Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Fabrication of Paper-Based Electrodes
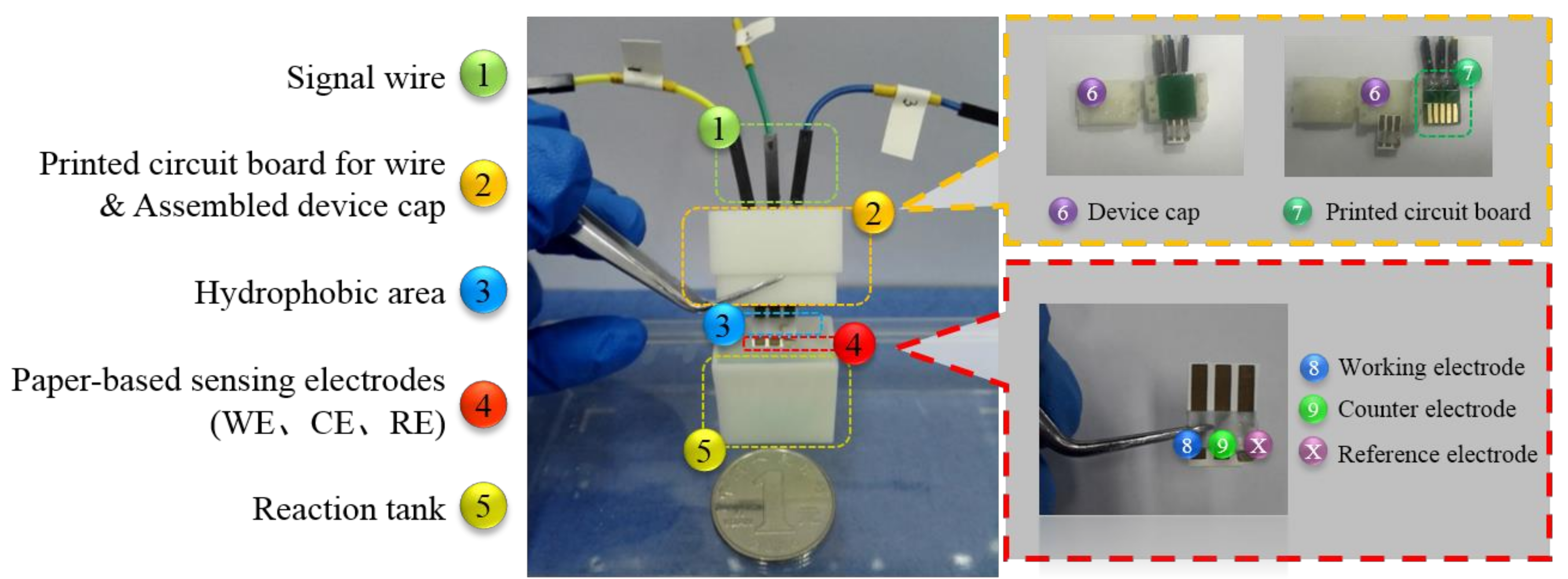
2.4. Package of the Electrode System
2.5. Square-Wave Stripping Voltammetry Measurement of Cu2+
3. Results and Discussion
3.1. Characterization of Paper-Based Electrodes
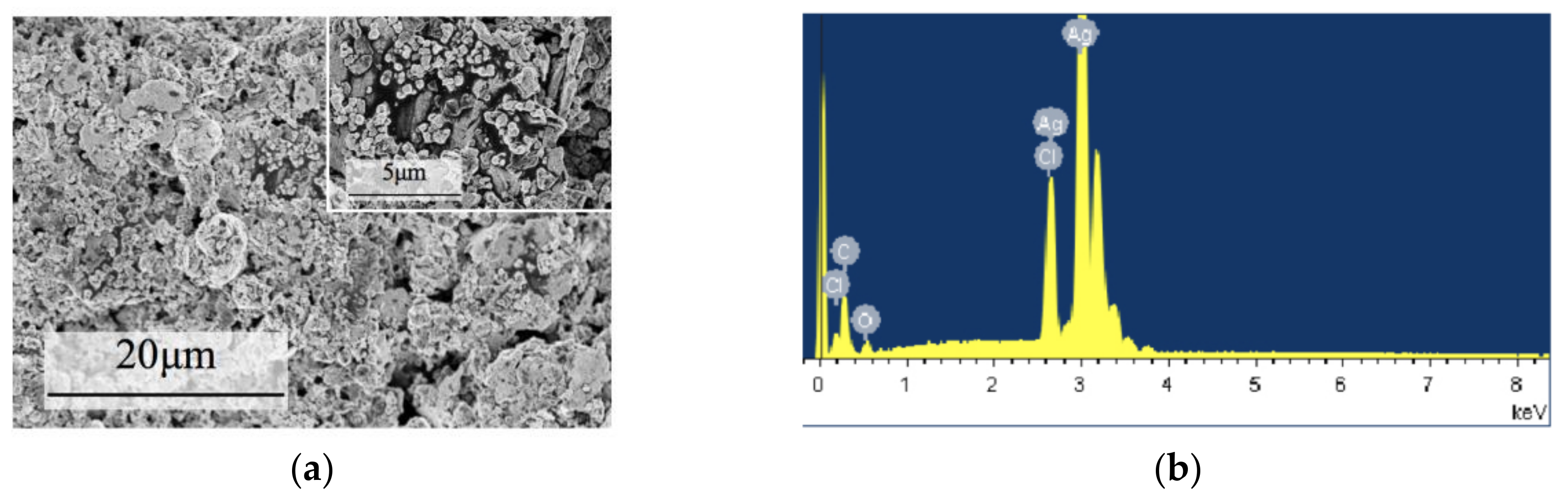
3.1.1. SEM and EDS Characterization
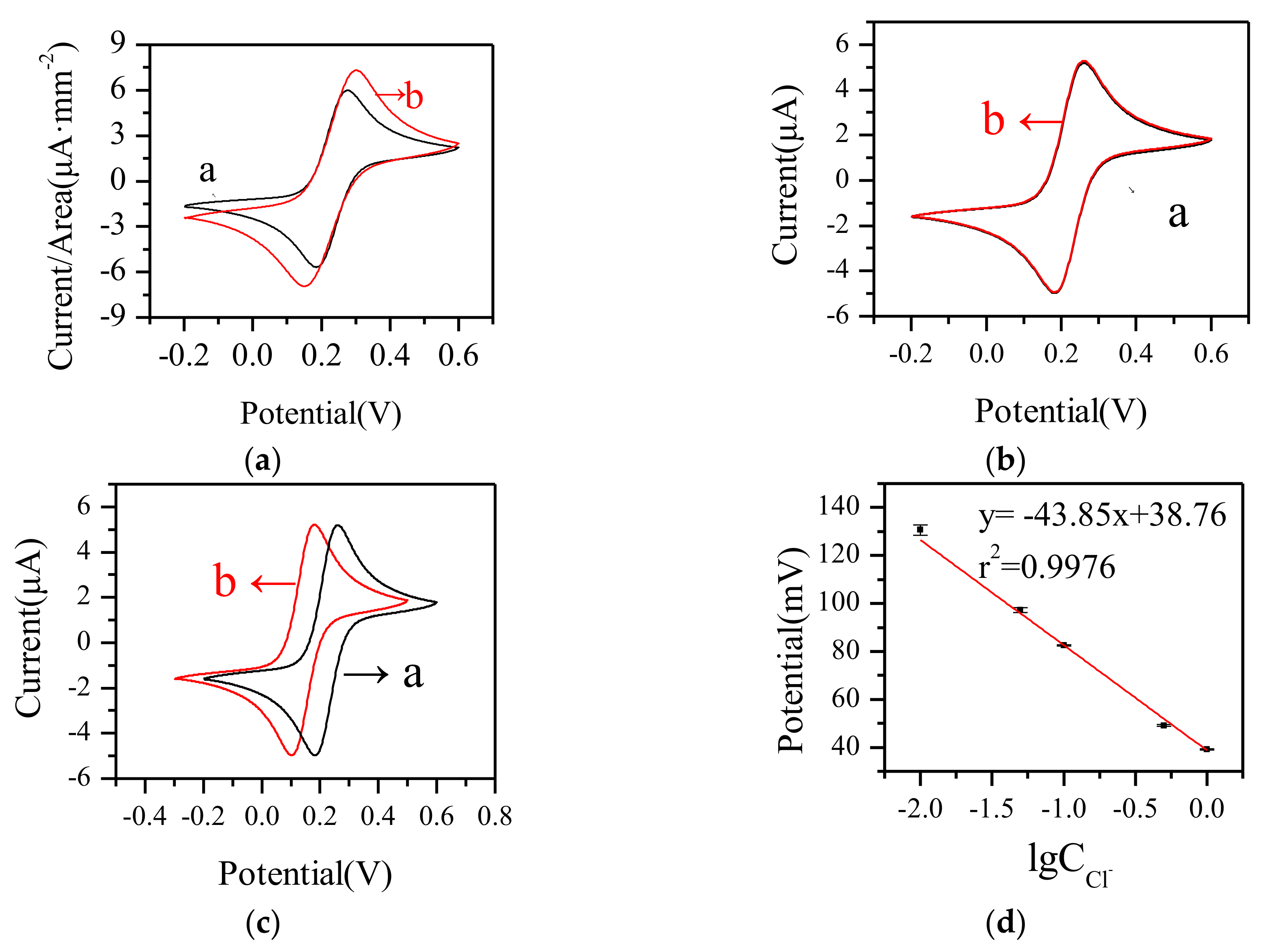
3.1.2. Electrochemical Characteristics
3.2. Optimization of Experimental Conditions
3.2.1. Optimization of Accumulation Potential
3.2.2. Optimization of Accumulation Time
3.2.3. Optimization of pH Value
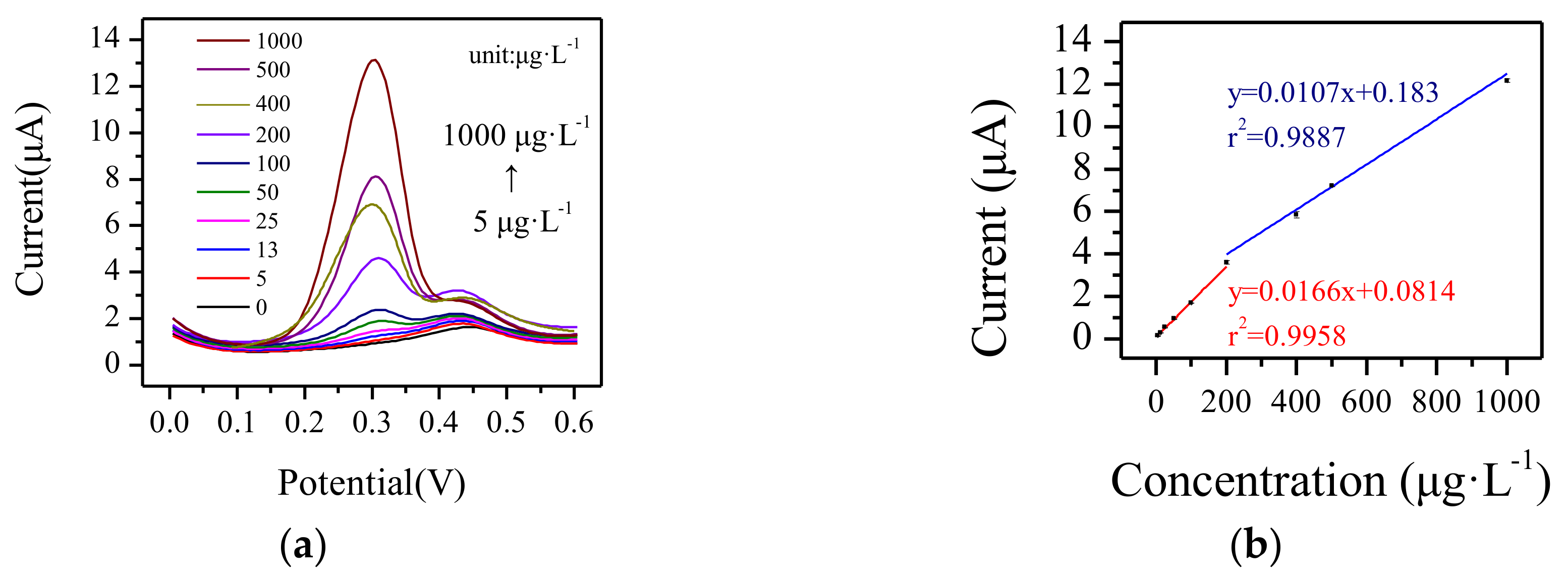
3.3. Electrochemical Response to Cu2+ by Paper-Based Sensor Chip
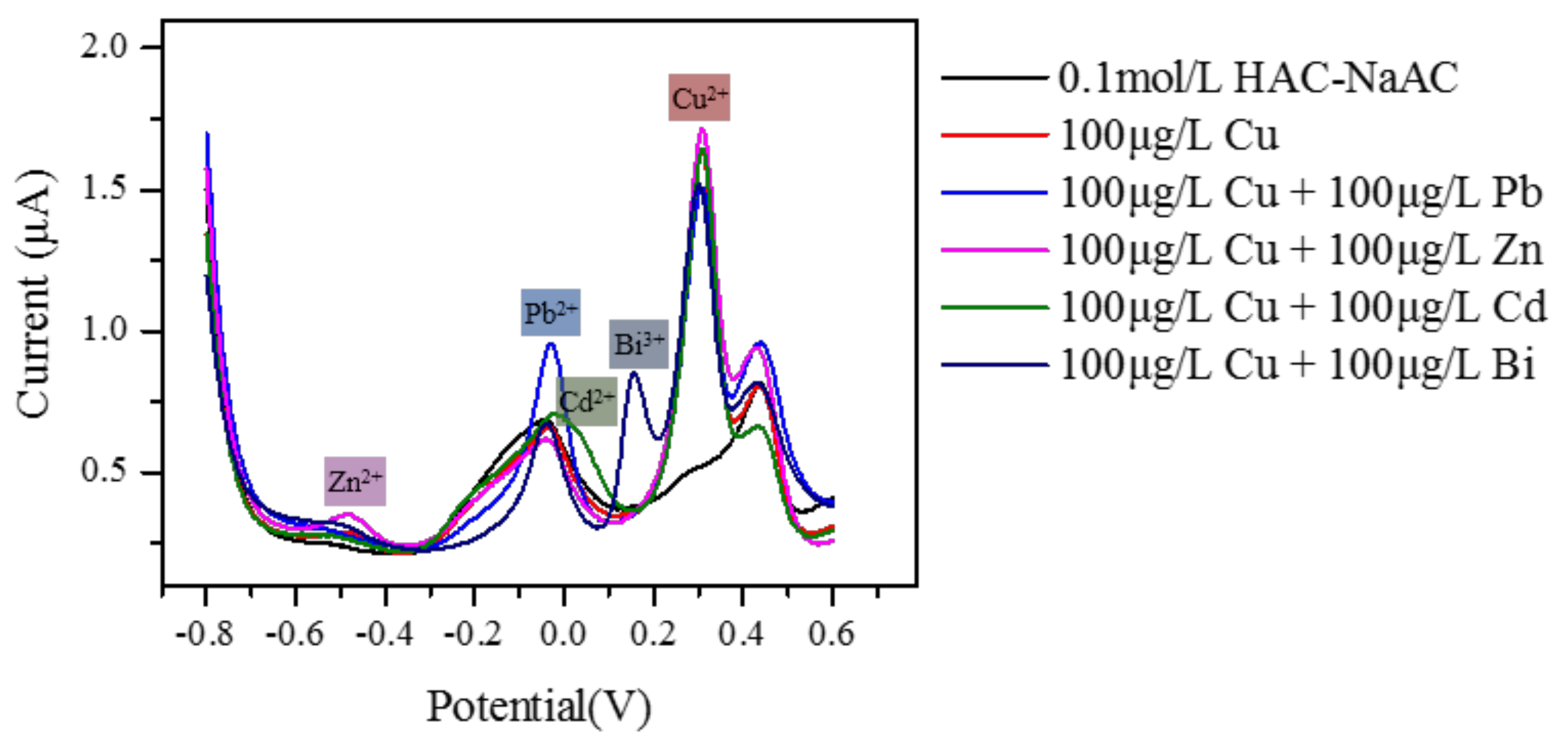
3.4. Interference Studies
3.5. Recovery Measurement in Real Samples (Cu2+)
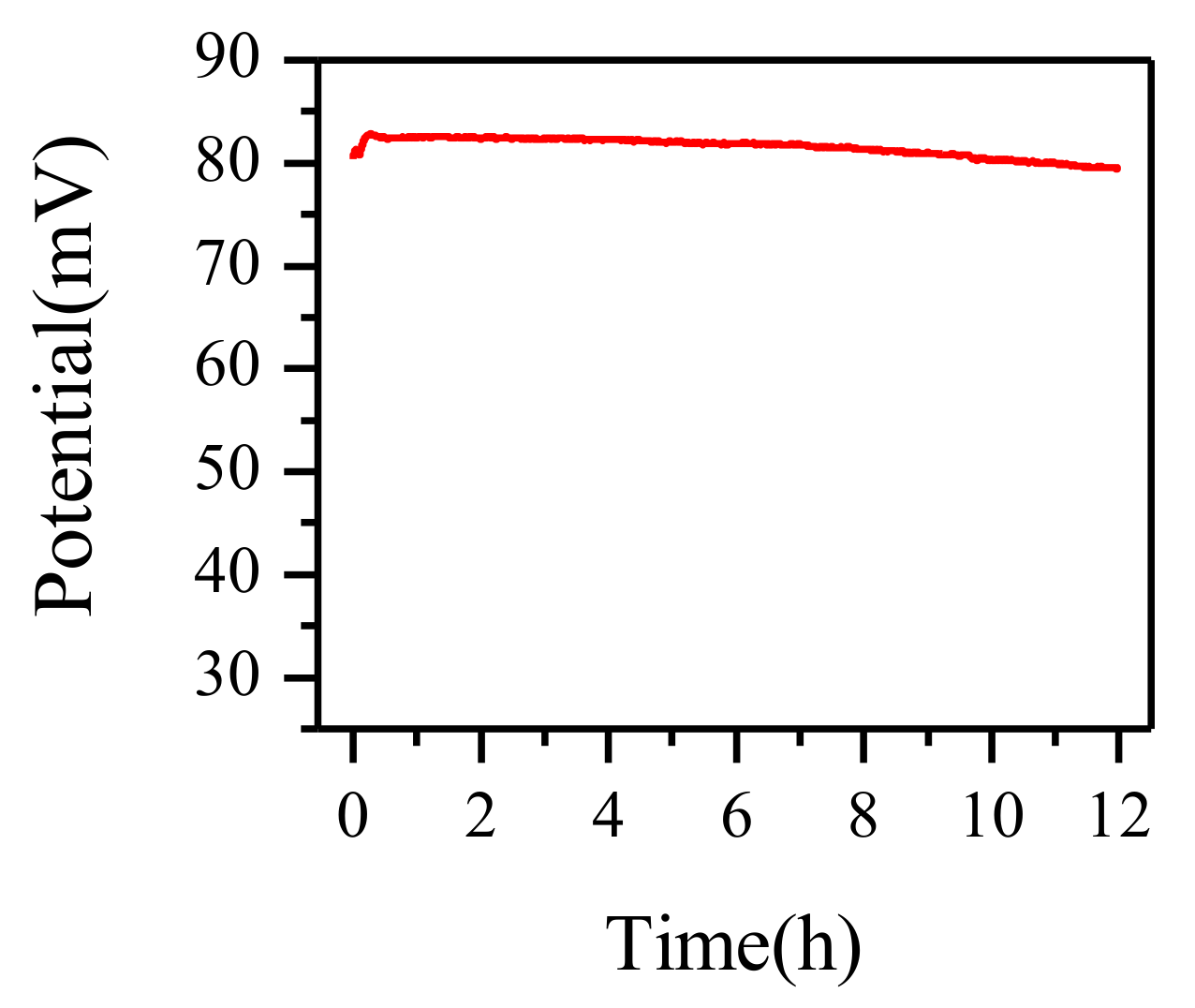
3.6. Reproducibility Study
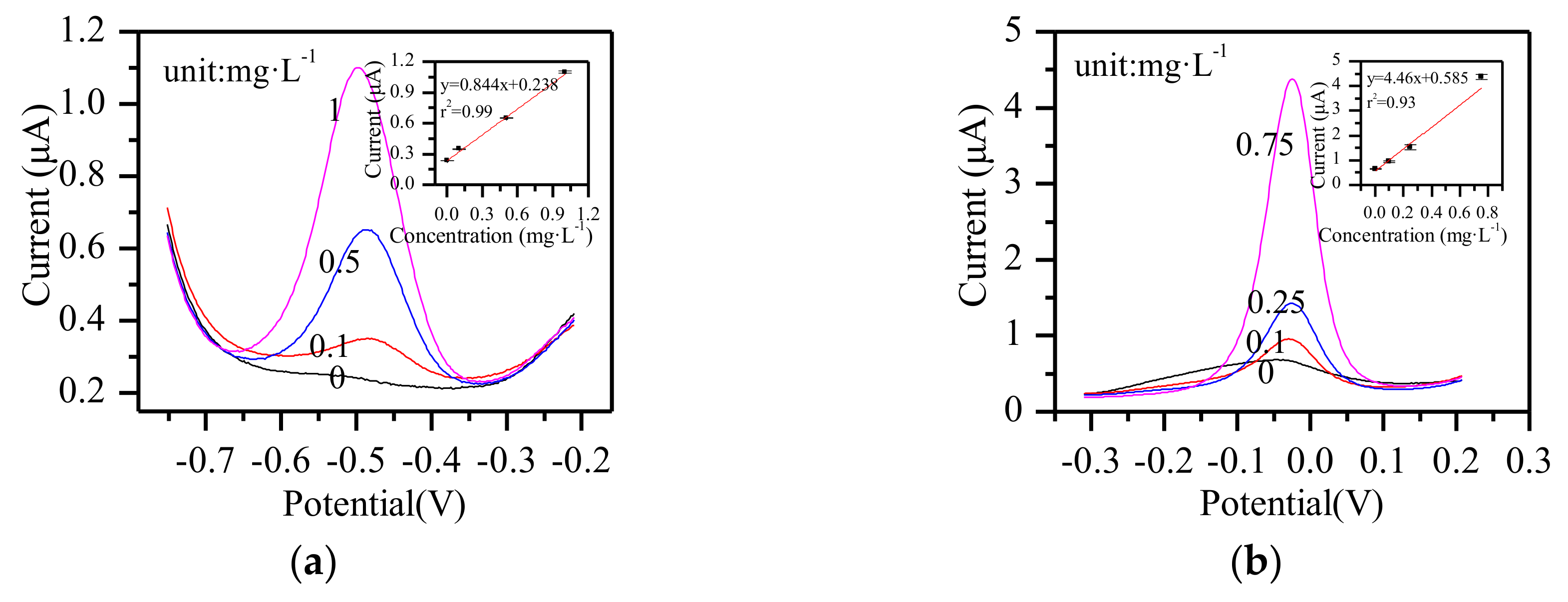
