On-Chip Facile Preparation of Monodisperse Resorcinol Formaldehyde (RF) Resin Microspheres
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
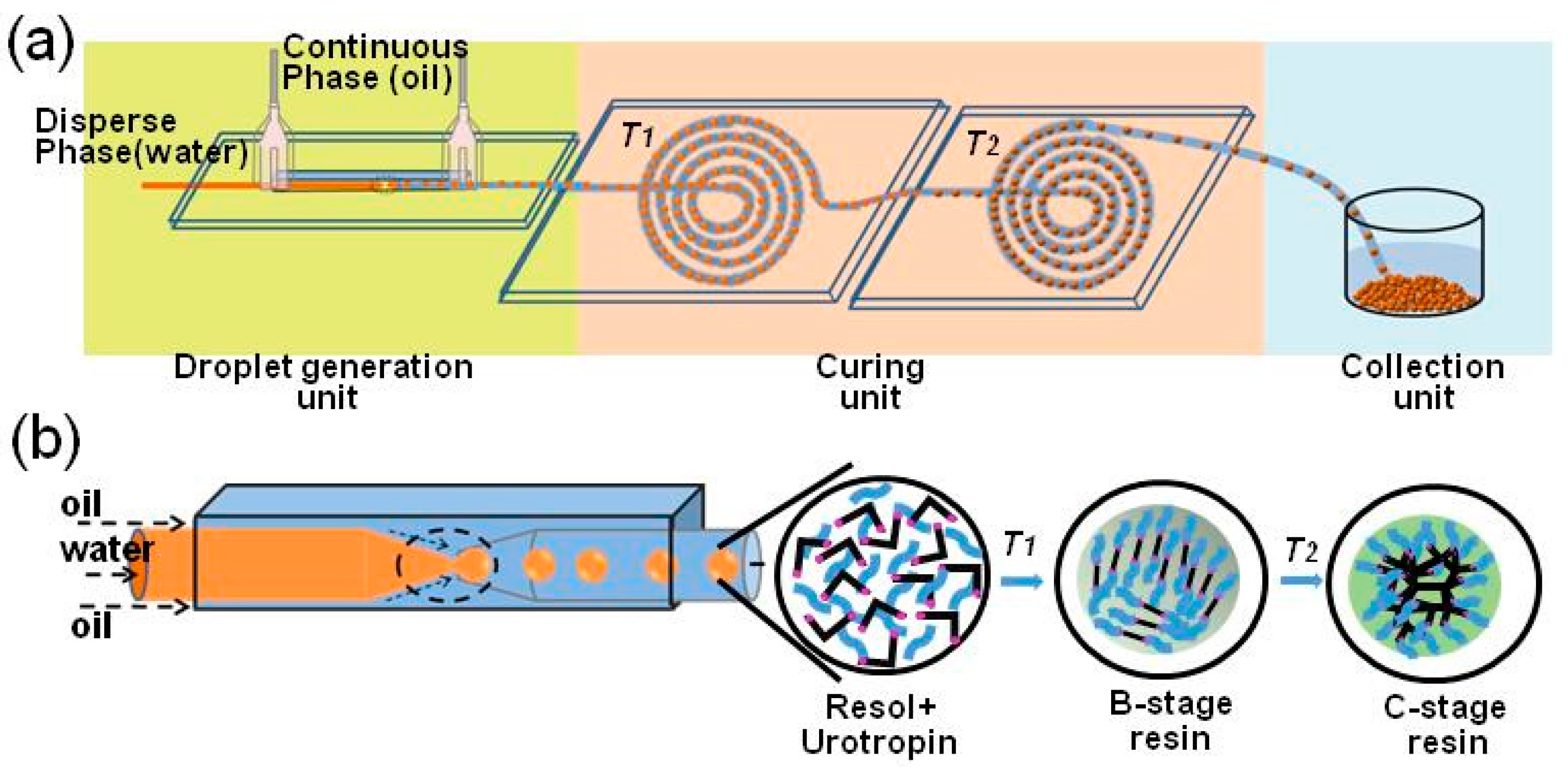
3.1. Experimental Setup
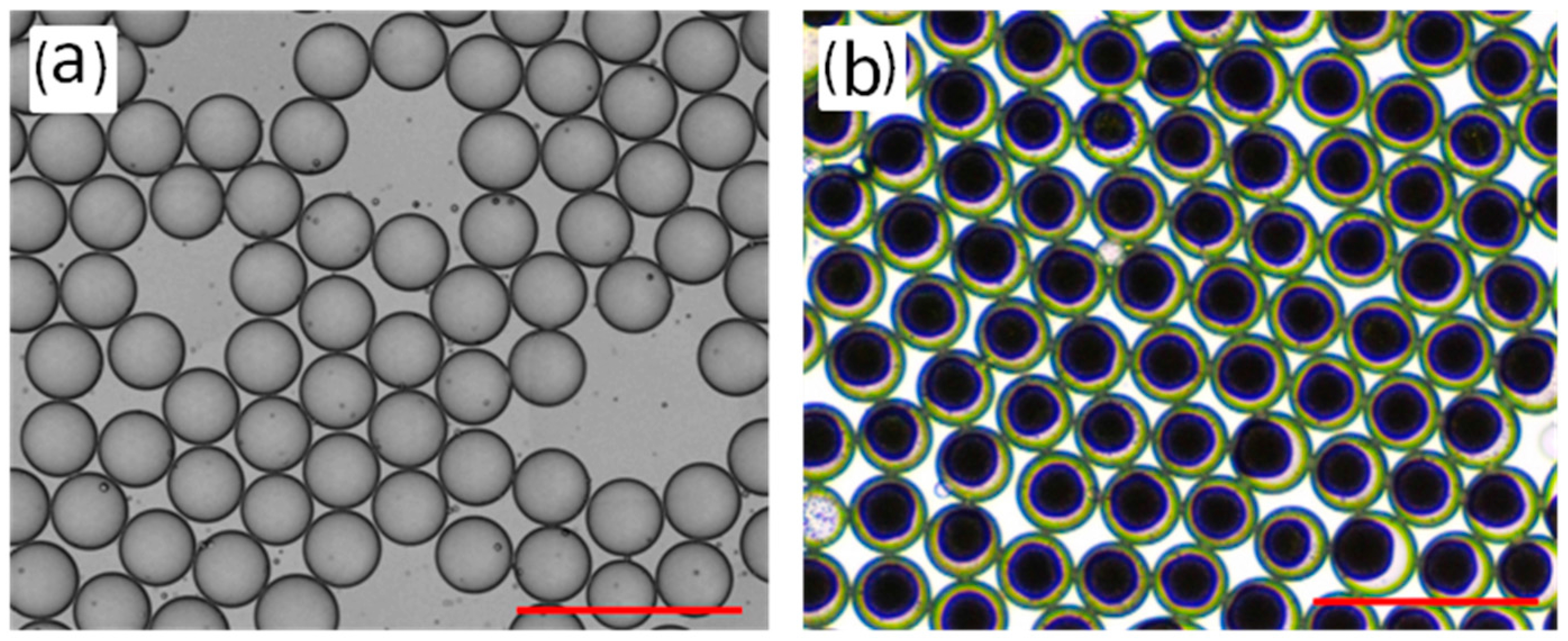
