The Viability of Single Cancer Cells after Exposure to Hydrodynamic Shear Stresses in a Spiral Microchannel: A Canine Cutaneous Mast Cell Tumor Model
Abstract
:1. Introductory Background
2. Materials and Methods
2.1. Theoretical Background for Spiral Microchannel Design
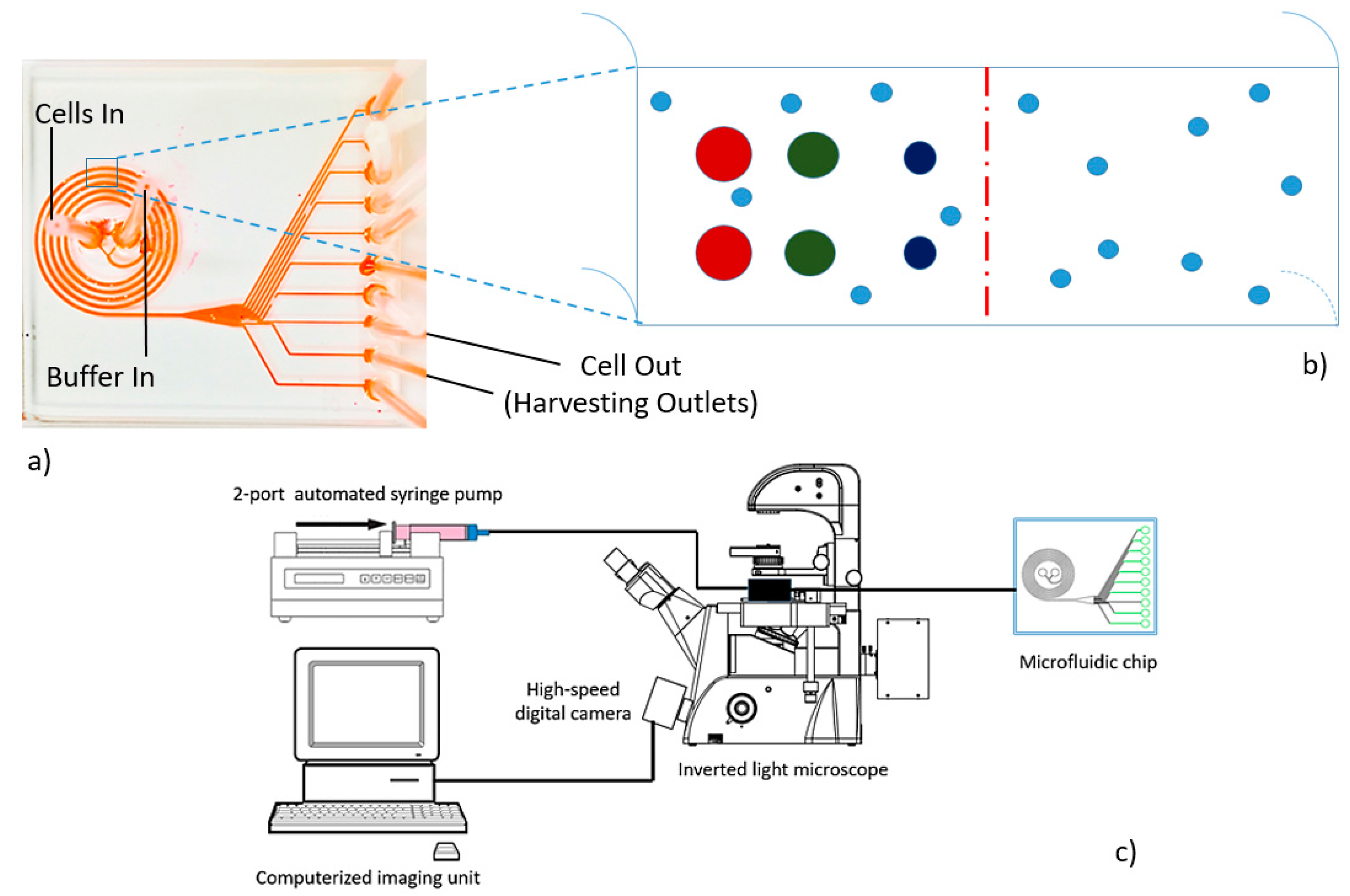
2.2. Microdevice Fabrication and Instrumentation
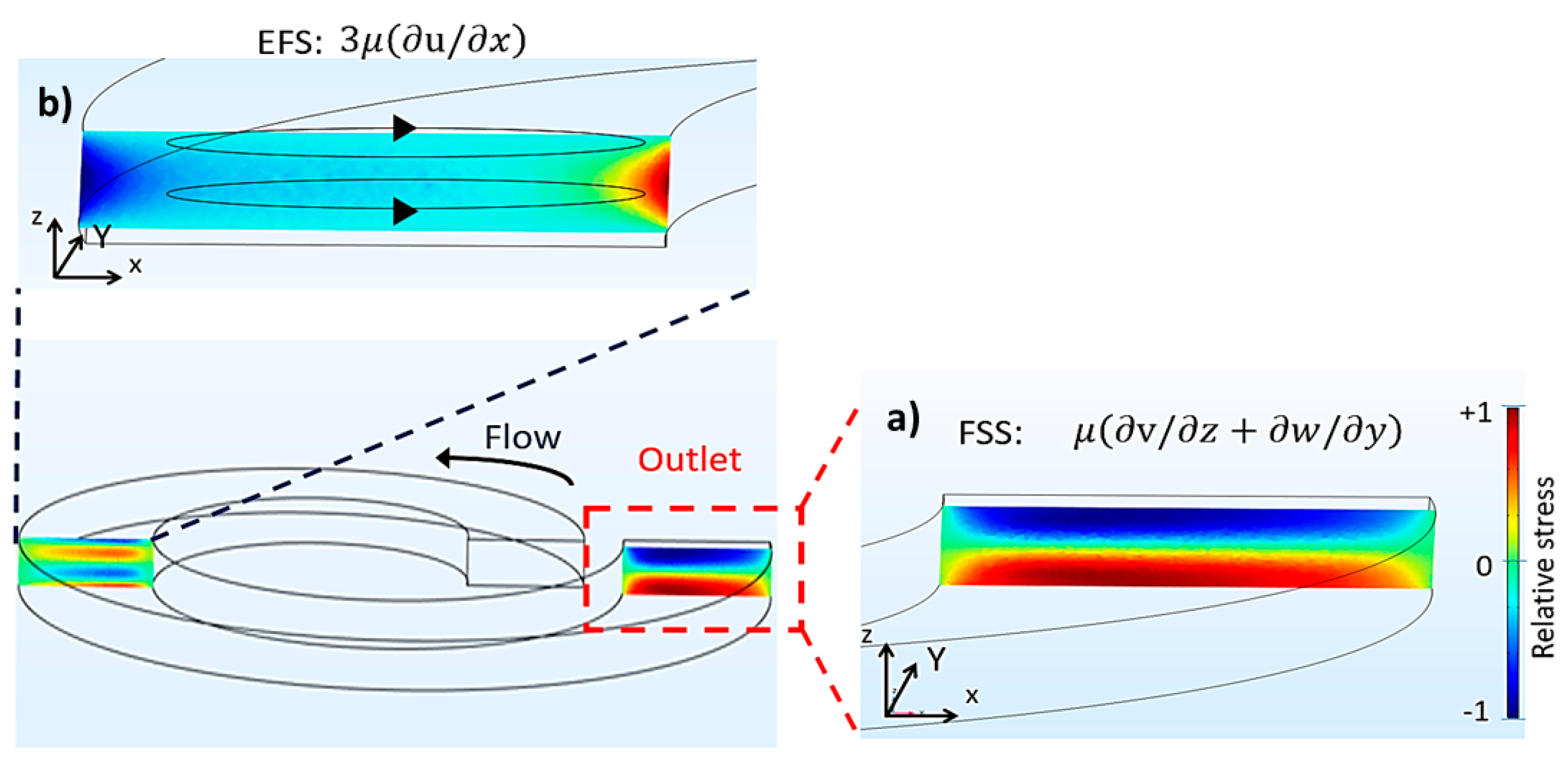
2.3. Computational Emulation of Fluid Shear Stress and Extensional Flow Stress
2.4. Single MCT Cell Isolation
2.5. Systematic Biomedical Assays for Mast Cell Tumor (MCT) Cell Viability
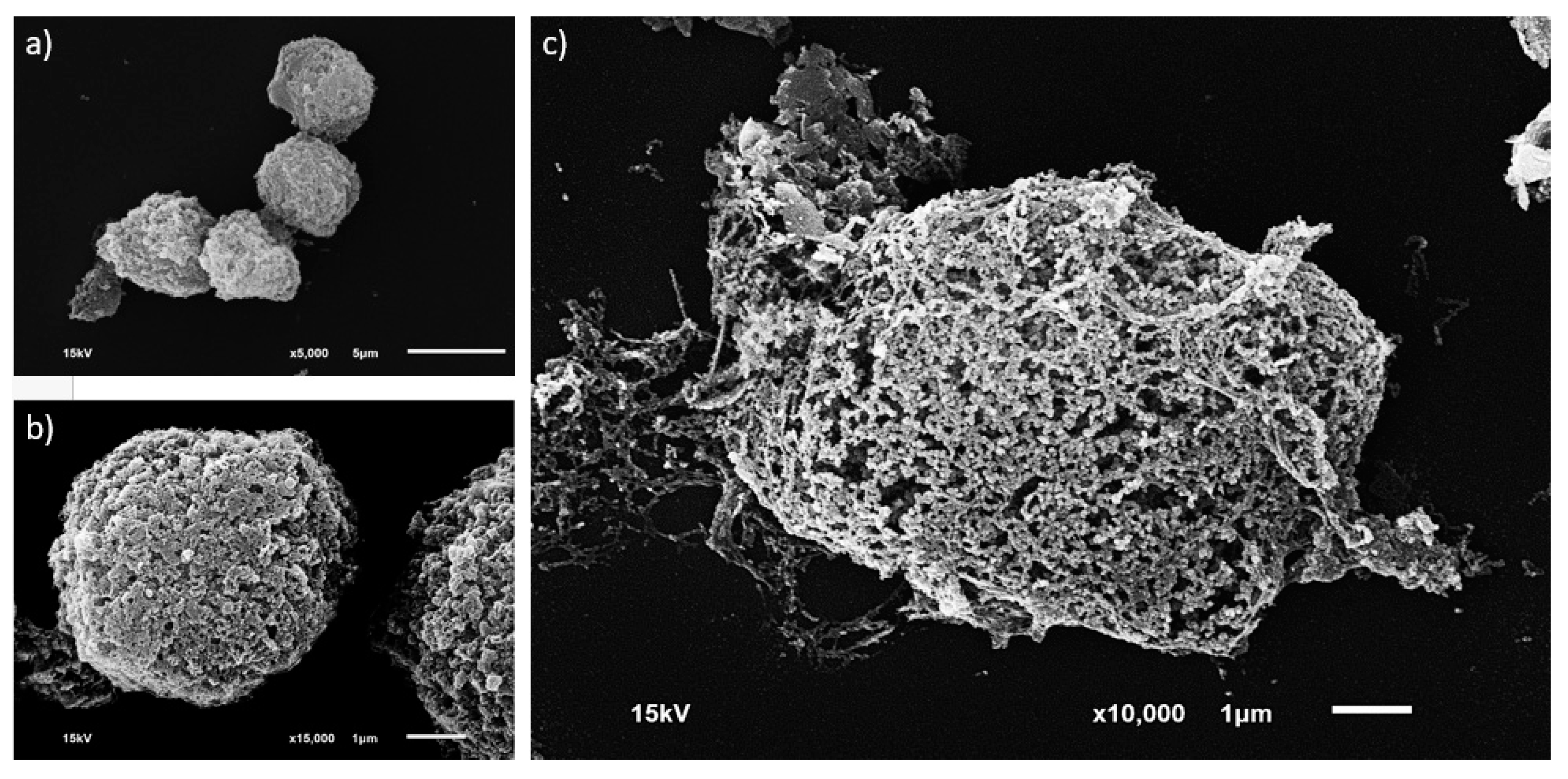
2.5.1. Morphological Investigation
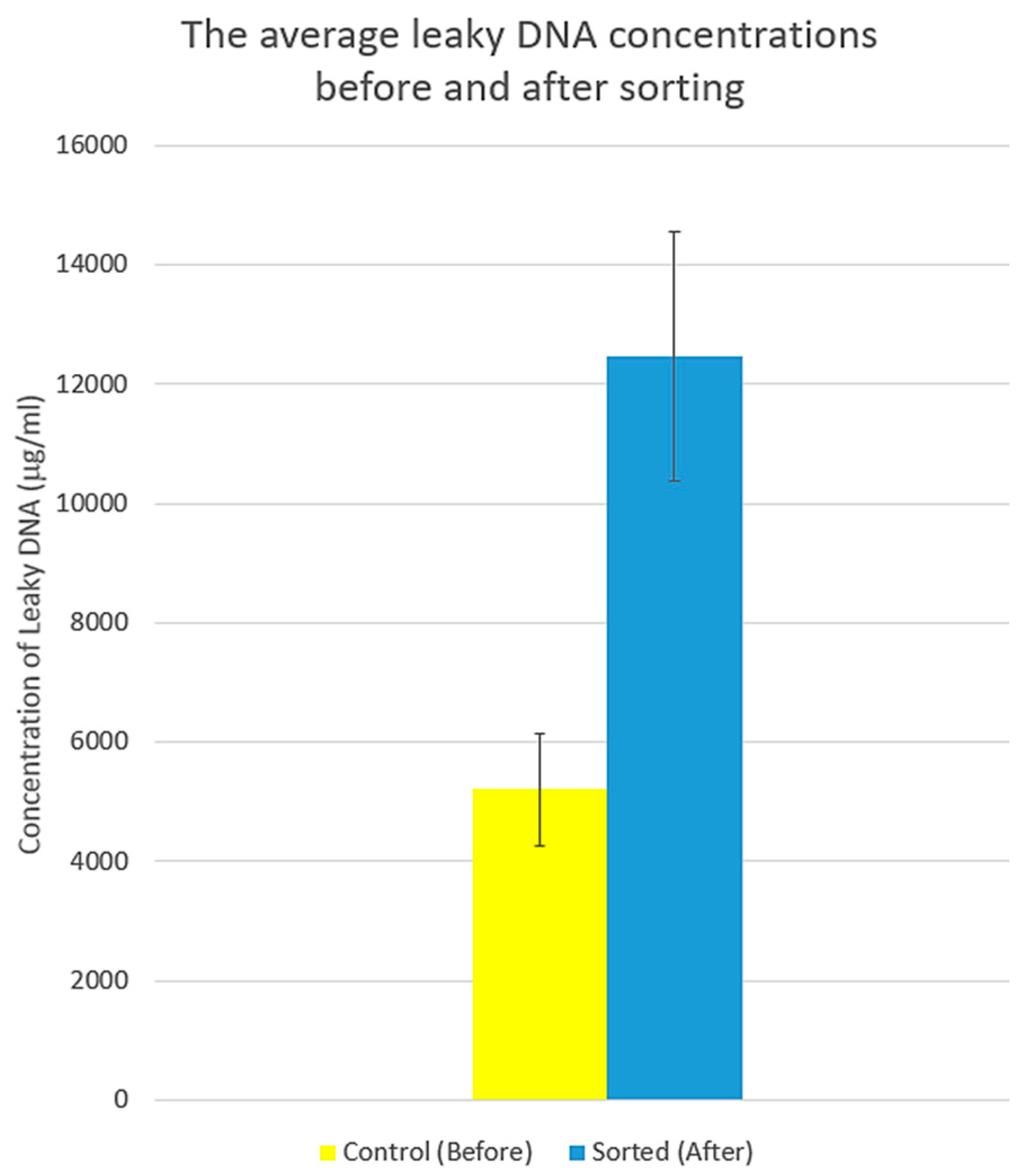
2.5.2. Assessments of Cell Membrane Disintegration
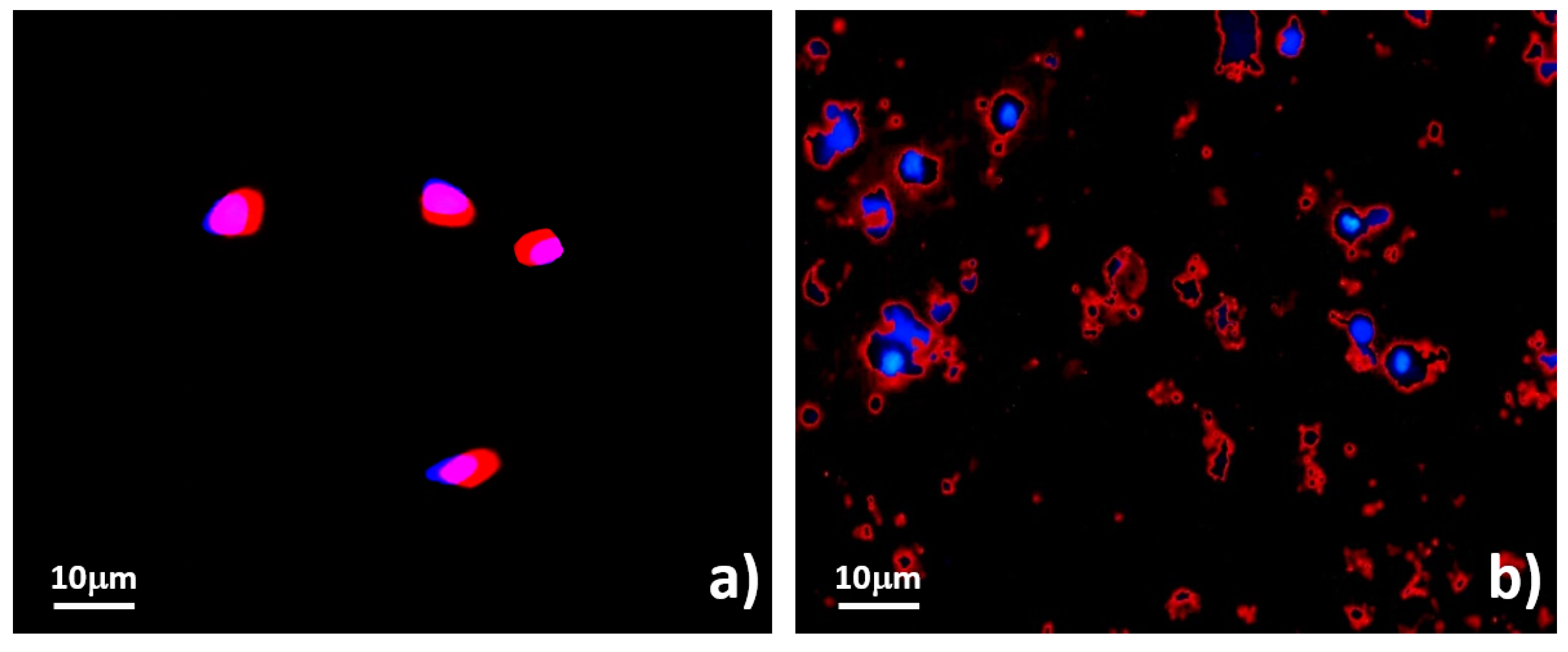
2.5.3. Evaluation of Functional Viability
3. Results
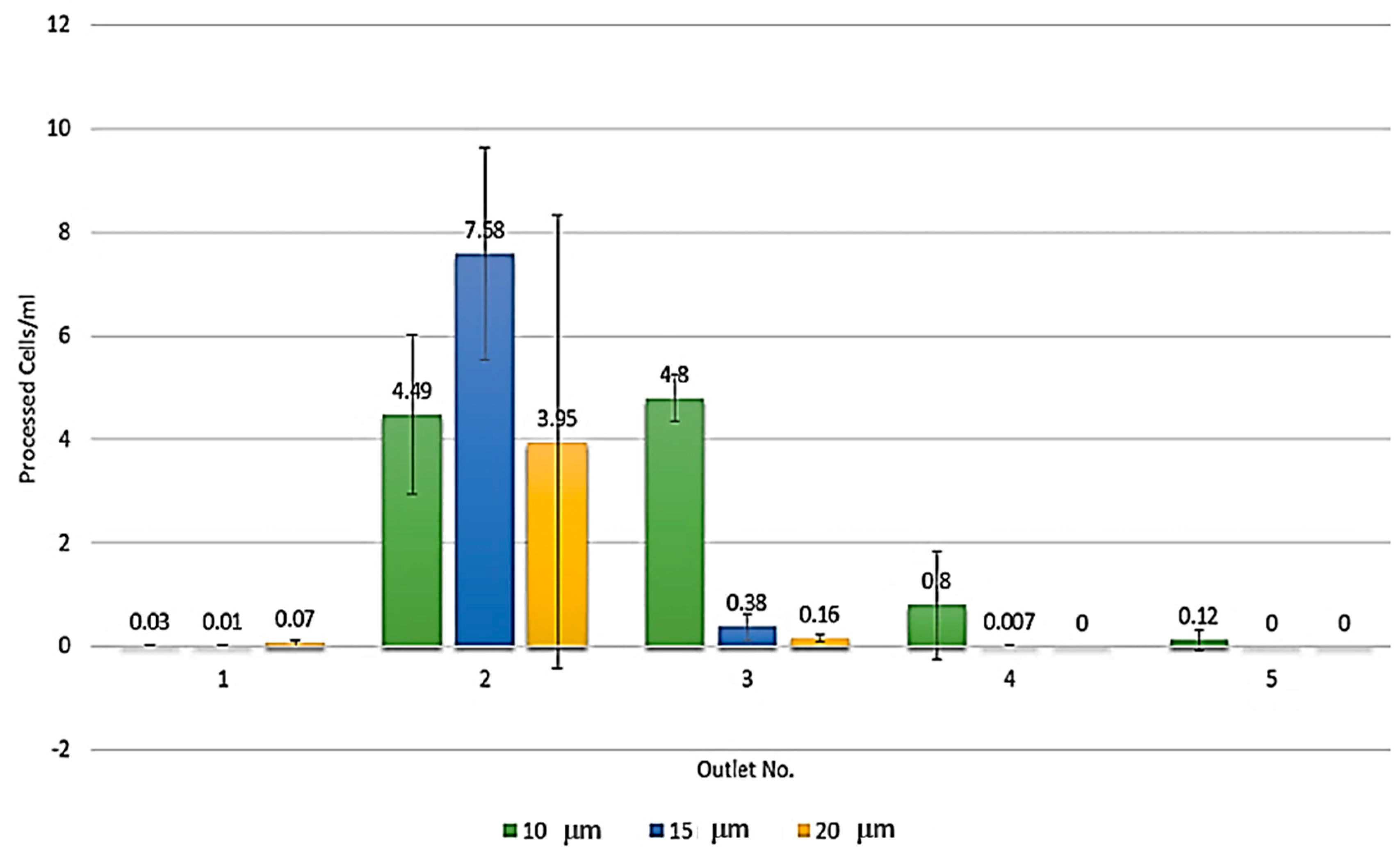
3.1. Microdevice Architecture
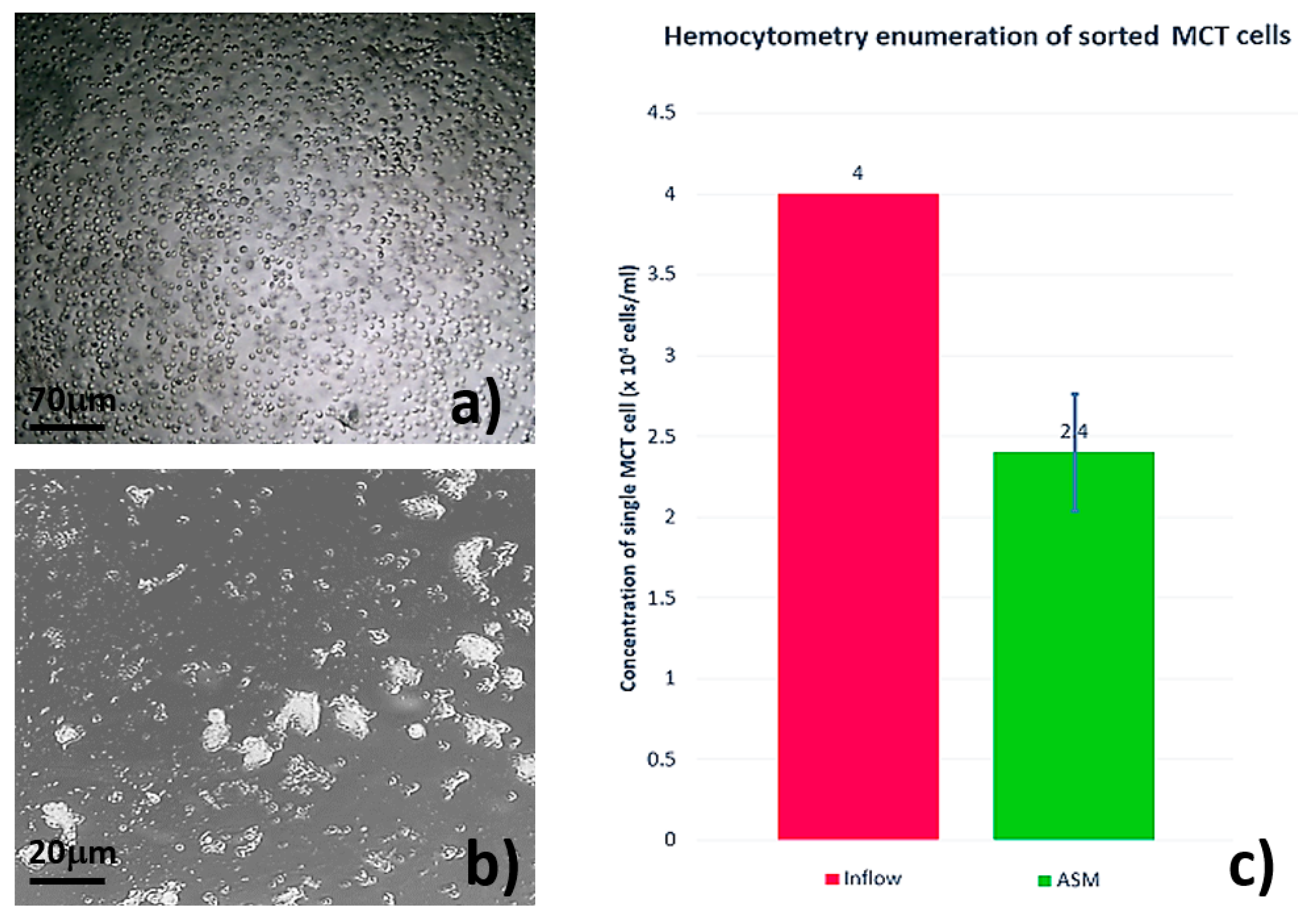
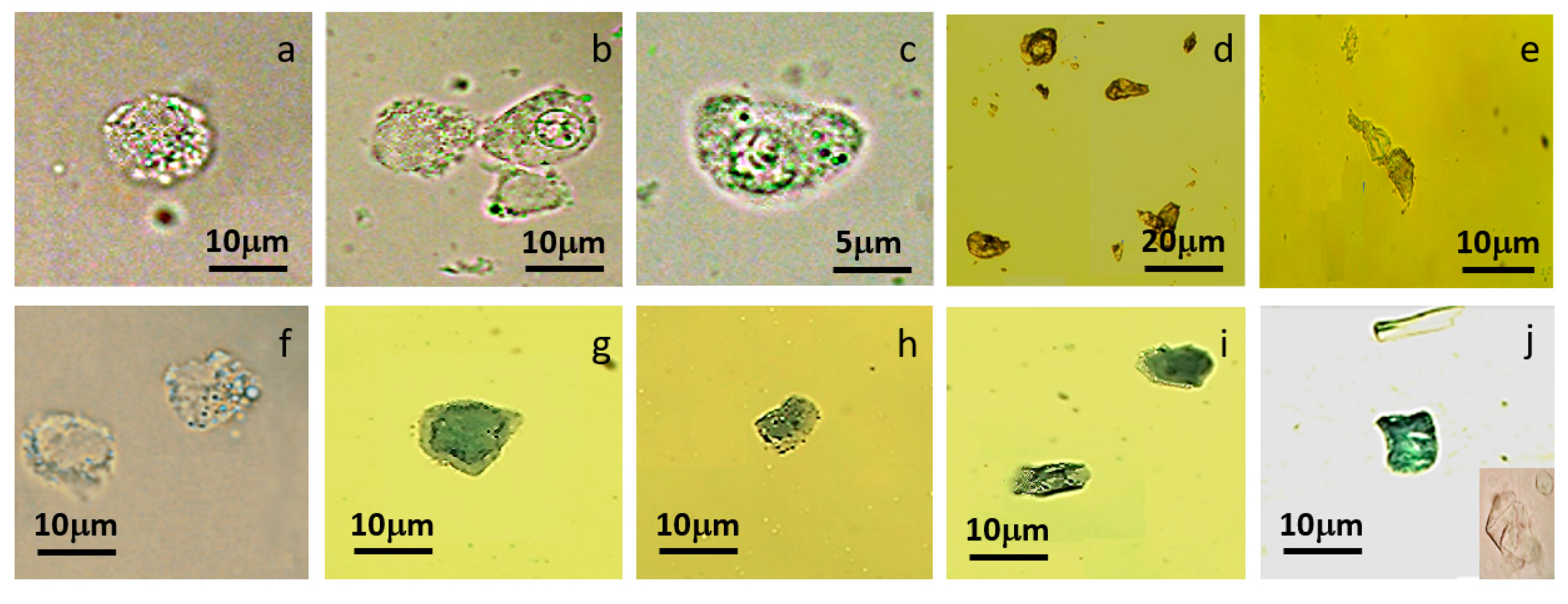
3.2. Single MCT Cell Isolation and Morphological Characterization of Unsorted MCT Cells
3.3. Systematic Biomedical Engineering Assays of the Viability of the Sorted MCT Cells
4. Discussion
5. Recapitulation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wyatt Shields Iv, C.; Reyes, C.D.; Lopez, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Qiao, Y.; Xu, M.; Li, J.; Liang, F.; Duan, M.; Ju, A.; Lu, Z. A cell sorting and trapping microfluidic device with an interdigital channel. AIP Adv. 2016, 6, 125042. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.N. Cancer stem cells: Understanding tumor hierarchy and heterogeneity. Medicine 2016, 95, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Rycaj, K.; Chen, X.; Tang, D.G. Cancer stem cells and cell size: A causal link? Semin. Cancer Biol. 2015, 35, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Saadatpour, A.; Lai, S.; Guo, G.; Yuan, G.-C. Single-cell analysis in cancer genomics. Trends Genet. 2015, 31, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tzanakakis, E.S. Deconstructing stem cell population heterogeneity: Single-cell analysis and modeling approaches. Biotechnol. Adv. 2013, 31, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.L.; Chaudhuri, P.K.; Ramalingam, N.; Tan, D.S.W.; Lim, C.T.; Warkiani, M.E. Single-cell profiling approaches to probing tumor heterogeneity. Int. J. Cancer 2016, 139, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishikawa, T.; Numayama-Tsuruta, K.; Imai, Y.; Ueno, H.; Yoshimoto, T.; Matsuki, N.; Yamaguchi, T. Inertial migration of cancer cells in blood flow in microchannels. Biomed. Microdevices 2012, 14, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for single-cell isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef] [PubMed]
- Lecault, V.; White, A.K.; Singhal, A.; Hansen, C.L. Microfluidic single cell analysis: From promise to practice. Curr. Opin. Chem. Biol. 2012, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zheng, W.; Sun, J.; Zhang, W.; Jiang, X. Microfluidics for manipulating cells. Small 2013, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Toner, M. Inertial focusing dynamics in spiral microchannels. Phys. Fluids 2012, 24, 32001. