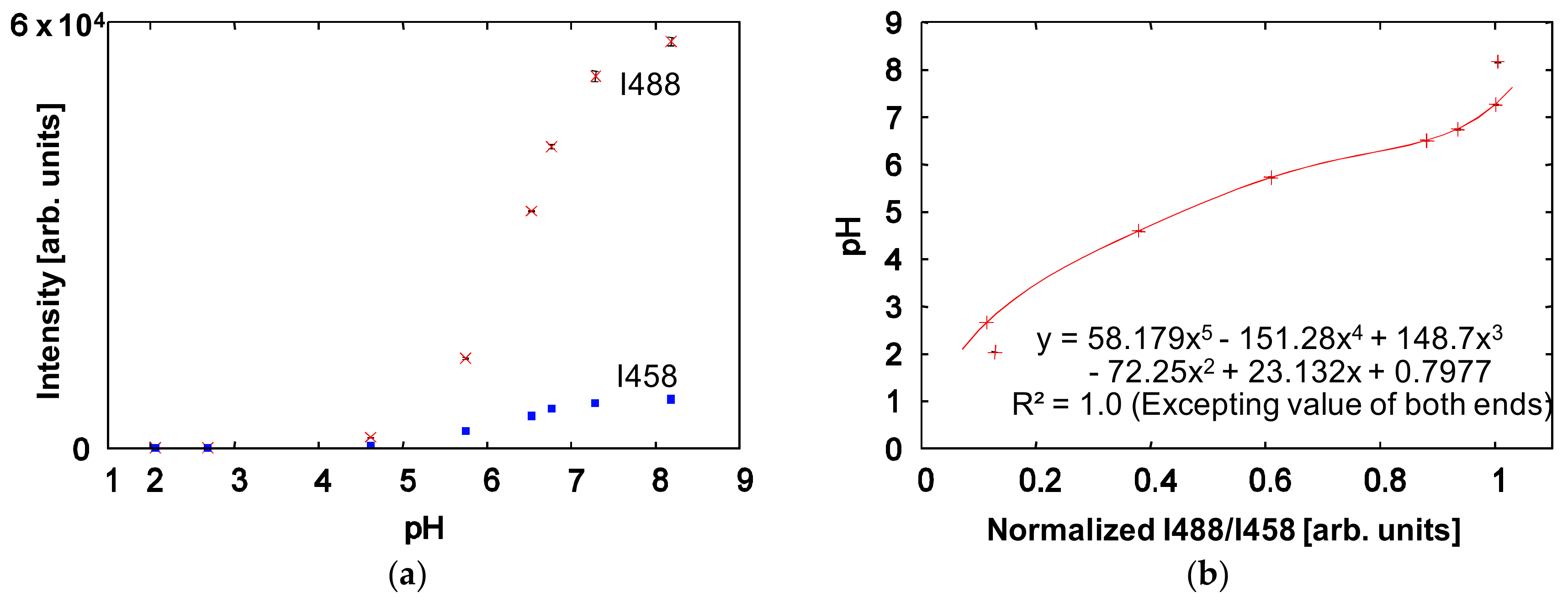
2.3. Standard Solutions for pH Calibration
3. Experimental Section
3.1. Experimental Setup
3.2. Device Fabrication
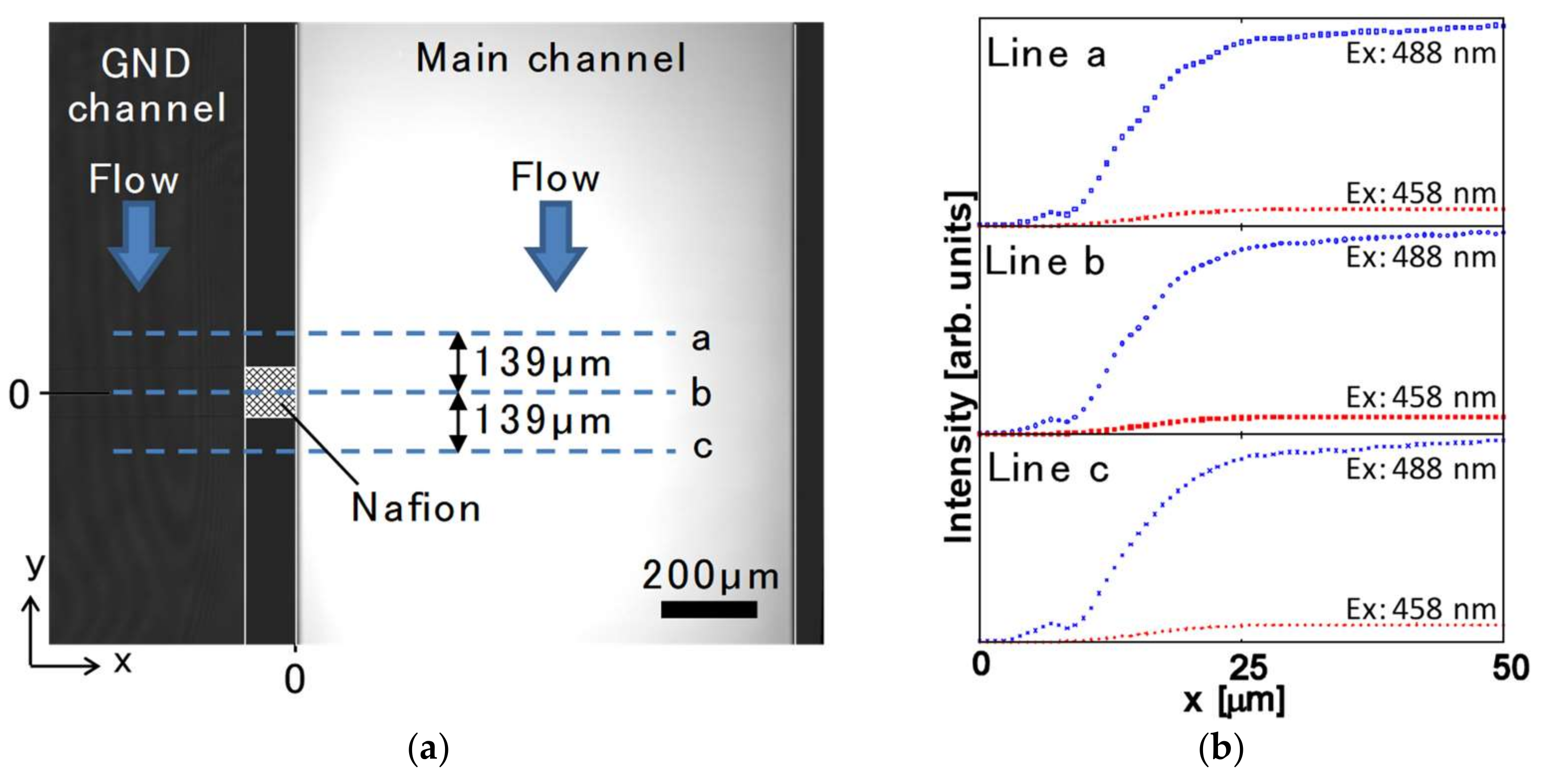
4. Results and Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Medintz, I.L.; Paegel, B.M.; Mathies, R.A. Microfabricated capillary array electrophoresis DNA analysis systems. J. Chromatogr. A 2001, 924, 265–270. [Google Scholar] [CrossRef]
- Chou, H.P.; Spence, C.; Scherer, A.; Quake, S. A microfabricated device for sizing and sorting DNA molecules. Proc. Natl. Acad. Sci. USA 1999, 96, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.; Shirley, S.G.; Schnelle, T.; Fuhr, G. Dielectrophoretic sorting of particles and cells in a microsystem. Anal. Chem. 1998, 70, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.W.; Reyes, C.D.; Lopez, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.W.; Yang, W.H. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes—Static runs. J. Membr. Sci. 2001, 183, 193–200. [Google Scholar]
- Bhattacharjee, S.; Chen, J.C.; Elimelech, M. Coupled model of concentration polarization and pore transport in crossflow nanofiltration. AIChE J. 2001, 47, 2733–2745. [Google Scholar] [CrossRef]
- Kim, J.; Cho, I.; Lee, H.; Kim, S.J. Ion concentration polarization by bifurcated current path. Sci. Rep. 2017, 7, 5091. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Lee, H.; Kang, K.H.; Lim, G. Ion concentration polarization-based continuous separation device using electrical repulsion in the depletion region. Sci. Rep. 2013, 3, 3483. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct seawater desalination by ion concentration polarization. Nat. Nanotechnol. 