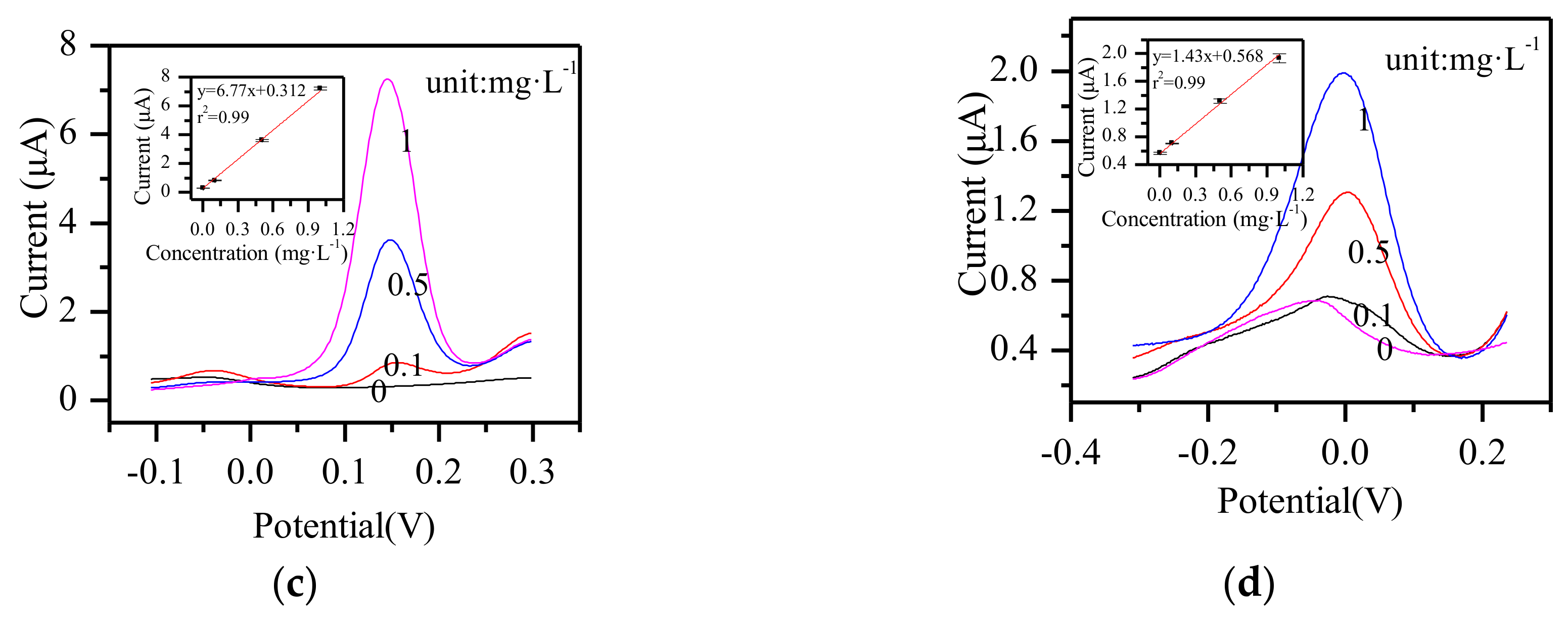
3.7. Other Metal Ions Detection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, G.; Wang, H.; Liu, G. Electrochemical determination of trace cadmium in soil by a bismuth film/graphene-beta-cyclodextrin-nafion composite modified electrode. Int. J. Electrochem. Sci. 2016, 11, 1840–1851. [Google Scholar]
- Zhan, S.S.; Wu, Y.G.; Wang, L.M.; Zhan, X.J.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, H. A selective fluorescence-on reaction of spiro form fluorescein hydrazide with Cu(II). Anal. Chim. Acta 2006, 575, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J. Determination of heavy metal ions in waste water by the plame atomic absorption spectrometry. Chin. J. Spectrosc. Lab. 2010, 27, 247–249. [Google Scholar]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan-Investigated by spectrophotometry and SEM-EDX analysis. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 275–282. [Google Scholar] [CrossRef]