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Toner, M. Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef] [PubMed]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C. Cell injury by electric forces. Ann. N. Y. Acad. Sci. 2005, 1066, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Sluyter, R.; Li, W.; Alici, G.; Nguyen, N.-T. Inertial particle separation by differential equilibrium positions in a symmetrical serpentine micro-channel. Sci. Rep. 2014, 4, 4527. [Google Scholar] [CrossRef] [PubMed]
- Salieb-Beugelaar, G.B.; Simone, G.; Arora, A.; Philippi, A.; Manz, A. Latest developments in microfluidic cell biology and analysis systems. Anal. Chem. 2010, 82, 4848–4864. [Google Scholar] [CrossRef] [PubMed]
- Thanormsridetchai, A.; Ketpun, D.; Pimpin, A.; Srituravanich, W.; Piyaviriyakul, P.; Sailasuta, A.; Jeamsaksiri, W.; Sripumkhai, W.; Jantawong, J.; Supadech, J. Sorting of multiple-size cells using spiral microchannels. In Proceedings of the 27th International Microprocesses and Nanotechnology Conference, Fukuoka, Japan, 4–7 November 2014; JJAP: Fukuoka, Japan, 2014. [Google Scholar]
- Suwannaphan, T.; Pimpin, A.; Srituravanich, W.; Jeamsaksiri, W.; Sripumkhai, W.; Ketpun, D.; Sailasuta, A.; Piyaviriyakul, P. Investigation of shear stress and cell survival in a microfluidic chip for a single cell study. In Proceedings of the 2015 8th Biomedical Engineering International Conference (BMEiCON), Pattaya, Thailand, 25–27 November 2015; pp. 1–5. [Google Scholar]
