Dielectrophoretic Microfluidic Device for in Vitro Fertilization
Abstract
:1. Introduction
2. Materials and Methods
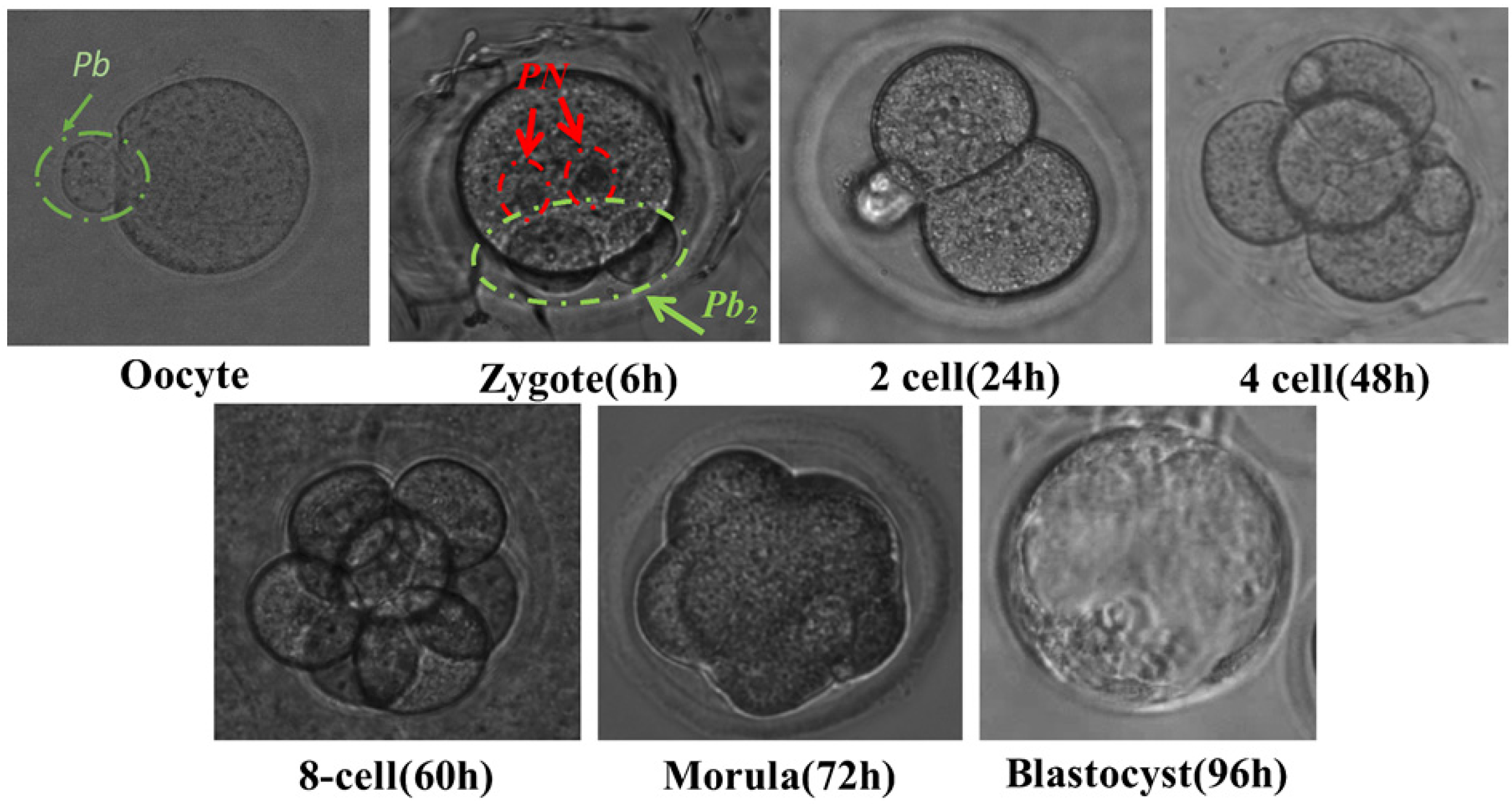
2.1. Gamete Collection
2.2. Theory
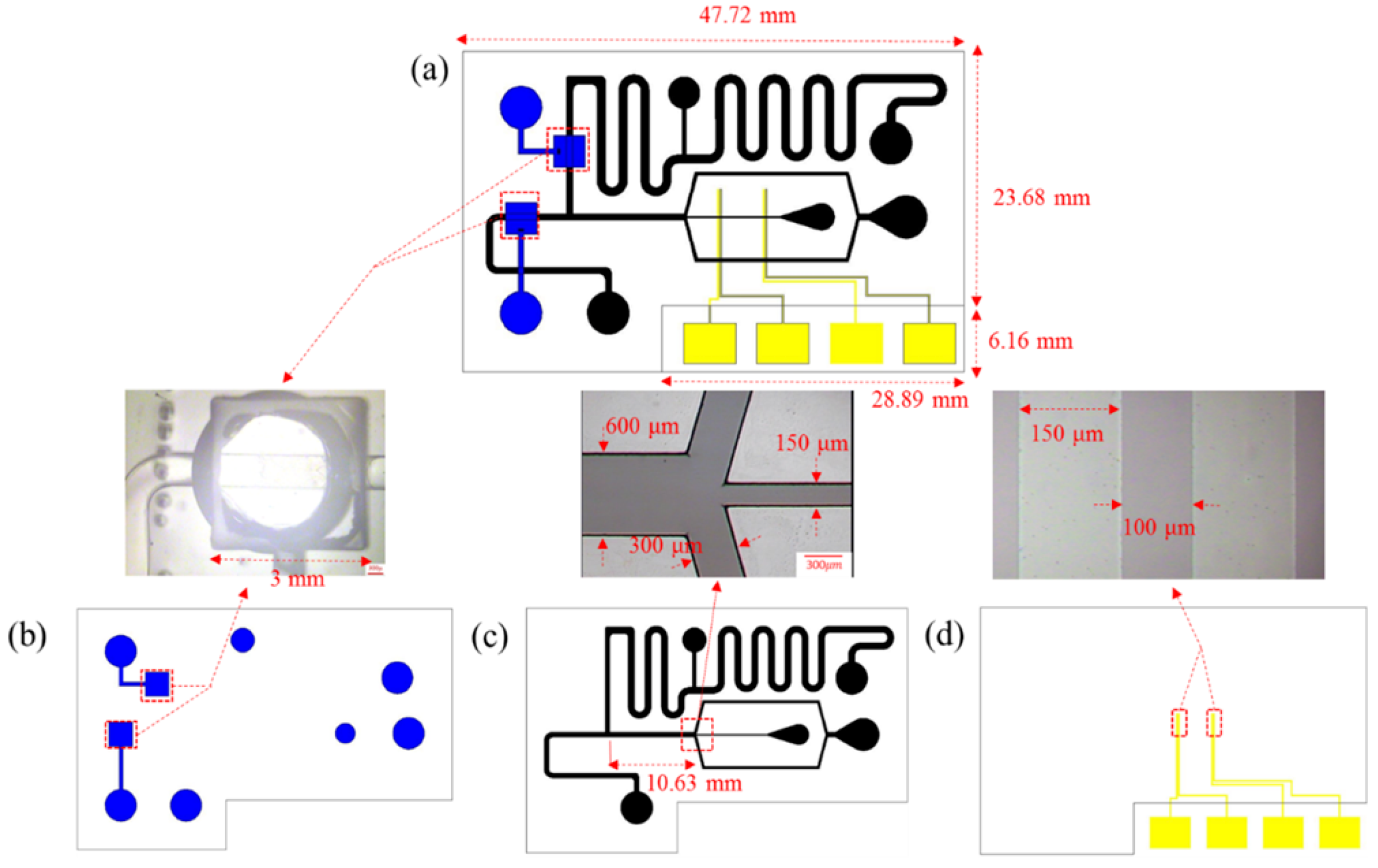
2.3. Chip Design
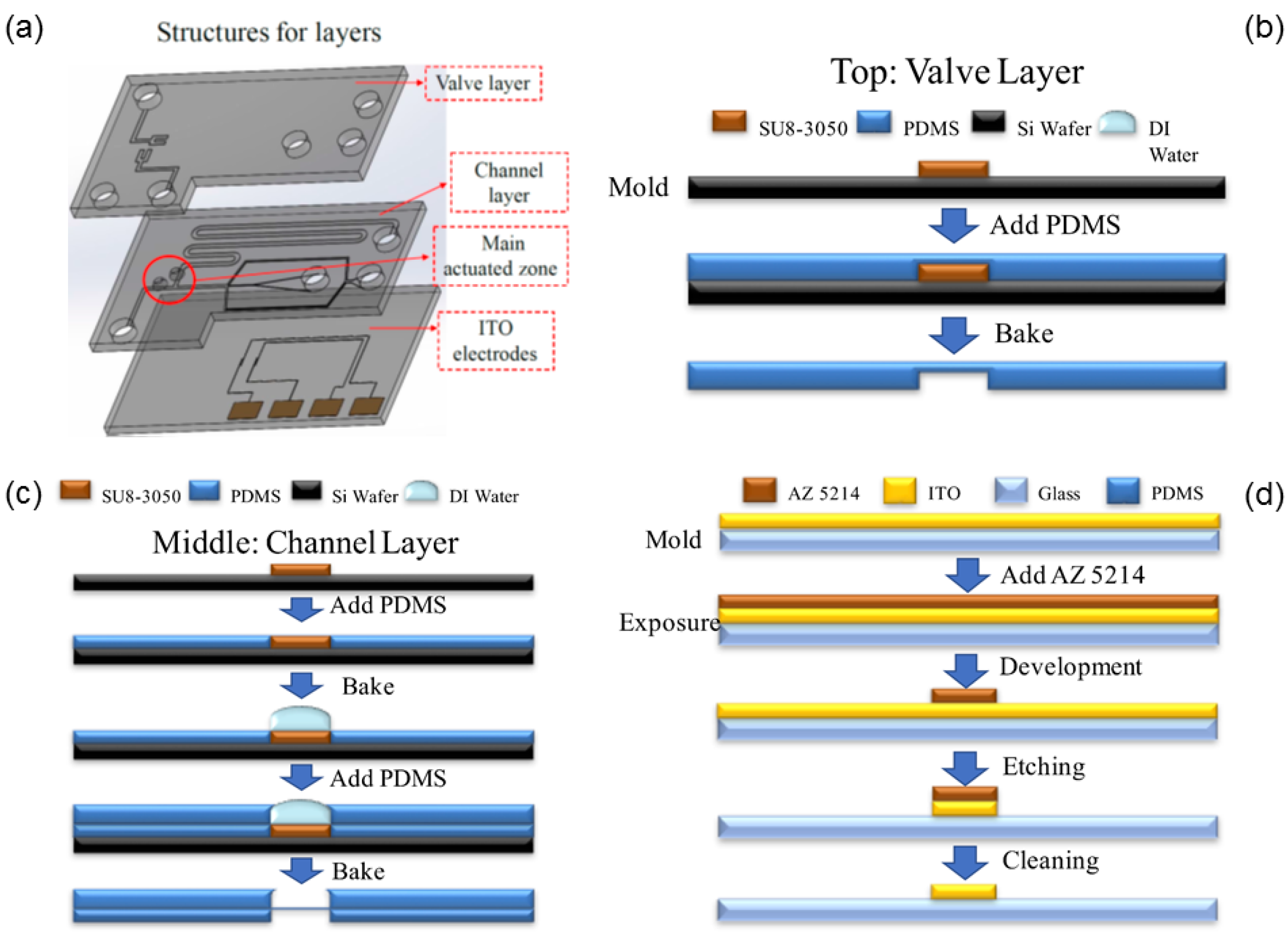
2.4. Chip Fabrication
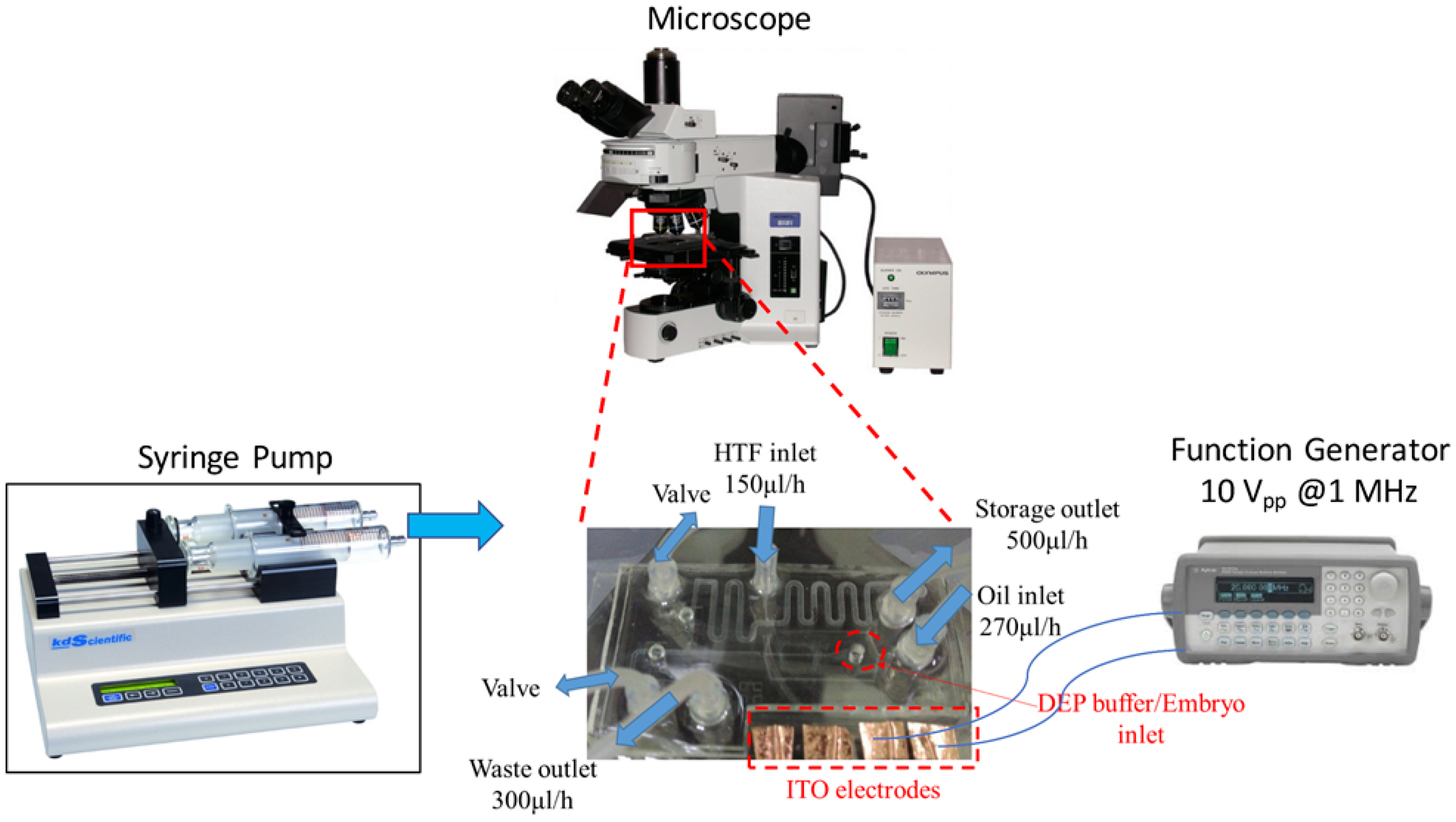
2.5. Experimental Process
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 312, 366. [Google Scholar] [CrossRef]
- Biggers, J.D.; McGinnis, L.K.; Lawitts, J.A. One-step versus two-step culture of mouse preimplantation embryos: Is there a difference? Hum. Reprod. 2005, 20, 3376–3384. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Gardner, D.K. Embryo culture medium: Which is the best? Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 27, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Palermo, G.D.; Goldstein, M.; Menendez, S.; Zaninovic, N.; Veeck, L.L.; Rosenwaks, Z. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology 1998, 49, 435–440. [Google Scholar] [CrossRef]
- Jain, T.; Gupta, R.S. Trends in the use of intracytoplasmic sperm injection in the United States. N. Engl. J. Med. 2007, 357, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Bundorf, M.K.; Behr, B.; McCallum, S.W. Use and outcomes of intracytoplasmic sperm injection for non-male factor infertility. Fertil. Steril. 