Fabrication of 3D Capillary Vessel Models with Circulatory Connection Ports
Abstract
:1. Introduction
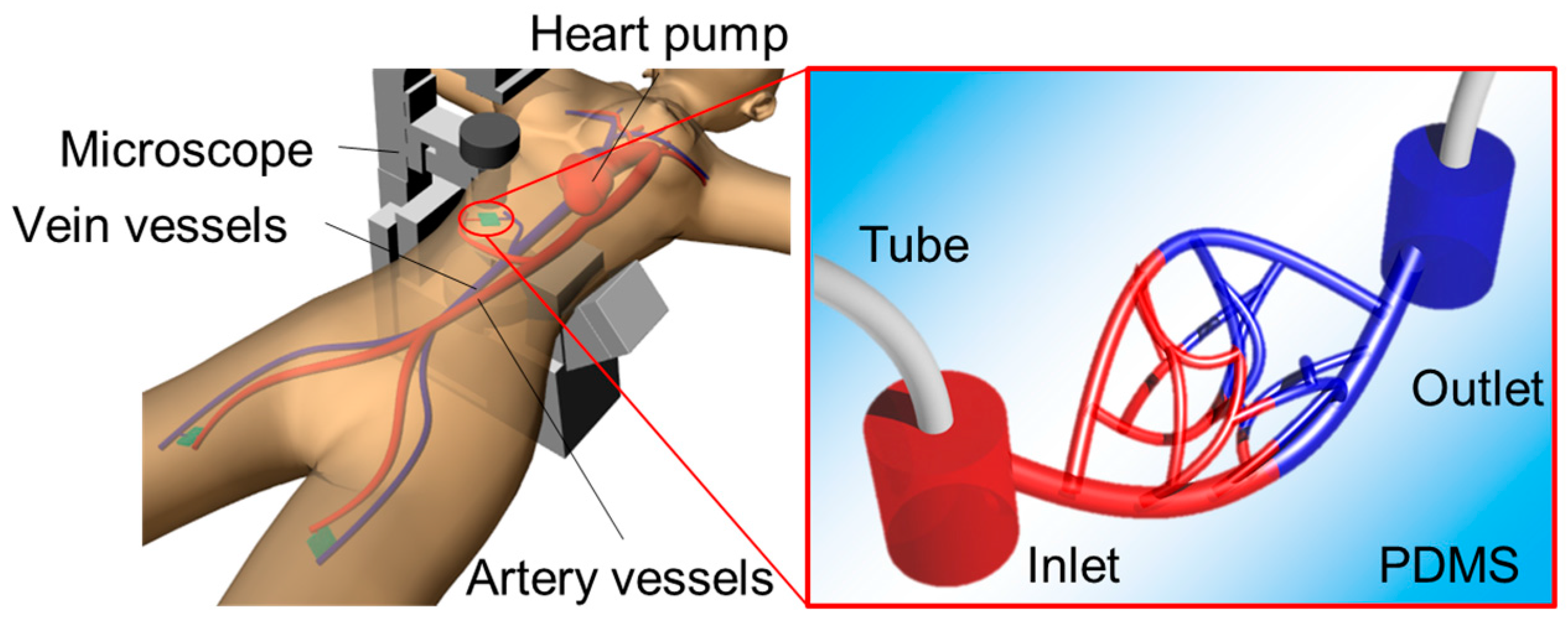
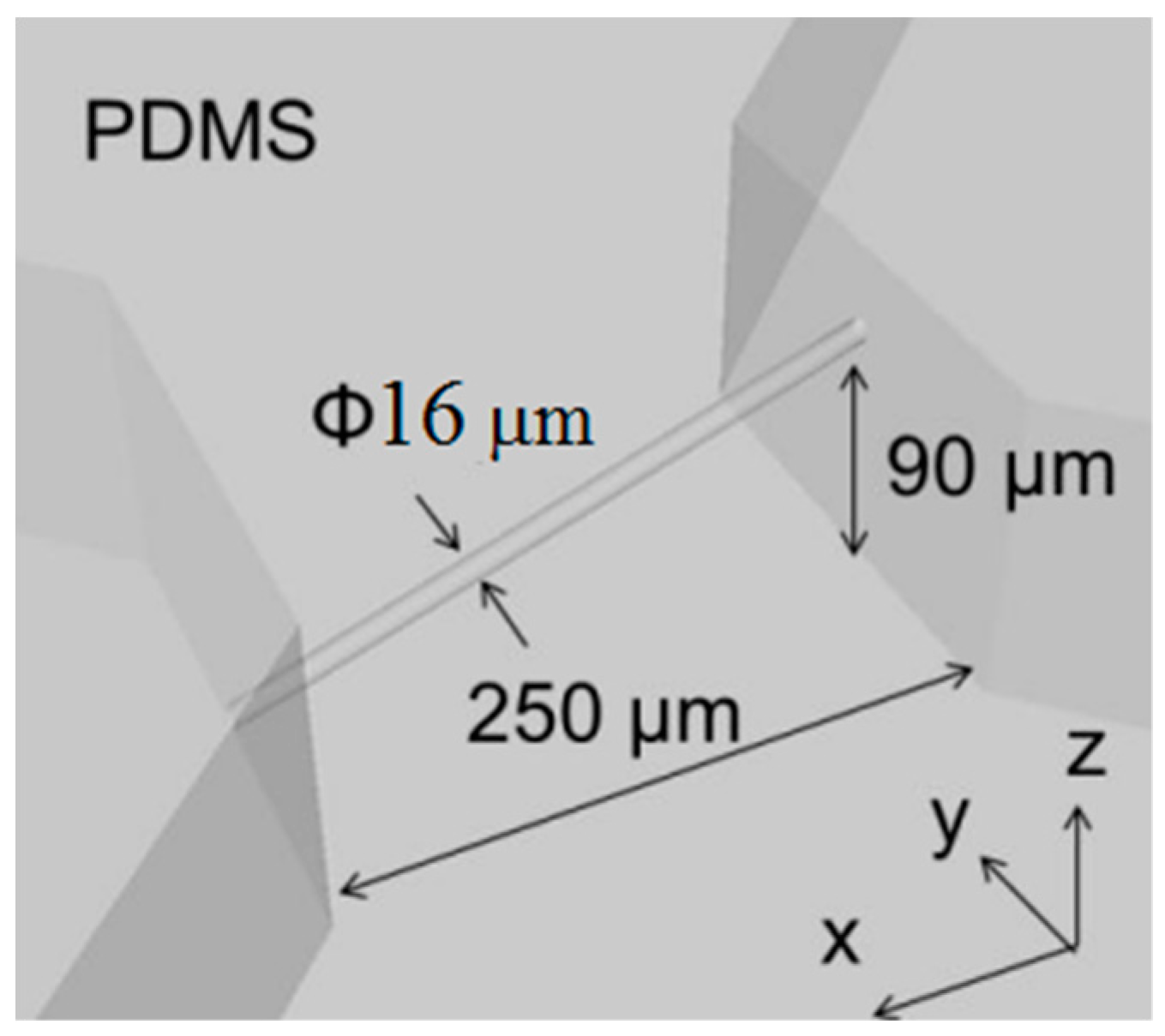
2. Microfluidic Channel Design and Concept
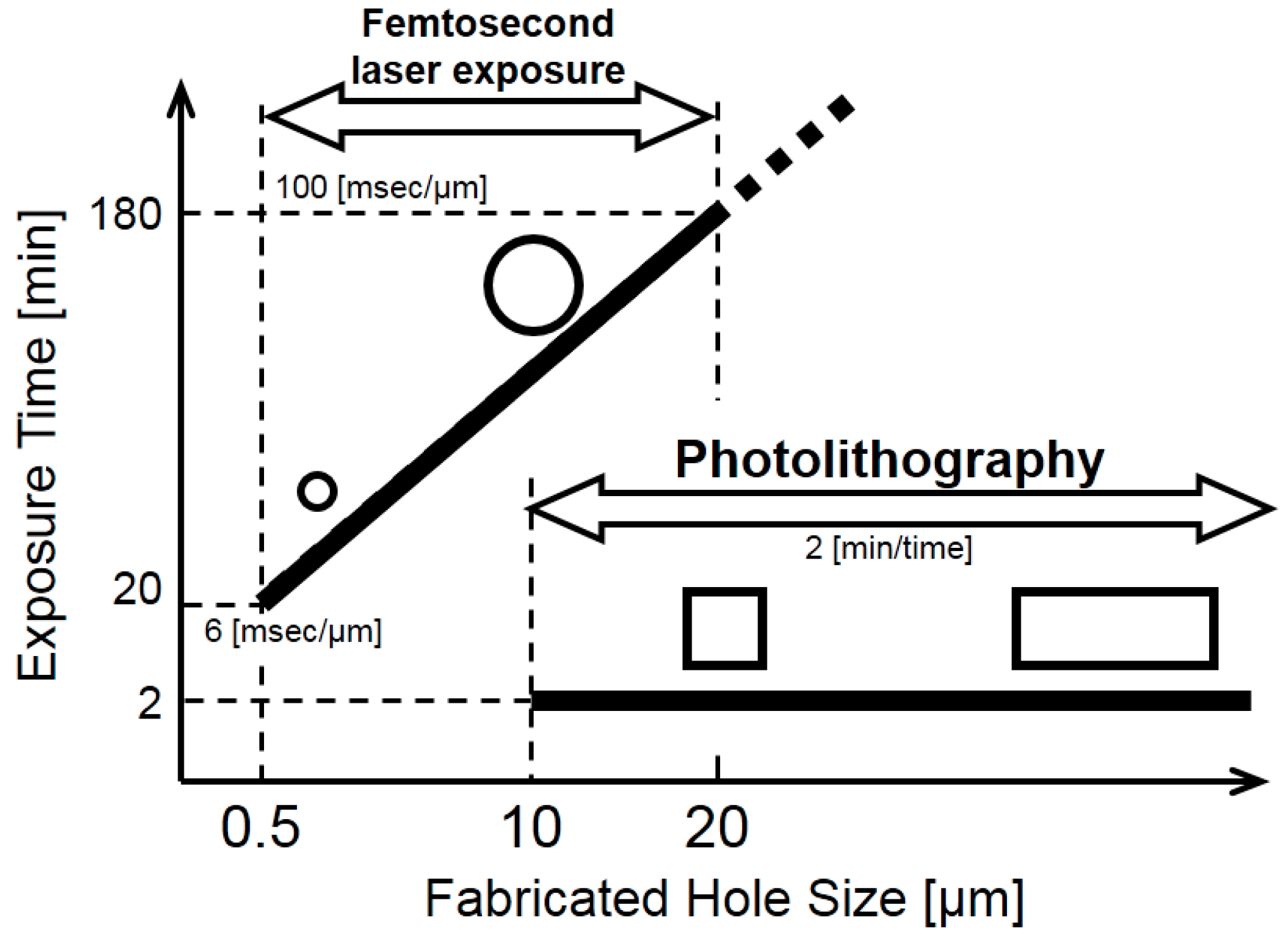
2.1. Photolithography Method
2.2. Femtosecond Laser Exposure Method
3. Fabrication methods
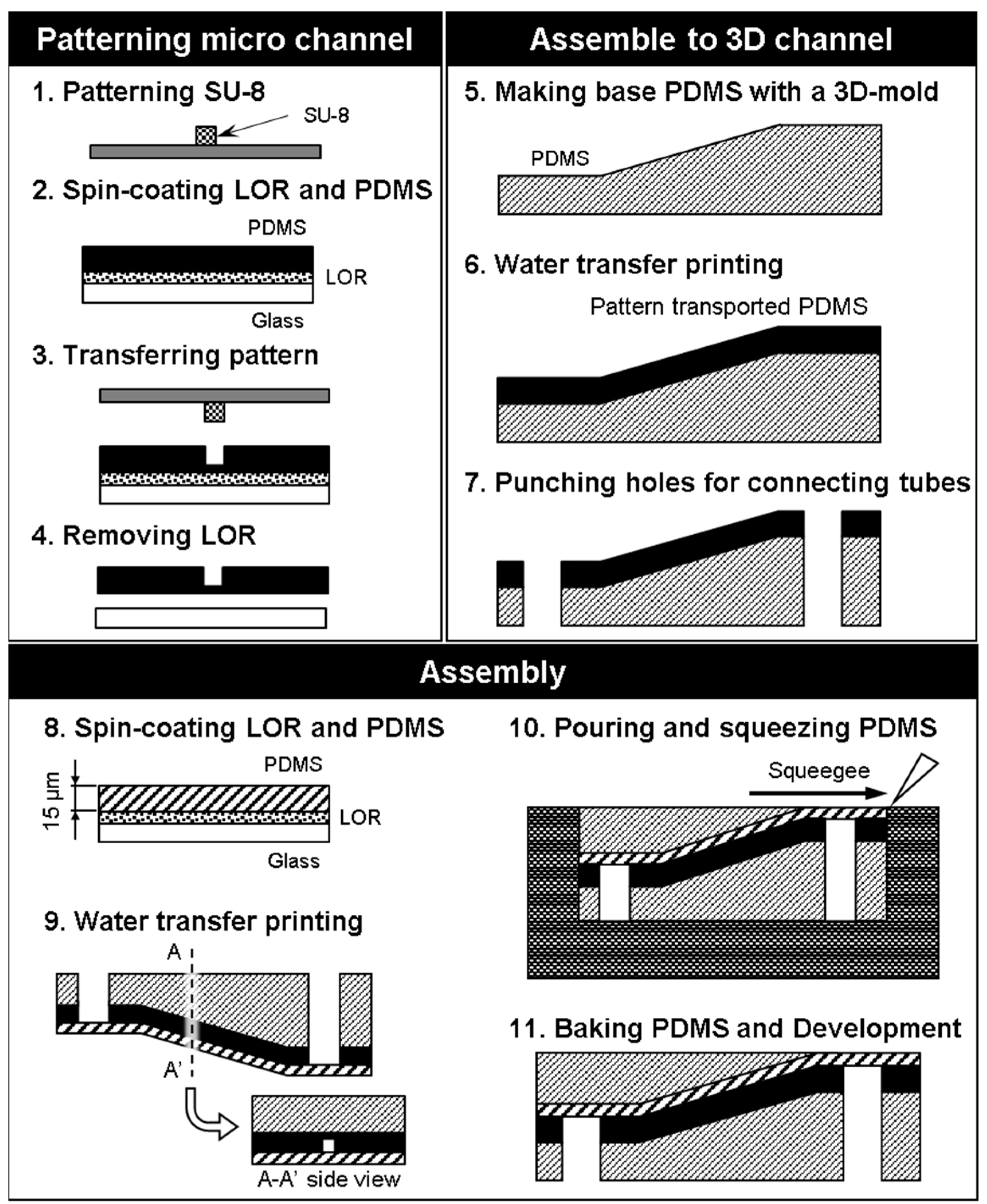
3.1. Photolithography Exposure Method
- Laser lithography (MA-6, SUSS Micro Tec KK, Kanagawa, Japan) was used to form a pattern in the SU-8 