- Thanormsridetchai, A.; Ketpun, D.; Srituravanich, W.; Piyaviriyakul, P.; Sailasuta, A.; Jeamsaksiri, W.; Sripumkhai, W.; Pimpin, A. Focusing and sorting of multiple-sized beads and cells using low-aspect-ratio spiral microchannels. J. Mech. Sci. Technol. 2017, 31, 5397–5405. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Fundamentals of inertial focusing in microchannels. Lab Chip 2013, 13, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J. Cell viability assays: Introduction. In Mammalian Cell Viability: Methods and Protocols; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–6. [Google Scholar]
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Hwang, Y.S.; Suh, H. Viability evaluation of engineered tissues. Yonsei Med. J. 2000, 41, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Voldman, J. A cell-based sensor of fluid shear stress for microfluidics. Lab Chip 2015, 15, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.B.; Jang, H.K.; Shin, T.H.; Phukan, G.; Tran, T.T.; Lee, G.; Hwang, W.R.; Kim, J.M. Microfluidic assessment of mechanical cell damage by extensional stress. Lab Chip 2016, 16, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Tanzeglock, T.; Soos, M.; Stephanopoulos, G.; Morbidelli, M. Induction of mammalian cell death by simple shear and extensional flows. Biotechnol. Bioeng. 2009, 104, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Nivedita, N.; Papautsky, I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 2013, 7, 54101. [Google Scholar] [CrossRef] [PubMed]
