SF6 Optimized O2 Plasma Etching of Parylene C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parylene C Preparation
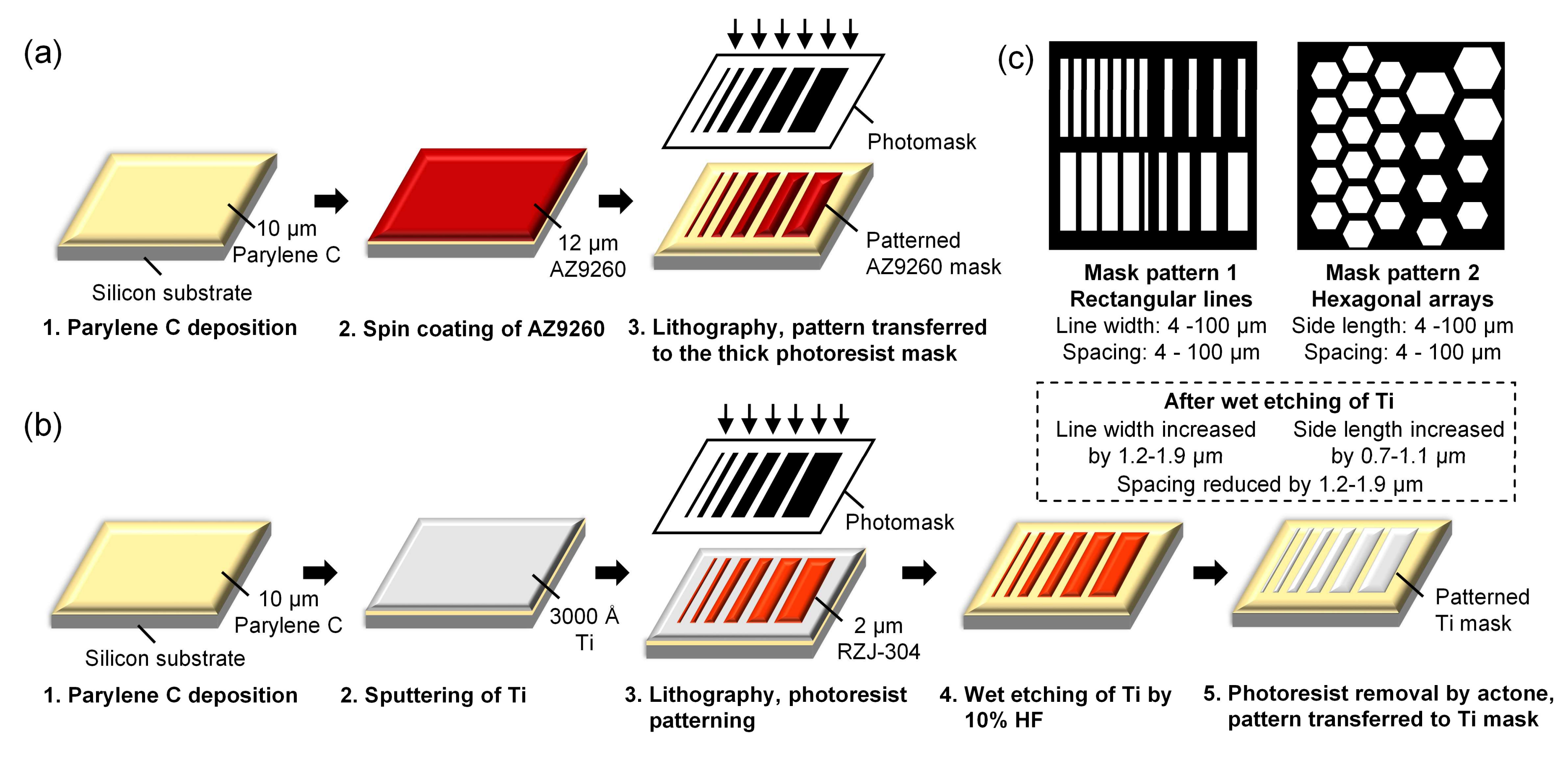
2.2. Mask for Etching
2.3. Dry Etching Conditions
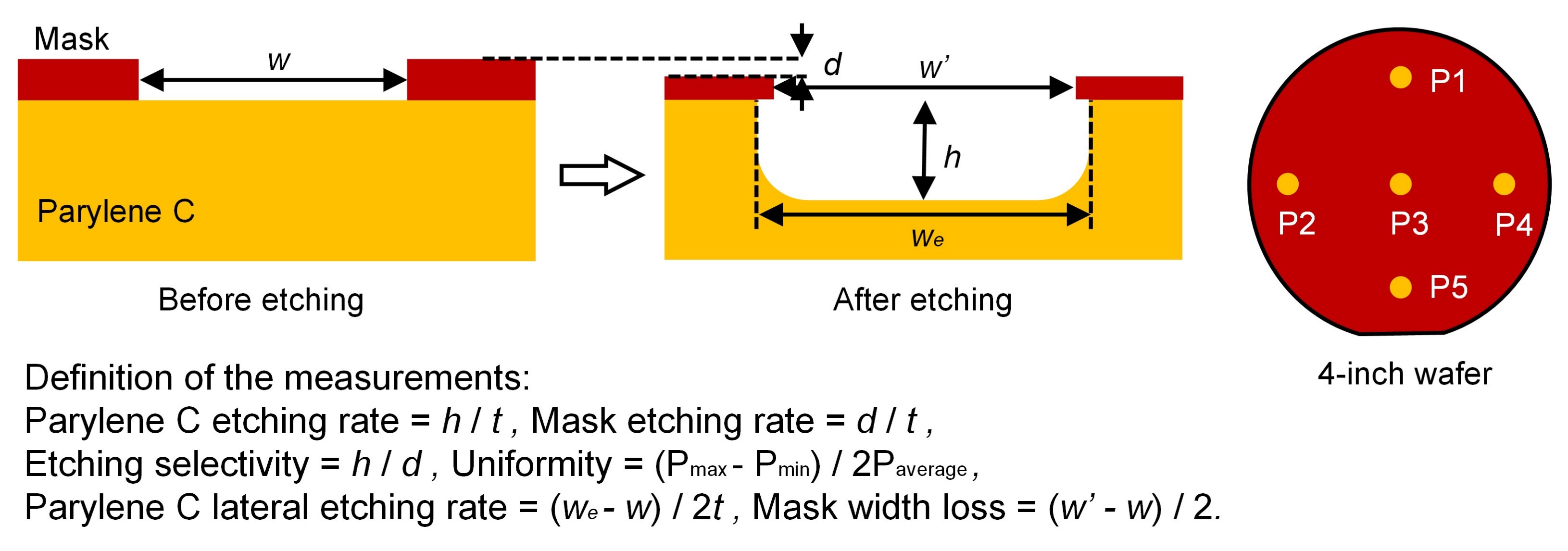
2.4. Etching Performance Measurements
3. Results and Discussions
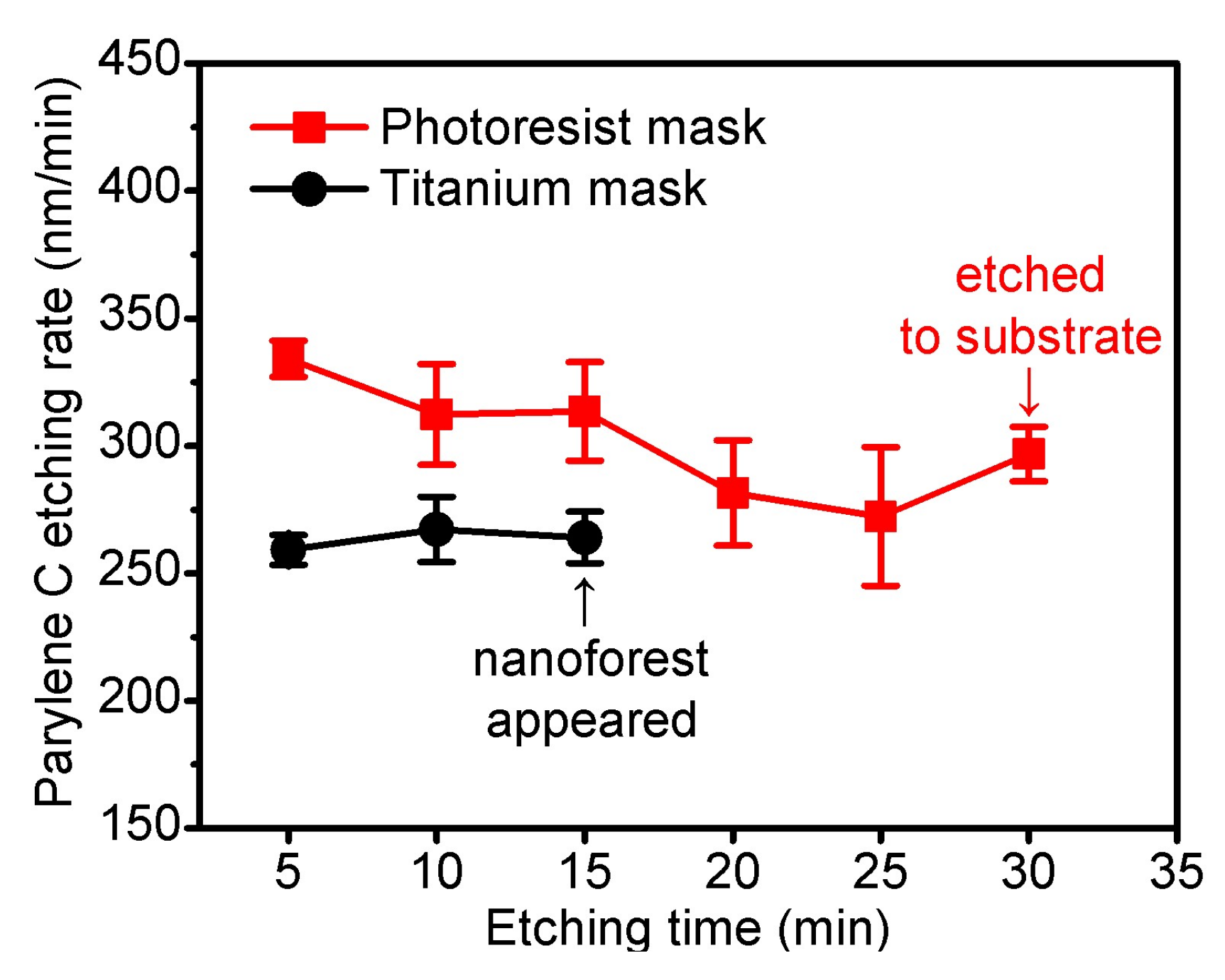
3.1. Reactive Ion Etching of Parylene C by Pure O2 Plasma
3.2. SF6 Optimized O2 Plasma Etching (SOOE)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, P.J.; Tai, Y.C. Floating-disk parylene micro check valve. In Proceedings of the 20th International Conference on Micro Electro Mechanical Systems, Kobe, Japan, 21–25 January 2007; pp. 453–456. [Google Scholar]
- Suzuki, Y.S.; Tai, Y.C. Micromachined high-aspect-ratio Parylene spring and its application to low-frequency accelerometers. J. Microelectromech. Syst. 2006, 15, 1364–1370. [Google Scholar] [CrossRef]
- Rodger, D.C.; Fong, A.J.; Li, W.; Ameri, H.; Ahuja, A.K.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.R.; Meng, E.; et al. Flexible Parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B 2008, 132, 449–460. [Google Scholar] [CrossRef]
- Trantidou, T.; Tariq, M.; Terracciano, C.M.; Toumazou, C.; Prodromakis, T. Parylene C-based flexible electronics for pH monitoring applications. Sensors 2014, 14, 11629–11639. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.T.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel flexible Parylene neural probe with 3D sheath structure for enhancing tissue integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zheng, N.; Wang, W.; Wang, S.; Zheng, X.; Li, Z. Electroplated nickel multielectrode microprobes with flexible Parylene cable for neural recording and stimulation. J. Microelectromech. Syst. 2013, 22, 1199–1206. [Google Scholar] [CrossRef]
- Solvent Resistance of the Parylenes. Available online: https://scscoatings.com/corporate/technical-library/ (accessed on 10 January 2018).