photoresist (Nippon Kayaku Co. Ltd., Tokyo, Japan) on a silicon surface. The exposure time is 9.7 s. The mold was heated using a hot plate at 65 °C for 1 min, and 95 °C for 3 min. Then, the part was developed with propylene glycol monomethyl ether (PM) for 120 s and rinsed with 2-propanol for 60 s. This pattern was used as a mold for the microchannels, and the size of the microchannels could be locally controlled by adjusting the exposure conditions.
- Spinning coat (2000 rpm for 30 s) was used to apply Lift-off resist (LOR) (Nippon Kayaku Co. Ltd., Tokyo, Japan) and then baked on a hotplate for 10 min at 95 °C. Spin coat PDMS (3000 rpm for 30 s) (Silpot 184, Dow Corning Toray Co. Ltd., Tokyo, Japan) was applied over LOR onto a glass substrate, and then baked on a hotplate for 10 min at 95 °C.
- The SU-8 mold was pushed onto the spin-coated PDMS and the ensemble was heated to 85 °C for 10 min with a hot plate.
- The LOR was dissolved with ethanol to free the PDMS sheet from the substrate.
- A PDMS base was created using a 3D printer (EDEN250, Stratasys Ltd., Eden Prairie, MN, USA).
- The PDMS sheet and base were treated with O2 plasma to activate their surfaces for bonding, and the PDMS sheet was transferred to the PDMS base.
- Holes were punched into the connection channel to connect external tubes from the bottom side of the model.
- A thin sheet of PDMS was created by spin-coating to serve as a cover layer for the channel.
- The thin PDMS sheet was placed over the PDMS sheet containing the microchannel.
- PDMS was poured and squeezed to form a slab over the angled surface.