- Jian-Ying, Q.I.; Xiang-Ping, L.I.; Chen, Y.H.; Liu, J.; Wang, C.L.; Chang, X.Y. Determination of heavy metals in soil by dynamic reaction cell-inductively coupled plasma mass spectrum. Chin. J. Anal. Lab. 2008, 27, 30–33. [Google Scholar]
- Ensafi, A.A.; Khayamian, T.; Benvidi, A.; Mirmomtaz, E. Simultaneous determination of copper, lead and cadmium by cathodic adsorptive stripping voltammetry using artificial neural network. Anal. Chim. Acta 2006, 561, 225–232. [Google Scholar] [CrossRef]
- Lopez-Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoci, A. High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2+ detection in drinking waters. Biosens. Bioelectron. 2013, 47, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Chang, G.; Su, J.; Cao, L.; Huang, Q.; Zhang, Y.; Xia, T.; He, Y. Single-step electrochemical deposition of high performance Au-graphene nanocomposites for nonenzymatic glucose sensing. Sens. Actuator B Chem. 2015, 220, 331–339. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Tang, L.; Wang, J.; Pan, H.; Du, M. Electrochemical sensor for detection of hydrazine based on Au@Pd core-shell nanoparticles supported on amino-functionalized TiO2 nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, D.; Wang, J.; Zhao, Y.; Dong, S.; Wang, X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sens. Actuator B Chem. 2017, 238, 427–433. [Google Scholar] [CrossRef]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Wang, W.; Zhang, Y.; Yang, T. Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J. Electroanal. Chem. 2016, 760, 52–58. [Google Scholar] [CrossRef]
- Wang, J.F.; Chao, B.; Tong, J.H.; Sun, J.Z.; Xia, S.H. Comparison of mercury-free microsensors based on gold nanoparticles for heavy metals detection. Chin. J. Anal. Chem. 2012, 40, 1791–1796. [Google Scholar] [CrossRef]