2010, 5, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Hyashida, K.; Mogi, K.; Yamamoto, T. Development of separation-and-concentration device for micro/nano particles by ion concentration polarization. In Proceedings of the 6th JSME Micro-Nano Symposium, Matsue, Shimane, Japan, 20–22 October 2014. [Google Scholar]
- Hyashida, K.; Mogi, K.; Honda, A.; Yamamoto, T. Separation and condensation of bio-nanoparticles using ion depletion effect. In Proceedings of the 7th JSME Micro-Nano Symposium, Niigata, Japan, 28–30 October 2015. [Google Scholar]
- Kwak, R.; Kim, S.J.; Han, J. Continuous-flow biomolecule and cell concentrator by ion concentration polarization. Anal. Chem. 2011, 83, 7348–7355. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yeh, C.P.; Yang, R.J. Ion concentration polarization near microchannel-nanochannel interfaces: Effect of pH value. Electrophoresis 2012, 33, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Fuest, M.; Rangharajan, K.K.; Boone, C.; Conlisk, A.T.; Prakash, S. Cation dependent surface charge regulation in gated nanofluidic devices. Anal. Chem. 2017, 89, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, H. Effect of nanochannel dimension on the transport of water molecules. J. Phys. Chem. B 2012, 116, 5925–5932. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.H.; Zhang, M.; Qian, S. Ion transport in a pH-regulated nanopore. Anal. Chem. 2013, 85, 7527–7534. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Lindqvist, L. PH-dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Mogi, K.; Sugii, Y.; Yamamoto, T.; Fujii, T. Rapid fabrication technique of nano/microfluidic device with high mechanical stability utilizing two-step soft lithography. Sens. Actuators B Chem. 2014, 201, 407–412. [Google Scholar] [CrossRef]