- Suwannaphan, T. Applications for a Single-Cell Study: Improving Cell Viability in Experimental Setups and Designing of a Single Cell Releasing Device; Chulalongkorn University: Bangkok, Thailand, 2015. [Google Scholar]
- London, C.A.; Thamm, D.H. Mast cell tumors. In Withrow and Macewen’s Small Animal Clinical Oncology, 5th ed.; Vail, D.M., Page, R.L., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2013; pp. 335–355. [Google Scholar]
- Thamm, D.H.; Vail, D.M. Mast cell tumors. In Withrow & Macewen’s Small Animal Clinical Oncology, 4th ed.; W.B. Saunders: Saint Louis, MO, USA, 2007; pp. 402–424. [Google Scholar]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. Microfluidic device for single-cell analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Marshall, D. Microfluidics for single cell analysis. Curr. Opin. Biotechnol. 2012, 23, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zandi, M.; Ho, C.C.; Kaval, N.; Papautsky, I. Single stream inertial focusing in a straight microchannel. Lab Chip 2015, 15, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.-W.; Lim, W.-T.; Han, J.; Bhagat, A.A.S.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Hasni, A.E.; Göbbels, K.; Thiebes, A.L.; Bräunig, P.; Mokwa, W.; Schnakenberg, U. Focusing and sorting of particles in spiral microfluidic channels. Procedia Eng. 2011, 25, 1197–1200. [Google Scholar] [CrossRef]
- Burkhardt, E. Scanning electron microscopy of peripheral blood leukocytes of the chicken. Cell Tissue Res. 1979, 204, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tamai, S.; Iida, H.; Yokota, S.; Sayano, T.; Kiguchiya, S.; Ishihara, N.; Hayashi, J.