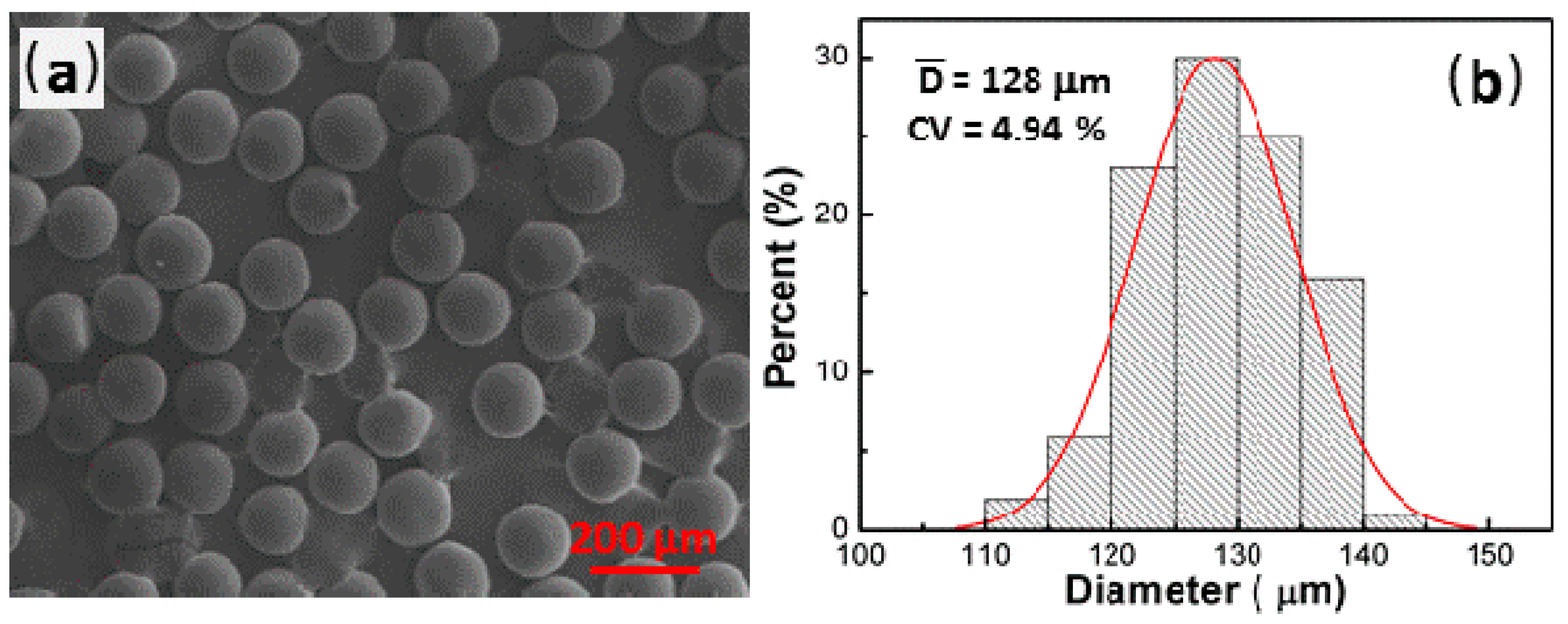
3.2. Fabrication of the RF Microspheres
3.2.1. The Effect of the Gelation Temperature and Holding Time
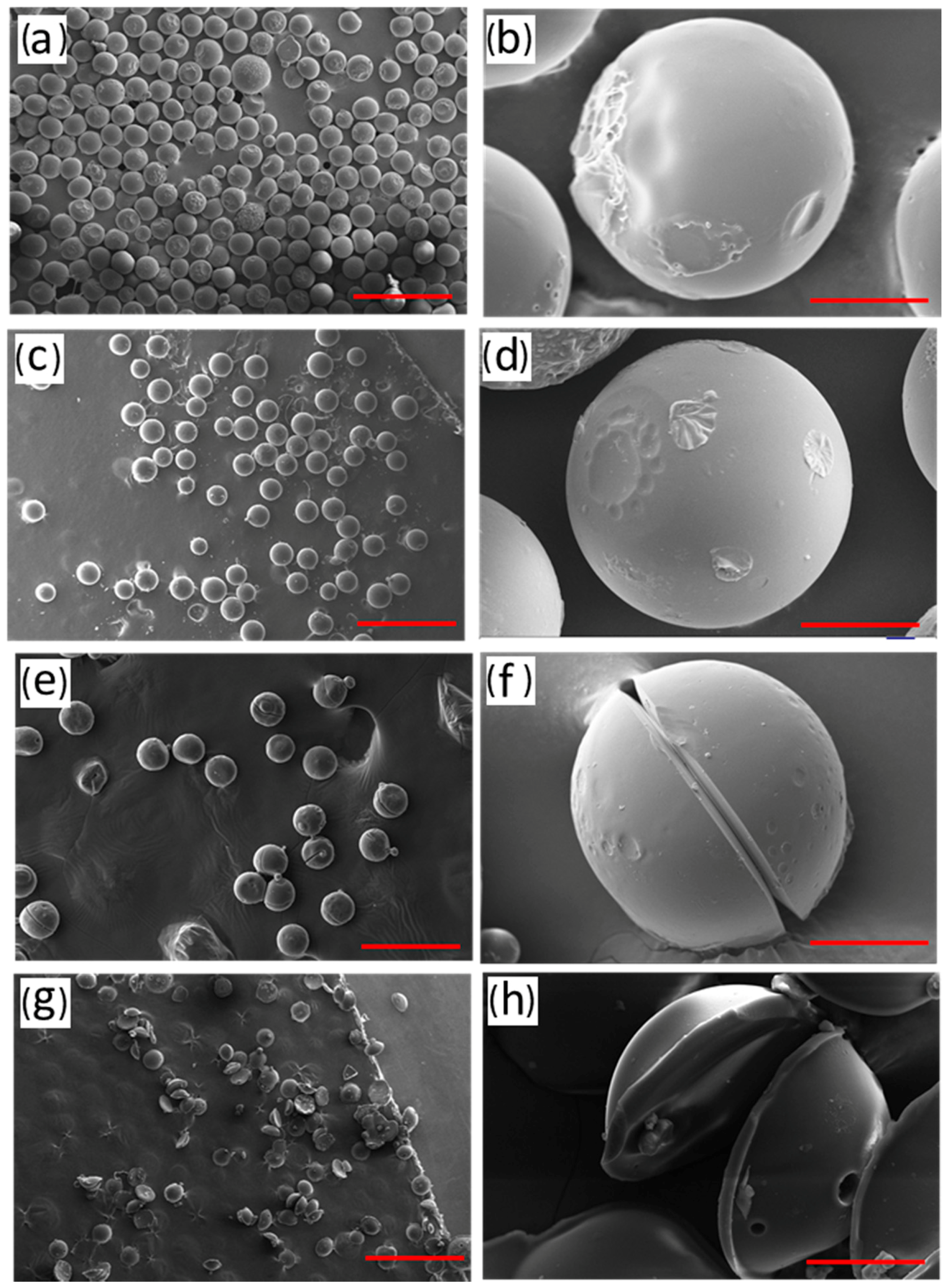
3.2.2. The Effect of the Solidification Temperature
3.2.3. Effect of the Solidification Time