- The assembled PDMS model was baked in the oven for 20 min at 85 °C, and the mold was removed.
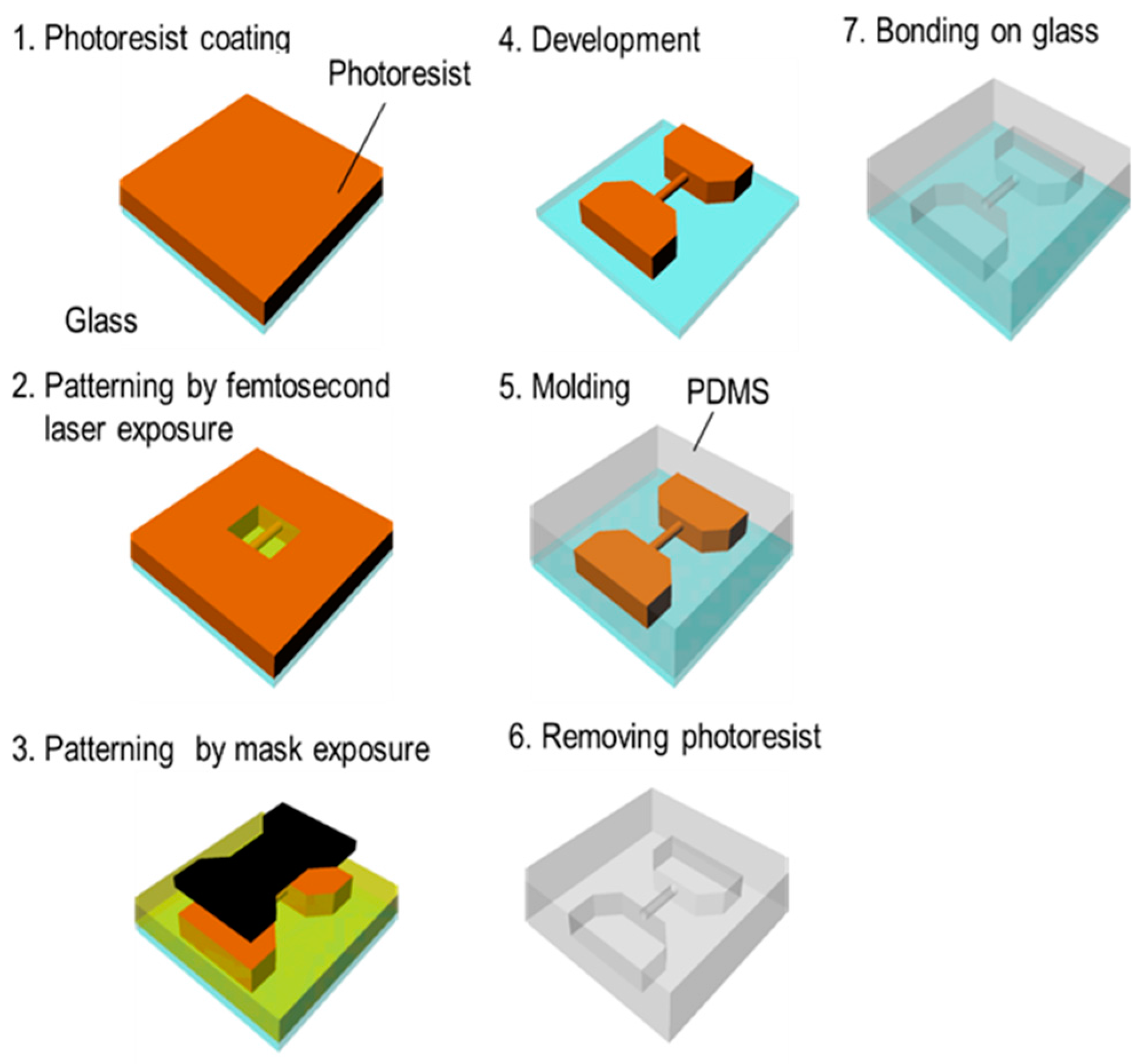
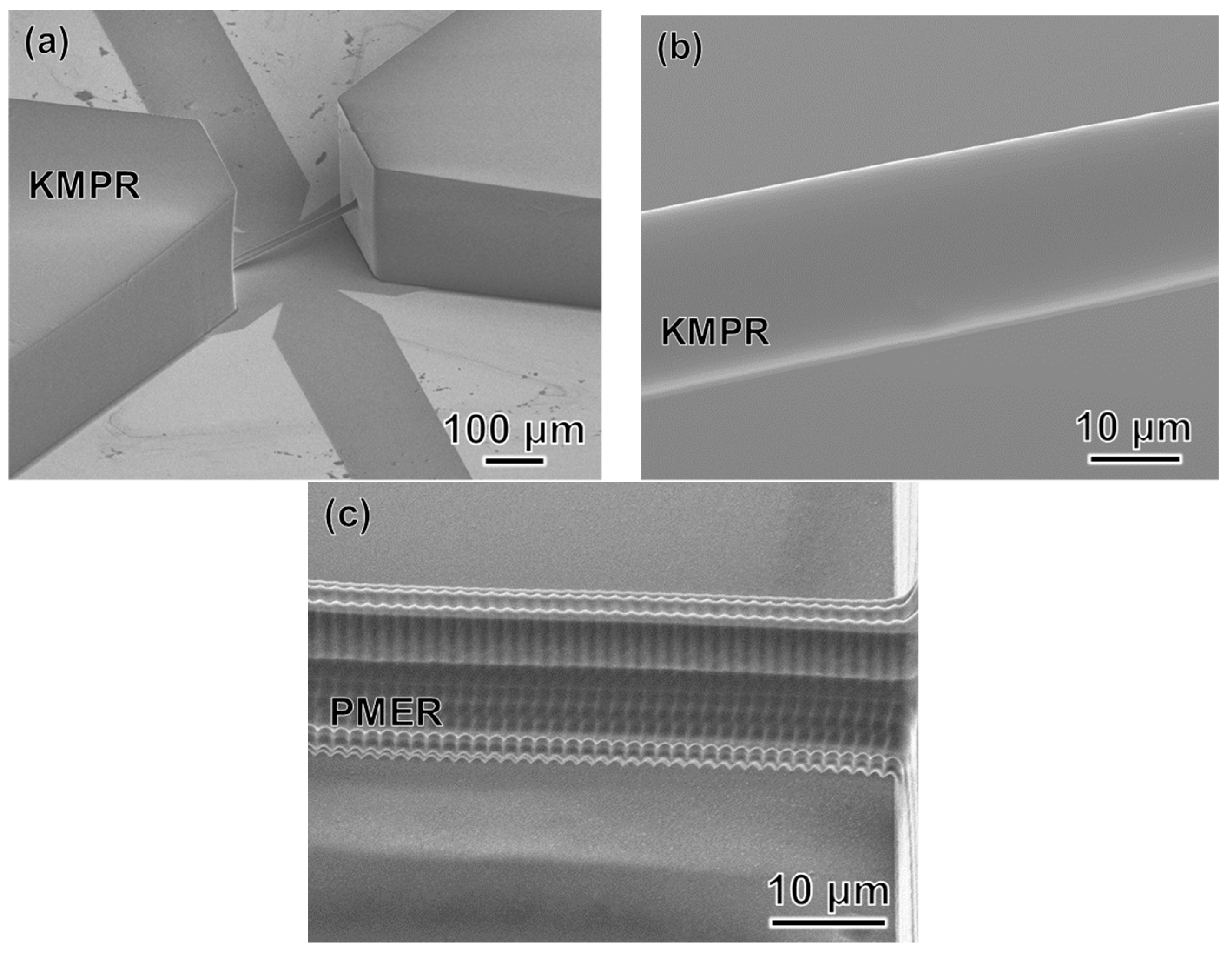
3.2. Femtosecond Laser and Mask Hybrid Exposure (FMEx)
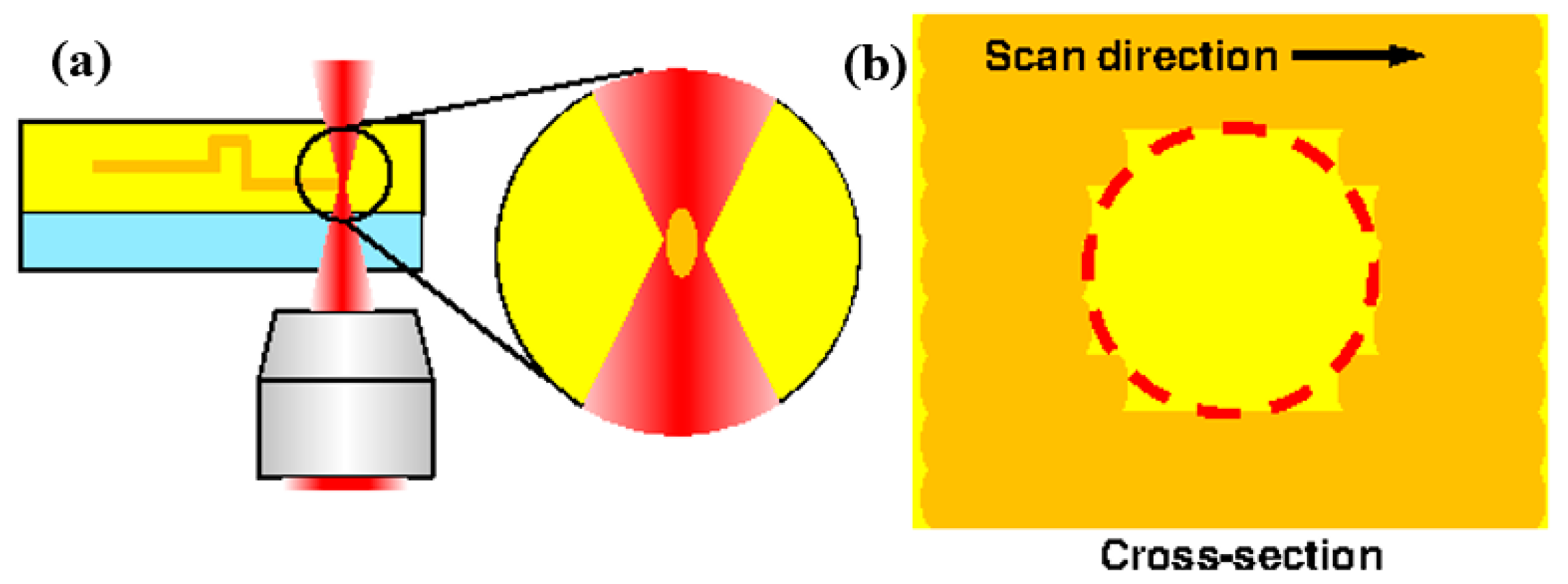
3.2.1. Femtosecond Laser Exposure System
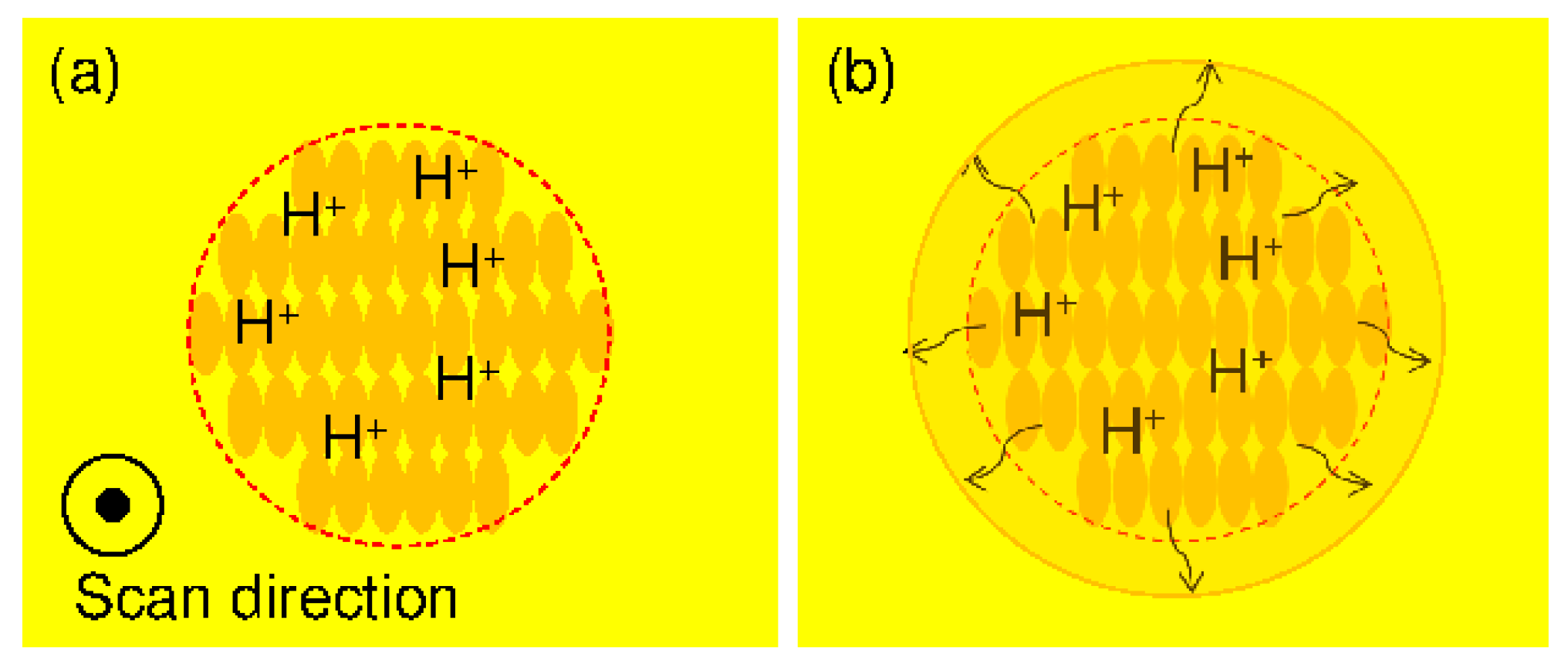
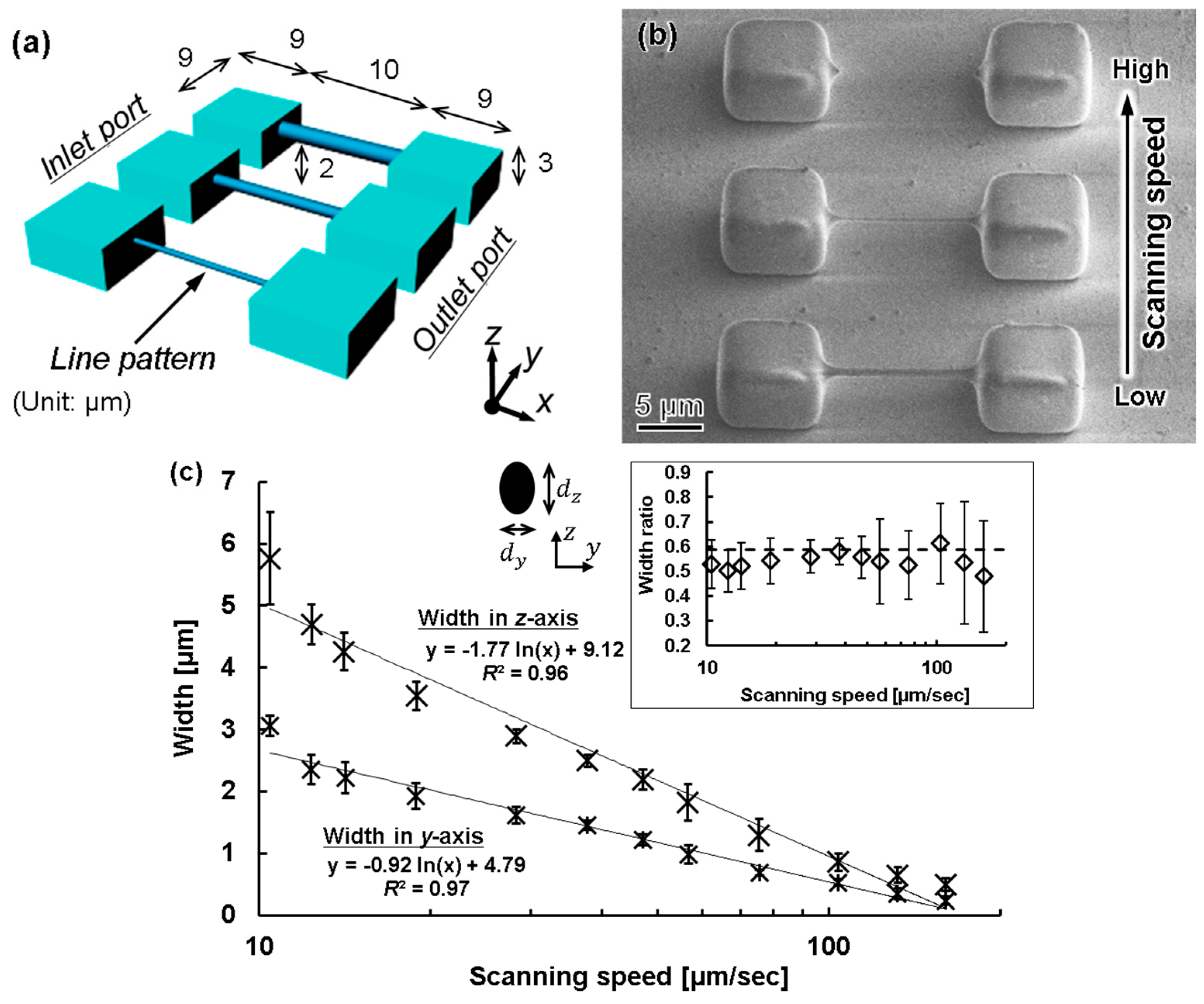
3.2.2. Line Width Processing
3.2.3. Experimental Procedure for FMEx
- A 20-μm-thick film of KMPR or PMER was formed by spin-coating and pre-baking on a hot plate at 100 °C for 30 min.