-I.; Mihara, K.; Oka, T. Characterization of the mitochondrial protein letm1, which maintains the mitochondrial tubular shapes and interacts with the aaa-atpase bcs1l. J. Cell Sci. 2008, 121, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- De Mora, F.; Puigdemont, A.; Torres, R. The role of mast cells in atopy: What can we learn from canine models? A thorough review of the biology of mast cells in canine and human systems. Br. J. Dermatol. 2006, 155, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Martin, H. A review of mast cell biology. Vet. Dermatol. 1998, 9, 145–166. [Google Scholar]
- Sailasuta, A.; Ketpun, D.; Piyaviriyakul, P.; Theerawatanasirikul, S.; Theewasutrakul, P.; Rungsipipat, A. The relevance of cd117-immunocytochemistry staining patterns to mutational exon-11 in c-kit detected by pcr from fine-needle aspirated canine mast cell tumor cells. Vet. Med. Int. 2014, 2014, 787498. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.R.; Prasse, K.W. Cytology of canine cutaneous round cell tumors. Vet. Pathol. 1979, 16, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a probe of mitochondrial membrane potential: Evaluation of proton flux through f(0) during atp synthesis. Biochim. Biophys. Acta 2003, 1606, 137–146. [Google Scholar] [CrossRef]
- Joshi, D.C.; Bakowska, J.C. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J. Vis. Exp. 2011, 2704. [Google Scholar] [CrossRef] [PubMed]
- Scaduto, R.C., Jr.; Grotyohann, L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 1999, 76, 469–477. [Google Scholar] [CrossRef]