- Mogi, K.; Sugii, Y.; Fujii, T.; Matsumoto, Y. A microfluidic culturing system for observation of free-floating microorganisms. In Proceedings of the 11th International Conference on Nanochannels, Microchannels, and Minichannels, Sapporo, Japan, 16–19 June 2013. [Google Scholar]
- Ichiyanagi, M.; Sato, Y.; Hishida, K. Optically sliced measurement of velocity and pH distribution in microchannel. Exp. Fluids 2007, 43, 425–435. [Google Scholar] [CrossRef]
- Kazoe, Y.; Mawatari, K.; Sugii, Y.; Kitamori, T. Development of a measurement technique for ion distribution in an extended nanochannel by super-resolution-laser-induced fluorescence. Anal. Chem. 2011, 83, 8152–8157. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.N.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.Y.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Fujii, T. A novel assembly technique with semi-automatic alignment for pdms substrates. Lab Chip 2013, 13, 1044–1047. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogi, K. A Visualization Technique of a Unique pH Distribution around an Ion Depletion Zone in a Microchannel by Using a Dual-Excitation Ratiometric Method. Micromachines 2018, 9, 167. https://doi.org/10.3390/mi9040167
Mogi K. A Visualization Technique of a Unique pH Distribution around an Ion Depletion Zone in a Microchannel by Using a Dual-Excitation Ratiometric Method. Micromachines. 2018; 9(4):167. https://doi.org/10.3390/mi9040167
Chicago/Turabian StyleMogi, Katsuo. 2018. "A Visualization Technique of a Unique pH Distribution around an Ion Depletion Zone in a Microchannel by Using a Dual-Excitation Ratiometric Method" Micromachines 9, no. 4: 167. https://doi.org/10.3390/mi9040167