- A fine 3D microchannel was formed by femtosecond laser exposure.
- The connection port, which introduces the liquid flow path, was exposed using the mask aligner.
- The mold was heated using a hot plate at 65 °C for 1 min, 95 °C for 3 min, and 65 °C for 1 min. Then, the part was developed with PM thinner and rinsed with 2-propanol.
- After replacing the mold with t-butyl alcohol, it was dried with a vacuum dryer and the pattern was transferred by pouring PDMS into the mold.
- Remover PG was applied to remove the KMPR or PMER resist and form a hollow structure.
- The finished microchannel was bonded to a glass substrate with plasma treatment.
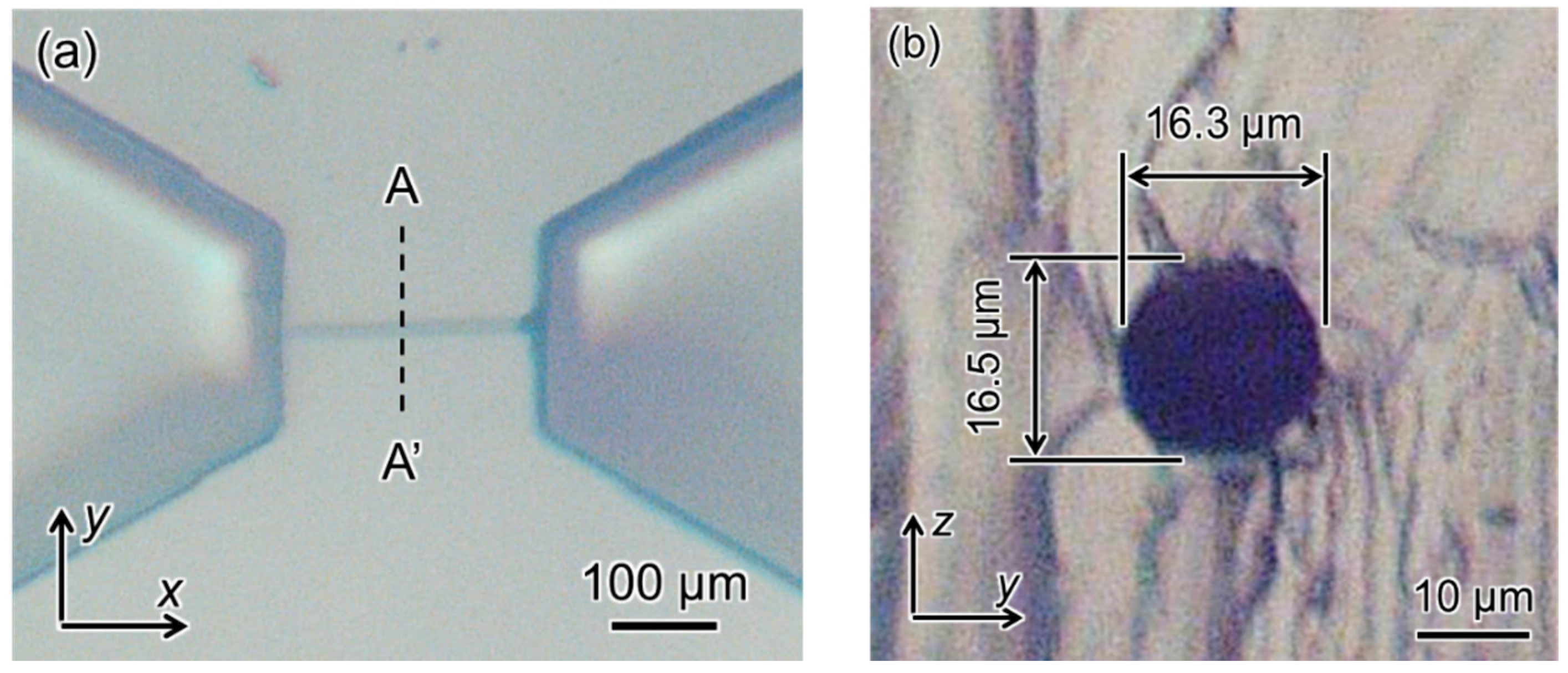
4. Experimental Results
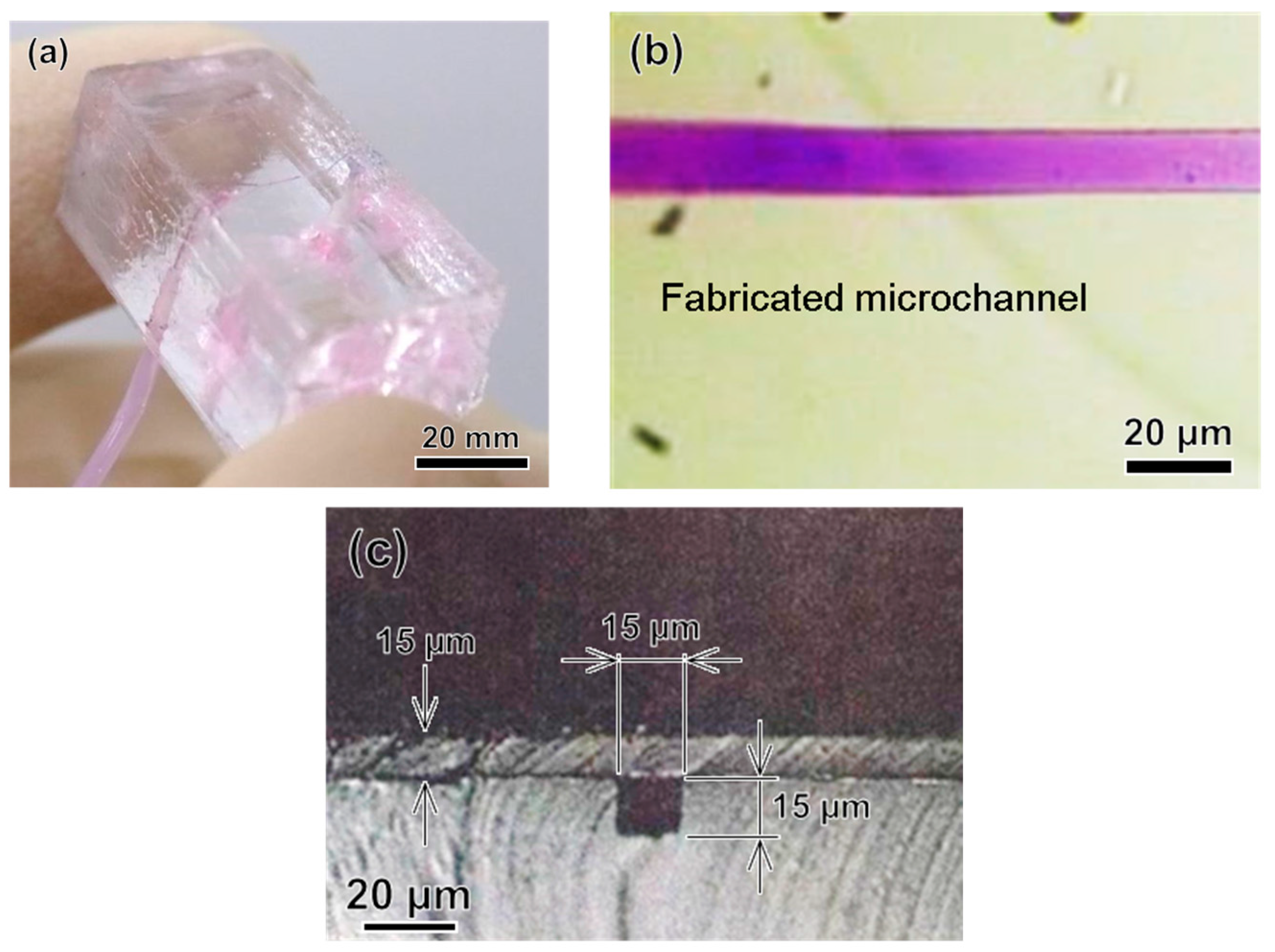
4.1. Photolithography Method
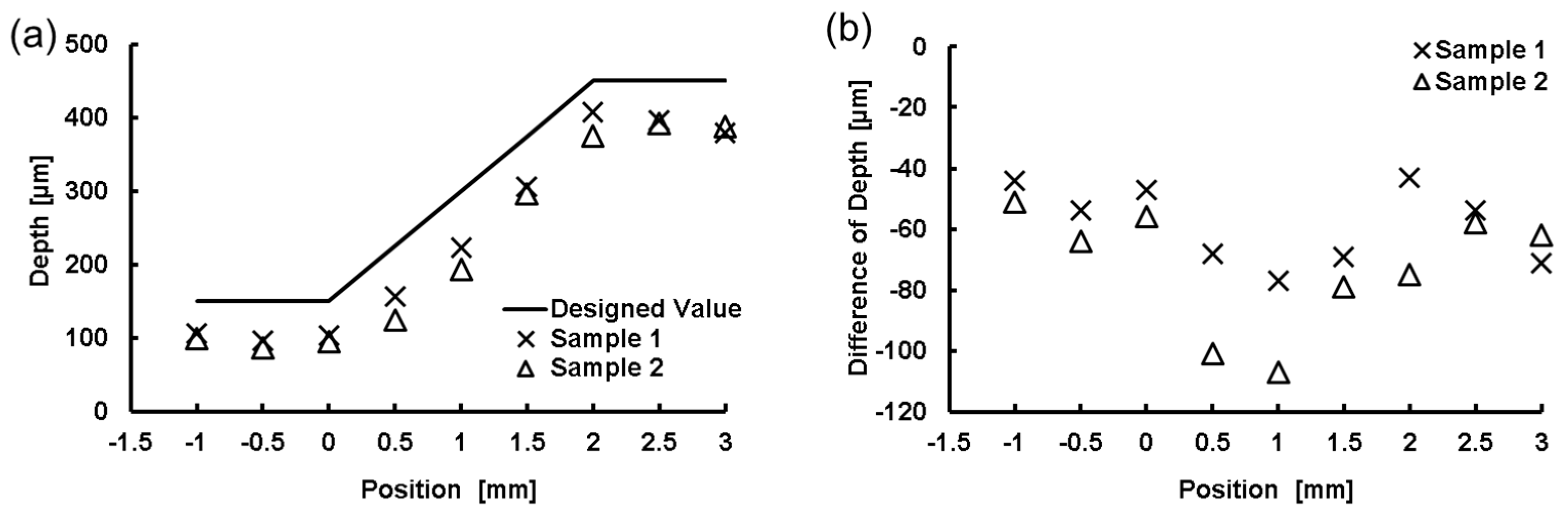
4.2. FMEx Method
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shinbane, J.S.; Wood, M.A.; Jensen, D.N.; Ellenbogen, K.A.; Fitzpatrick, A.P.; Scheinman, M.M. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J. Am. Coll. Cardiol. 1997, 29, 709–715. [Google Scholar] [CrossRef]
- Kneebone, R. Simulation in surgical training: Educational issues and practical implications. Med. Educ. 2003, 37, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Sugita, N.; Ueta, T.; Tamaki, Y.; Tanimoto, K.; Mitsuishi, M. Microsurgical robotic system for vitreoretinal surgery. Int. J. Comput. Assist. Radiol. Surg. 2012, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Stergiopulos, N.; Young, D.; Rogge, T. Computer simulation of arterial flow with applications to arterial and aortic stenoses. J. Biomech. 1992, 25, 1477–1488. [Google Scholar] [CrossRef]
- Seymour, N.E.; Gallagher, A.G.; Roman, S.A.; Obrien, M.K.; Bansal, V.K.; Andersen, D.K.; Satava, R.M. Virtual reality training improves operating room performance: Results of a randomized, double-blinded study. Ann. Surg. 2002, 236, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Arai, F.; Fukuda, T.; Negoro, M.; Irie, K. An in vitro patient specific biological model of the cerebral artery reproduced with a membranous configuration for simulating endovascular intervention. J. Robot Mechatron. 2005, 17, 327–333. [Google Scholar] [CrossRef]
- Matsushima, M.; Tercero, C.; Ikeda, S.; Fukuda, T.; Arai, F.; Negoro, M.; Takahashi, I. Photoelastic stress analysis in blood vessel phantoms: Three-dimensional visualization and saccular aneurysm with bleb. Int. J. Med. Robot. Comput. Assist. Surg. 2011, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Itoyama, T.; Yoshida, K.; Sawada, Y.; Ikeda, S.; Fukuda, T.; Matsuda, T.; Negoro, M.; Arai, F. Multiscale fabrication of a transparent circulation type blood vessel simulator. Biomicrofluidics 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Harada, K.; Ida, Y.; Tomita, K.; Kato, I.; Arai, F.; Ueta, T.; Noda, Y.; Sugita, N.; Mitsuishi, M. Quantitative assessment of manual and robotic microcannulation for eye surgery using new eye model. Int. J. Med. Robot. Comput. Assist. Surg. 2015, 11, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3625–3627. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. Moving from millifluidic to truly microfluidic sub-100-μm cross-section 3D printed devices. Anal. Bioanal. Chem. 2017, 409, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Noda, D.; Matsumoto, Y.; Setomoto, M. Fabrication of Coil Lines with High Aspect Ratio for Electromagnetic Actuators. In Proceedings of the IEEE 7th Annual International Symposium on Micro-Nano Mechatronics and Human Science, Nagoya, Japan, 11–14 November 2007; pp. 436–441. [Google Scholar]
- Itoga, K.; Yamato, M.; Kobayashi, J.; Kikuchi, A.; Okano, T. Cell micropatterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials 2004, 25, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, J.T.; Terai, H.; King, K.R.; Weinberg, E.J.; Kaazempur, M.R.; Vacanti, J.P. Microfabrication technology for vascularized tissue engineering. Biomed. Microdevices 2002, 4, 167–175. [Google Scholar] [CrossRef]
- Ito, H.; England, W.P.; Ueda, M. Chemical amplification based on acid-catalyzed depolymerization. J. Photopolym. Sci. Technol. 1990, 3, 219–233. [Google Scholar] [CrossRef]
- Schlegel, L.; Ueno, T.; Hayashi, N.; Iwanaga, T. Determination of acid diffusion in chemical amplification positive deep ultraviolet resists. J. Vac. Sci. Technol. B 1991, 9, 278–289. [Google Scholar] [CrossRef]
- Asakawa, K. Diffusion of acid and activation energy of positive chemical amplification resist. J. Photopolym. Sci. Technol. 1993, 6, 505–514. [Google Scholar] [CrossRef]
- Asakawa, K.; Ushirogouchi, T.; Nakase, M. Effect of remaining solvent on sensitivity diffusion of acid and resolution in chemical amplification resists. J. Vac. Sci. Technol. B 1995, 13, 833–839. [Google Scholar] [CrossRef]
- Chen, Q.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. Curved SU-8 Structure Fabrication Based on the Acid-diffusion Effect. In Proceedings of the IEEE 24th Annual International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 225–228. [Google Scholar]
- Masuda, T.; Yamagishi, Y.; Takei, N.; Owaki, H.; Matsusaki, M.; Akashi, M.; Arai, F. Three-dimensional assembly of multilayered tissues using water transfer printing. J. Robot Mechatron. 2013, 25, 690–697. [Google Scholar] [CrossRef]
- Futai, N.; Gu, W.; Takayama, S. Rapid prototyping of microstructures with bell-shaped cross-sections and its application to deformation-based microfluidic valves. Adv. Mater. 2004, 16, 1320–1323. [Google Scholar] [CrossRef]