2007, 88, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Bigelow, C.; Duke, M.; Ruman, J.; Sandler, B.; Grunfeld, L.; Copperman, A.B. Should ICSI be recommended routinely in patients with four or fewer oocytes retrieved? J. Assist. Reprod. Genet. 2011, 28, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Hamilton, M.P.; Shaaban, M.; Khalaf, Y.; Seddler, M.; Ghobara, T.; Braude, P.; Kennedy, R.; Rutherford, A.; Hartshorne, G. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: A randomised controlled trial. Lancet 2001, 357, 2075–2079. [Google Scholar] [CrossRef]
- Bonduelle, M.; Van Assche, E.; Joris, H.; Keymolen, K.; Devroey, P.; Van Steirteghem, A.; Liebaers, I. Prenatal testing in ICSI pregnancies: Incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum. Reprod. 2002, 17, 2600–2614. [Google Scholar] [CrossRef] [PubMed]
- Gjerris, A.C.; Loft, A.; Pinborg, A.; Christiansen, M.; Tabor, A. Prenatal testing among women pregnant after assisted reproductive techniques in Denmark 1995-2000: A national cohort study. Hum. Reprod. 2008, 23, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Amor, D.J.; Halliday, J. A review of known imprinting syndromes and their association with assisted reproduction technologies. Hum. Reprod. 