- Glavan, A.C.; Niu, J.; Chen, Z.; Güder, F.; Cheng, C.M.; Liu, D.R.; Whitesides, G.M. Analytical devices based on direct synthesis of DNA on paper. Anal. Chem. 2016, 88, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.M.; Ainla, A.; Güder, F.; Christodouleas, D.C.; Fernández-Abedul, M.T.; Whitesides, G.M. Integrating electronics and microfluidics on paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Ainla, A.; Hamedi, M.M.; Güder, F.; Whitesides, G.M. Electrical textile valves for paper microfluidics. Adv. Mater. 2017, 29, 1702894. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.M.L.; Pons, J.; Blake, D.A.; Merkoci, A. All-Integrated and highly sensitive paper based device with sample treatment platform for Cd2+ immunodetection in drinking/tap waters. Anal. Chem. 2013, 85, 3532–3538. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiang, Y.; Lu, Y.; Crooks, R.M. Aptamer-based origami paper analytical device for electrochemical detection of adenosine. Angew. Chem. 2012, 51, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.; Zhu, S.; Yan, M.; Ge, S.; Yu, J. 3D origami electrochemical device for sensitive Pb2þ testing based on DNA functionalized iron-porphyrinic metal-organic framework. Biosens. Bioelectron. 2017, 87, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Khayamian, T.; Benvidi, A.; Mirmomtaz, E. Electrogeneration of gold nanoparticles on porous-carbon paper-based electrodes and application to inorganic arsenic analysis in white wines by chronoamperometric stripping. Anal. Chim. Acta 2006, 561, 225–232. [Google Scholar] [CrossRef]
- Feng, L.; Li, H.; Niu, L.Y.; Guan, Y.S.; Duan, C.F.; Guan, Y.F.; Tung, C.H.; Yang, Q.Z. A fluorometric paper-based sensor array for the discrimination of heavy-metal ions. Talanta 2013, 108, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, P.; Maattanen, A.; Vanamo, U.; Novell, M.; Ihalainen, P.; Andrade, F.J.; Bobacka, J.; Peltonen, J. Paper-based