- Meng, E.; Li, P.Y.; Tai, Y.C. Plasma removal of Parylene C. J. Micromech. Microeng. 2008, 18, 512–520. [Google Scholar] [CrossRef]
- Yeh, J.T.C.; Grebe, K.R. Patterning of poly-para-xylylenes by reactive ion etching. J. Vac. Sci. Technol. A 1983, 1, 604–608. [Google Scholar] [CrossRef]
- Callahan, R.R.A.; Pruden, K.G.; Raupp, G.B.; Beaudoin, S.P. Downstream oxygen etching characteristics of polymers from the Parylene family. J. Vac. Sci. Technol. B 2003, 21, 1496–1500. [Google Scholar] [CrossRef]
- Tacito, R.D. Fine-line patterning of Parylene-n by reactive ion etching for application as an interlayer dielectric. J. Electrochem. Soc. 1996, 143, 1974–1977. [Google Scholar] [CrossRef]
- Trantidou, T.; Terracciano, C.M.; Kontziampasis, D.; Humphrey, E.J.; Prodromakis, T. Biorealistic cardiac cell culture platforms with integrated monitoring of extracellular action potentials. Sci. Rep. 2015, 5, 11067. [Google Scholar] [CrossRef] [PubMed]
- Kontziampasis, D.; Trantidou, T.; Regoutz, A.; Humphrey, E.J.; Carta, D.; Terracciano, C.M.; Prodromakis, T. Effects of Ar and O2 plasma etching on Parylene C: Topography versus surface chemistry and the impact on cell viability. Plasma Process. Polym. 2016, 13, 324–333. [Google Scholar] [CrossRef]
- Trantidou, T.; Prodromakis, T.; Toumazou, C. Oxygen plasma induced hydrophilicity of parylene-C thin films. Appl. Surf. Sci. 2012, 261, 43–51. [Google Scholar] [CrossRef]
- Noh, H.S.; Huang, Y.; Hesketh, P.J. Parylene micromolding, a rapid and low-cost fabrication method for Parylene microchannel. Sens. Actuators B 2004, 102, 78–85. [Google Scholar] [CrossRef]
- Youn, S.W.; Goto, H.; Takahashi, M.; Takahashi, S.; Oshinomi, Y.; Matsutani, K.; Maeda, R. A replication process of metallic micro-mold by using Parylene embossing and electroplating. Microelectron. Eng. 2008, 85, 161–167. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Bak, M.J.; Christensen, P. Laser exposure of Parylene C insulated microelectrodes. J. Neurosci. Methods 1995, 62, 89–92. [Google Scholar] [CrossRef]
- Yoo, J.M.; Song, J.I.; Tathireddy, P.; Solzbacher, F.; Rieth, L.W. Hybrid laser and reactive ion etching of Parylene C for deinsulation of a Utah electrode array. J. Micromech. Microeng. 2012, 22, 105036. [Google Scholar] [CrossRef]
- Lu, P.L.; Fan, C.L.; Yang, L.J.; Lin, C.W.; Jaw, F.S. Novel fabrication of full Parylene-isolated neuroprobes. J. Bionanosci. 2009, 3, 58–60. [Google Scholar] [CrossRef]
- Callahan, R.; Raupp, G.; Beaudoin, S. Etching Parylene-N using a remote oxygen microwave plasma. J. Vac. Sci. Technol. B 2002, 20, 1870–1877. [Google Scholar] [CrossRef]
- Callahan, R.R.A.; Raupp, G.B.; Beaudoin, S.P. Effects of gas pressure and substrate temperature on the etching of Parylene-N using a remote microwave oxygen plasma. J. Vac. Sci. Technol. B 2001, 19, 725–731. [Google Scholar] [CrossRef]
- Shutov, D.A.; Kim, S.I.; Kwon, K.H. On the etching mechanism of Parylene C in inductively coupled O2 plasma. Trans. Electr. Electron. Mater. 2008, 9, 156–162. [Google Scholar] [CrossRef]
- Ham, Y.H.; Shutov, D.A.; Kwon, K.H. Surface characteristics of etched parylene-C films for low-damaged patterning process using inductively-coupled O2/CHF3 gas plasma. Appl. Surf. Sci. 2013, 273, 287–292. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for bioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Meng, E.; Aoyagi, S.; Tai, Y.C. High aspect ratio Parylene etching for microfluidics and bioMEMS. Proc. Micro Total Anal. Syst. 2004, 297, 401–403. [Google Scholar]
- Selvarasah, S.; Chao, S.H.; Chen, C.L.; Sridhar, S.; Busnaina, A.; Khademhosseini, A.; Dokmeci, M.R. A reusable high aspect ratio Parylene C shadow mask technology for diverse micropatterning applications. Sens. Actuators B 2008, 145, 306–315. [Google Scholar] [CrossRef]