2008, 23, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Nygren, K.G.; Iliadou, A.; Hultman, C.M.; Reichenberg, A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA 2013, 310, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Nejat, E.J.; Buyuk, E. Reproductive technologies and the risk of birth defects. N. Engl. J. Med. 2012, 367, 875–876. [Google Scholar] [PubMed]
- Zhu, J.L.; Basso, O.; Obel, C.; Bille, C.; Olsen, J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. Br. Med. J. 2006, 333, 679. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xie, L.; Han, C.; Su, K.; Qiu, T.; Wang, L.; Huang, G.; Xing, W.; Qiao, J.; Wang, J. In Vitro Fertilization on a Single-Oocyte Positioning System Integrated with Motile Sperm Selection and Early Embryo Development. Anal. Chem. 2011, 83, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Swain, J.E.; Bormann, C.L. Microfluidics for gametes, embryos, and embryonic stem cells. Semin. Reprod. Med. 2011, 29, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Smith, G.D. Advances in embryo culture platforms: Novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum. Reprod. Update 2011, 17, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Takayama, S.; Swain, J.E. Rethinking In Vitro Embryo Culture: New Developments in Culture Platforms and Potential to Improve Assisted Reproductive Technologies. Biol. Reprod. 2012, 86, 62. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Lai, D.; Takayama, S.; Smith, G.D. Thinking big by thinking small: Application of microfluidic technology to improve ART. Lab Chip 2013, 13, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Wu, T.L.; Huang, H.R.; Li, C.J.; Fu, H.T.; Soong, Y.K.; Lee, M.Y.; Yao, D.J. Isolation of motile spermatozoa with a microfluidic chip having a surface-modified microchannel. J. Lab. Autom. 2014, 19, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, J.S.; Lee, D.H.; Lee, K.K.; Koo, D.B.; Park, J.K. Dielectrophoretic oocyte selection chip for in vitro fertilization. Biomed. Microdevices 2008, 10, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Suh, R.S.; Zhu, X.; Phadke, N.; Ohl, D.A.; Takayama, S.; Smith, G.D. IVF within microfluidic channels requires lower total numbers and lower concentrations of sperm. Hum. Reprod. 2006, 21, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Huang, Y.H.; Kao, W.L.; Yao, D.J. Embryo formation from low sperm concentration by using dielectrophoretic force. Biomicrofluidics 2015, 9, 022404. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: Cambridge, UK, 1995; pp. 34–81. [Google Scholar]
- Slentz, B.E.; Penner, N.A.; Regnier, F.E. Capillary electrochromatography of peptides on microfabricated poly(dimethylsiloxane) chips modified by cerium(IV)-catalyzed polymerization. J. Chromatogr. A 2002, 948, 225–233. [Google Scholar] [CrossRef]