potentiometric ion sensors constructed on ink-jet printed gold electrodes. Sens. Actuator B Chem. 2016, 224, 325–332. [Google Scholar] [CrossRef]
- Xu, Y.H.; Lou, B.H.; Lv, Z.Z.; Zhou, Z.X.; Zhang, L.B.; Wang, E.K. Paper-based solid-state electrochemiluminescence sensor using poly(sodium 4-styrenesulfonate) functionalized graphene/nafion composite film. Anal. Chim. Acta 2013, 763, 20–27. [Google Scholar] [CrossRef] [PubMed]



| Interference Ions | Concentration of Interference Ions (μg·L−1) | Percentage of Peak Current Compared with 100 μg·L−1 Cu2+ (%) |
|---|---|---|
| Pb2+ | 100 | 86.17 ± 6.15 |
| 250 | 84.23 ± 7.44 | |
| Cd2+ | 100 | 103.46 ± 9.97 |
| 500 | 99.59 ± 7.38 | |
| Zn2+ | 100 | 107.62 ± 5.11 |
| 500 | 104.16 ± 6.65 | |
| Bi3+ | 100 | 142.95 ± 5.23 |
| 500 | 102.44 ± 6.02 | |
| Cl−, SO42−, CO32−, PO43−, Na+, K+ | 105, 105, 5 × 104, 5 × 104, 105, 1.1 × 105 | 92.42 ± 4.19 |
| Samples | Concentration of Added Cu2+ (μg·L−1) | Concentration Found (μg·L−1) | Recovery (%) |
|---|---|---|---|
| 1 | 0 | N | - |
| 20 | 21.52 ± 0.60 | 107.59 ± 2.99 | |
| 100 | 108.96 ± 1.16 | 108.96 ± 1.16 | |
| 2 | 0 | N | - |
| 40 | 43.42 ± 0.30 | 108.56% ± 0.74 | |
| 75 | 72.11 ± 1.13 | 96.14% ± 1.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sun, J.; Tong, J.; Guan, X.; Bian, C.; Xia, S. Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV. Micromachines 2018, 9, 150. https://doi.org/10.3390/mi9040150
Wang X, Sun J, Tong J, Guan X, Bian C, Xia S. Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV. Micromachines. 2018; 9(4):150. https://doi.org/10.3390/mi9040150
Chicago/Turabian StyleWang, Xiaoqing, Jizhou Sun, Jianhua Tong, Xin Guan, Chao Bian, and Shanhong Xia. 2018. "Paper-Based Sensor Chip for Heavy Metal Ion Detection by SWSV" Micromachines 9, no. 4: 150. https://doi.org/10.3390/mi9040150