- Hayakawa, T.; Fukada, S.; Arai, F. Fabrication of an on-chip nanorobot integrating functional nanomaterials for single-cell punctures. IEEE Trans. Robot. 2014, 30, 59–67. [Google Scholar] [CrossRef]
- Tomita, K.; Nakano, T.; Onda, K.; Fukuda, T.; Natsuda, T.; Negoro, M.; Arai, F. Fabrication of 3D capillary vessel simulator using femtosecond laser and mask hybrid exposure. In Proceedings of the IEEE 22th Annual International Symposium on Micro-Nano Mechatronics and Human Science, Nagoya, Japan, 6–9 November 2011; pp. 24–26. [Google Scholar]
- Sun, H.B.; Tanaka, T.; Kawata, S. Three-dimensional focal spots related to two-photon excitation. Appl. Phys. Lett. 2002, 80, 3673–3675. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Khuen, K.; Miwa, M.; Misawa, H. Two-photon lithography of nanorods in SU-8 photoresist. Nanotechnology 2005, 16, 846–849. [Google Scholar] [CrossRef]
- Hayakawa, T.; Kato, I.; Arai, F.; Mitsuishi, M.; Sugita, N.; Harada, K.; Tanaka, S.; Noda, Y.; Ueta, T. Retinal vessel model fabricated on a curved surface structure for a simulation of microcannulation. Robomech J. 2016, 3. [Google Scholar] [CrossRef]
- Yamguchi, H.; Saito, T.; Shiraishi, Y.; Arai, F.; Morimoto, Y.; Yuasa, A. Quantitative study on appearance of microvessels in spectral endoscopic imaging. J. Biomed. Opt. 2015, 20, 36005. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Kuroda, K.; Tsai, C.H.D.; Fukui, W.; Arai, F.; Kaneko, M. Red blood cell fatigue evaluation based on the close-encountering point between extensibility and recoverability. Lab Chip 2014, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]

| Method | Femtosecond Laser Exposure | Photolithography |
|---|---|---|
| Dimension | 3D | 3D |
| Microchannel width | Max. 20 µm | Over 10 µm |
| Process time | Hours | Seconds |
| Cross-sectional shape of microchannel | Circular cross-section Circularity: 0.95 | Rectangular |
| Total time of fabrication | Over 4 h | Less than 2 h |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallab, M.; Tomita, K.; Omata, S.; Arai, F. Fabrication of 3D Capillary Vessel Models with Circulatory Connection Ports. Micromachines 2018, 9, 101. https://doi.org/10.3390/mi9030101
Gallab M, Tomita K, Omata S, Arai F. Fabrication of 3D Capillary Vessel Models with Circulatory Connection Ports. Micromachines. 2018; 9(3):101. https://doi.org/10.3390/mi9030101
Chicago/Turabian StyleGallab, Mahmoud, Kyohei Tomita, Seiji Omata, and Fumihito Arai. 2018. "Fabrication of 3D Capillary Vessel Models with Circulatory Connection Ports" Micromachines 9, no. 3: 101. https://doi.org/10.3390/mi9030101