- Matsuura, K.; Hayashi, N.; Kuroda, Y.; Takiue, C.; Hirata, R.; Takenami, M.; Aoi, Y.; Yoshioka, N.; Habara, T.; Mukaida, T. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod. BioMed. Online 2010, 20, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Van Steirteghem, A.C.; Nagy, Z.; Joris, H.; Liu, J.; Staessen, C.; Smitz, J.; Wisanto, A.; Devroey, P. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum. Reprod. 1993, 8, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Strassburger, D.; Friedler, S.; Raziel, A.; Schachter, M.; Kasterstein, E.; Ron-el, R. Very low sperm count affects the result of intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2000, 17, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D. Sperm preparation techniques and iatrogenic failures of in-vitro fertilization. Hum. Reprod. 1991, 6, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Mehta, A.; Kissin, D.M.; Warner, L.; Kawwass, J.F.; Jamieson, D.J. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. J. Am. Med. Assoc. 2015, 313, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, S.; He, X.; Sun, R.; He, Q.; Gan, Y.; Liu, S.; Funahashi, H.; Li, Y. Application of a microfluidic sperm sorter to in vitro production of dairy cattle sex-sorted embryos. Theriogenology 2016, 85, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z.; Su, L.; Nedambale, T.L.; Zhang, J.; Schenk, J.; Moreno, J.F.; Dinnyés, A.; Ji, W.; Tian, X.C. Developmental potential of vitrified holstein cattle embryos fertilized in vitro with sex-sorted sperm. J. Dairy Sci. 2006, 89, 2510–2518. [Google Scholar] [CrossRef]




| Sperm-Oocyte Ratio | Fertilization Rate [23] | Fertilization Rate by Traditional IVF | Fertilization Rate by Microfluidic System |
|---|---|---|---|
| 200 | 26.3% | ||
| 400/500 | 27.1% | 33–38% | ~39% |
| 750 | 18.0% | ||
| 2500/2000 | 8.1% | 44–56% | ~50% |
| 5000 | 12.0% | 45–53% | ~53% |
| 10,000 | N/A* | 53–62% | ~58% |
| 25,000 | N/A | 66–74% | ~65% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-Y.; Lai, Y.-L.; Yao, D.-J. Dielectrophoretic Microfluidic Device for in Vitro Fertilization. Micromachines 2018, 9, 135. https://doi.org/10.3390/mi9030135
Huang H-Y, Lai Y-L, Yao D-J. Dielectrophoretic Microfluidic Device for in Vitro Fertilization. Micromachines. 2018; 9(3):135. https://doi.org/10.3390/mi9030135
Chicago/Turabian StyleHuang, Hong-Yuan, Yun-Li Lai, and Da-Jeng Yao. 2018. "Dielectrophoretic Microfluidic Device for in Vitro Fertilization" Micromachines 9, no. 3: 135. https://doi.org/10.3390/mi9030135