- Lecomte, A.; Lecestre, A.; Bourrier, D.; Blatché, M.C.; Jalabert, L.; Descamps, E.; Bergaud, C. Deep plasma etching of Parylene C patterns for biomedical applications. Microelectron. Eng. 2017, 177, 70–73. [Google Scholar] [CrossRef]
- Mao, H.; Wu, D.; Wu, W.; Xu, J.; Hao, Y. The fabrication of diversiform nanostructure forests based on residue nanomasks synthesized by oxygen plasma removal of photoresist. Nanotechnology 2009, 20, 445304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; She, D.; Chen, Q.; Li, Y.; Wu, W. Fabrication of amorphous silica nanowires via oxygen plasma treatment of polymers on silicon. J. Micromech. Microeng. 2017, 28, 024003. [Google Scholar] [CrossRef]
- Gogolides, E.; Constantoudis, V.; Kokkoris, G.; Kontziampasis, D.; Tsougeni, K.; Boulousis, G.; Vlachopoulou, M.; Tserepi, A. Controlling roughness: From etching to nanotexturing and plasma-directed organization on organic and inorganic materials. J. Phys. D Appl. Phys. 2011, 44, 174021. [Google Scholar] [CrossRef]
- Vourdas, N.; Kontziampasis, D.; Kokkoris, G.; Constantoudis, V.; Goodyear, A.; Tserepi, A.; Cooke, M.; Gogolides, E. Plasma directed assembly and organization: Bottom-up nanopatterning using top-down technology. Nanotechnology 2010, 21, 85302. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: From stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Measurements | |||||
|---|---|---|---|---|---|---|
| Mask Type | O2 Flow (sccm) | Power (W) | Parylene C Etching Rate (nm/min) | Mask Etching Rate (nm/min) | Selectivity | Uniformity |
| Photoresist | 60 | 150 | 218.4 ± 5.9 1 | 199.4 ± 17.1 | 1.10 ± 0.09 | 3.7% |
| 60 | 250 | 334.2 ± 7.1 | 321.1 ± 34.9 | 1.05 ± 0.11 | 2.6% | |
| 60 | 350 | 414.7 ± 4.7 | 426.9 ± 24.8 | 0.97 ± 0.05 | 1.4% | |
| 50 | 350 | 359.9 ± 5.5 | 366.7 ± 61.8 | 1.00 ± 0.17 | 2.1% | |
| 55 | 350 | 381.0 ± 12.5 | 392.1 ± 14.9 | 0.97 ± 0.05 | 1.7% | |
| 65 | 350 | 435.4 ± 4.2 | 445.2 ± 14.2 | 0.98 ± 0.04 | 1.0% | |
| Titanium | 60 | 350 | 358.3 ± 3.6 | <20 | >100 | 1.0% |
| 60 | 250 | 259.3 ± 5.9 | <20 | >100 | 2.8% | |
| Parameters | Measurements | ||||||
|---|---|---|---|---|---|---|---|
| Mask Type | O2 Flow (sccm) | SF6 Flow (sccm) | Power (W) | Parylene C Etching Rate (nm/min) | Mask Etching Rate (nm/min) | Selectivity | Uniformity |
| Titanium | 60 | 0 | 250 | 259.3 ± 5.9 1 | <2 | >100 | 2.8% |
| 60 | 5 | 250 | 352.4 ± 11.4 | 8.7 ± 0.5 | 40.4 ± 1.1 | 3.9% | |
| 60 | 8 | 250 | 349.6 ± 3.2 | 13.0 ± 0.7 | 27.1 ± 0.8 | 1.1% | |
| Mask Type | Etching Method | Minimum Feature Size | Parylene C Thickness | Aspect Ratio | Residuals |
|---|---|---|---|---|---|
| Aluminum (Ref. [27]) | O2 plasma, ICP (inductively coupled plasma) | 6 μm | 10–55 μm | 9:1 | Unavoidable residuals on the substrate (>1 μm in width & height) |
| Nickel (Ref. [28]) | O2 plasma, ICP-RIE | 50 μm | 23 μm | 1:2 | Residuals found in the opening & prevented the carved Parylene pieces from proper peeling |
| Titanium (this work) | O2 plasma, RIE | 2 μm | 4 μm (not etched to substrate) | 2:1 | Nanoforest residuals appeared on the surface & prevented the etching from proceeding normally |
| SF6 added (SOOE) | 2 μm | 10 μm | 5:1 | Residual free etching was achieved |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, Y.; Li, Z.; Wang, W. SF6 Optimized O2 Plasma Etching of Parylene C. Micromachines 2018, 9, 162. https://doi.org/10.3390/mi9040162
Zhang L, Liu Y, Li Z, Wang W. SF6 Optimized O2 Plasma Etching of Parylene C. Micromachines. 2018; 9(4):162. https://doi.org/10.3390/mi9040162
Chicago/Turabian StyleZhang, Lingqian, Yaoping Liu, Zhihong Li, and Wei Wang. 2018. "SF6 Optimized O2 Plasma Etching of Parylene C" Micromachines 9, no. 4: 162. https://doi.org/10.3390/mi9040162