- Kretzmer, G.; Schugerl, K. Response of mammalian cells to shear stress. Appl. Microbiol. Biotechnol. 1991, 34, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C. Influence of hydrodynamic shear stress on microcarrier-attached cell growth: Cell line dependency and surfactant protection. Bioprocess Eng. 1999, 21, 201–206. [Google Scholar] [CrossRef]
- Lee, S.S.; Ahn, K.H.; Lee, S.J.; Sun, K.; Goedhart, P.T.; Hardeman, M.R. Shear induced damage of red blood cells monitored by the decrease of their deformability. Korea-Aust. Rheol. J. 2004, 16, 141–146. [Google Scholar]
- Cheng, Y.H.; Chen, Y.C.; Brien, R.; Yoon, E. Scaling and automation of a high-throughput single-cell-derived tumor sphere assay chip. Lab Chip 2016, 16, 3708–3717. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.S.; Fujiwara, K.; Abe, J. Shear stress and atherosclerosis. Mol. Cells 2014, 37, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Neoplasia. In Robbins Basic Pathology, 9th ed.; Kumar, V., Abbas, A.K., Aster, J.C., Eds.; Saunders, Elsevier: Philadelphia, PA, USA, 2013; pp. 161–214. [Google Scholar]
- Strayer, D.S.; Rubin, E. Neoplasia. In Essentials of Rubin’s Pathology, 6th ed.; Reisner, H.M., Rubin, E., Eds.; Lippincott Williams & Wilkins: Hong Kong, China, 2014; pp. 93–122. [Google Scholar]
- Diz-Munoz, A.; Fletcher, D.A.; Weiner, O.D. Use the force: Membrane tension as an organizer of cell shape and motility. Trends Cell. Biol. 2013, 23, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Nauseef, J.T.; Henry, M.D. Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS ONE 2012, 7, e50973. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jung, H.I. Multiorifice flow fractionation: Continuous size-based separation of microspheres using a series of contraction/expansion microchannels. Anal. Chem. 2009, 81, 8280–8288. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; King, M.R. Computational and experimental models of cancer cell response to fluid shear stress. Front. Oncol. 2013, 3, 44. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketpun, D.; Sailasuta, A.; Suwannaphan, T.; Bhanpattanakul, S.; Pimpin, A.; Srituravanich, W.; Sripumkhai, W.; Jeamsaksiri, W.; Piyaviriyakul, P. The Viability of Single Cancer Cells after Exposure to Hydrodynamic Shear Stresses in a Spiral Microchannel: A Canine Cutaneous Mast Cell Tumor Model. Micromachines 2018, 9, 9. https://doi.org/10.3390/mi9010009
Ketpun D, Sailasuta A, Suwannaphan T, Bhanpattanakul S, Pimpin A, Srituravanich W, Sripumkhai W, Jeamsaksiri W, Piyaviriyakul P. The Viability of Single Cancer Cells after Exposure to Hydrodynamic Shear Stresses in a Spiral Microchannel: A Canine Cutaneous Mast Cell Tumor Model. Micromachines. 2018; 9(1):9. https://doi.org/10.3390/mi9010009
Chicago/Turabian StyleKetpun, Dettachai, Achariya Sailasuta, Thammawit Suwannaphan, Sudchaya Bhanpattanakul, Alongkorn Pimpin, Werayut Srituravanich, Witsaroot Sripumkhai, Wutthinan Jeamsaksiri, and Prapruddee Piyaviriyakul. 2018. "The Viability of Single Cancer Cells after Exposure to Hydrodynamic Shear Stresses in a Spiral Microchannel: A Canine Cutaneous Mast Cell Tumor Model" Micromachines 9, no. 1: 9. https://doi.org/10.3390/mi9010009