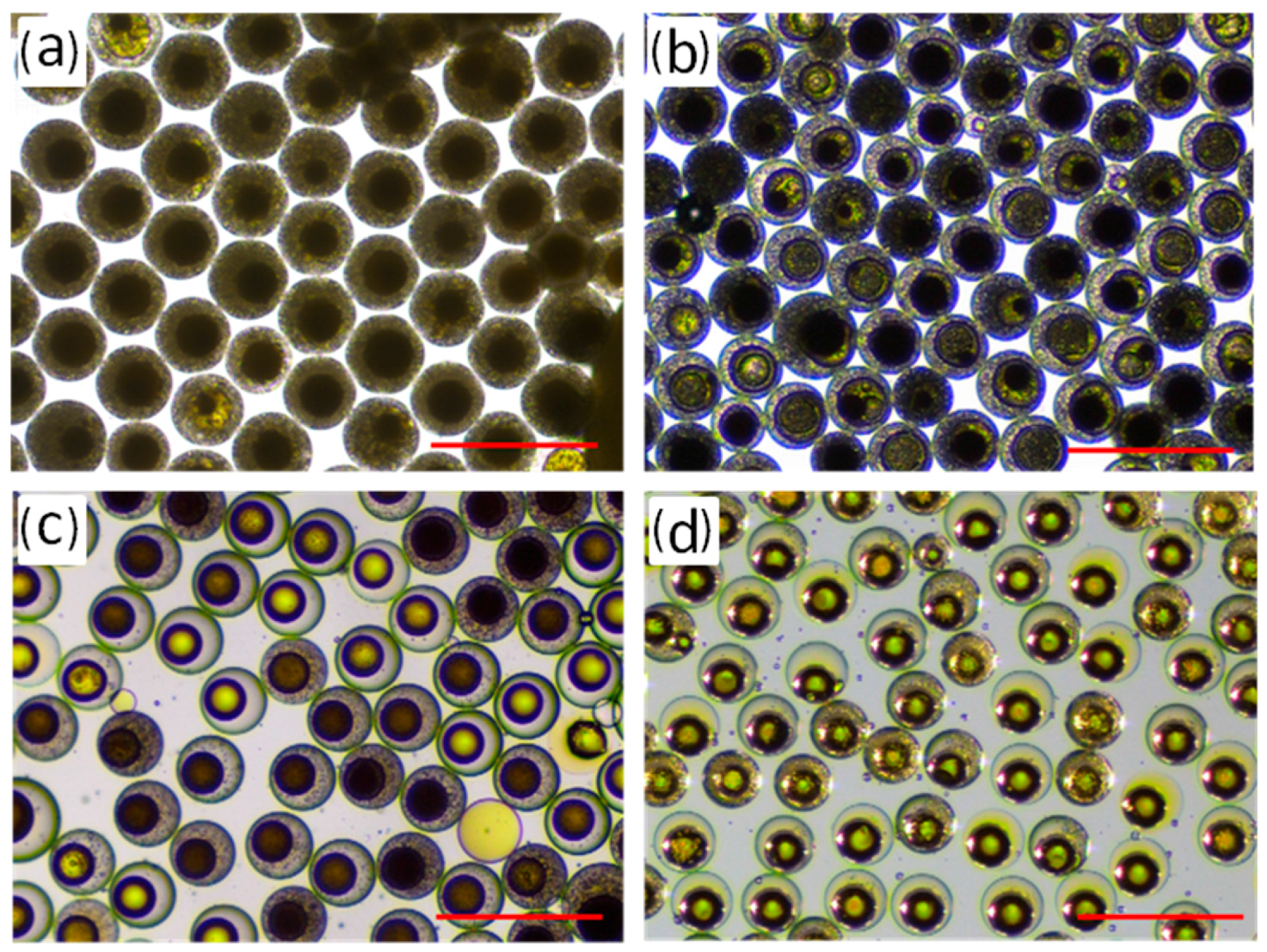
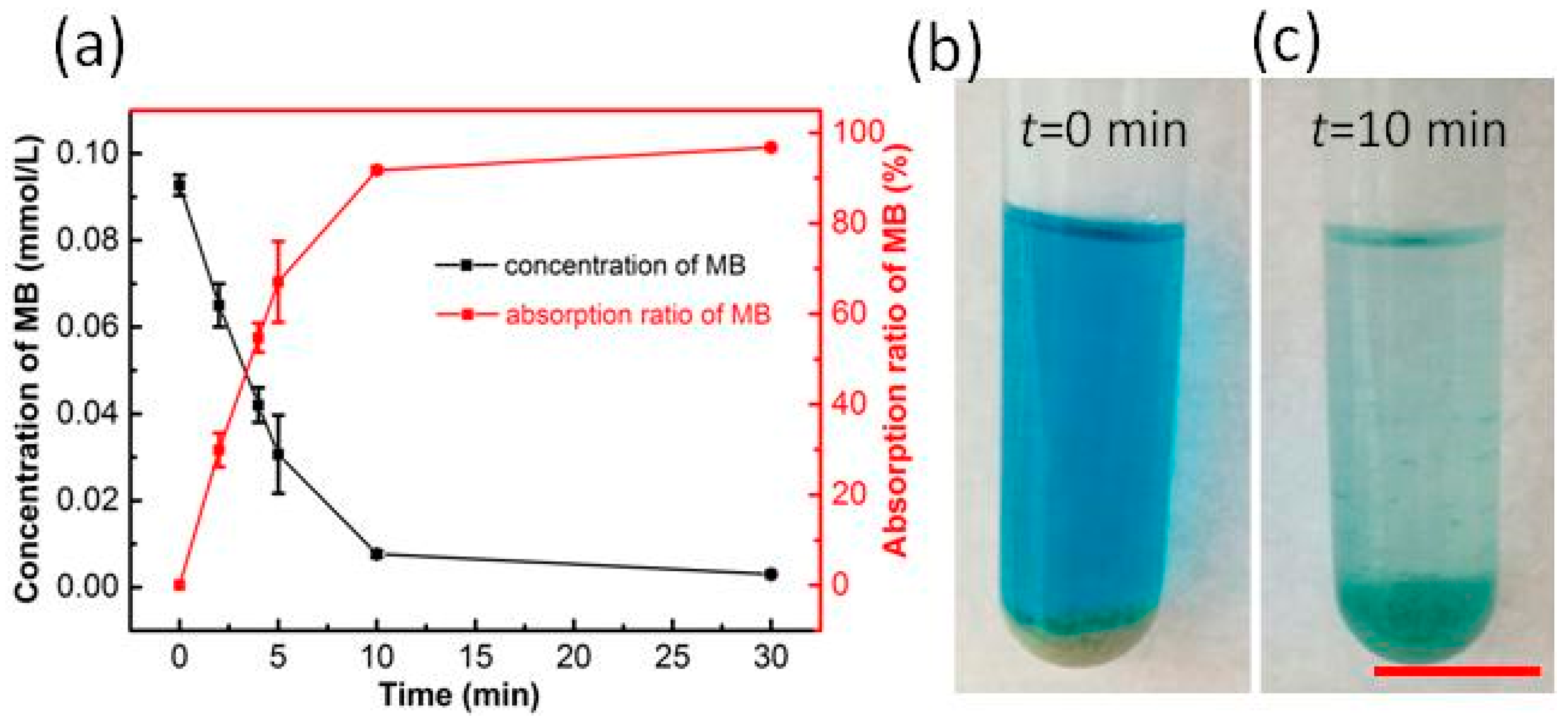
3.2.4. Adsorption Test of the Porous RF Microspheres
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seredych, M.; Ania, C.; Bandosz, T.J. Moisture insensitive adsorption of ammonia on resorcinol-formaldehyde resins. J. Hazard. Mater. 2016, 305, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, Y.; Yu, S.; Hu, S.; Wu, K. Morphology-tuned preparation of nanostructured resorcinol-formaldehyde carbonized polymers as highly sensitive electrochemical sensor for amaranth. J. Electroanal. Chem. 2016, 779, 169–175. [Google Scholar] [CrossRef]
- Zhong, Y.; Ni, Y.; Li, S.; Wang, M. Chain-like Fe3O4@resorcinol-formaldehyde resins–ag composite microstructures: Facile construction and applications in antibacterial and catalytic fields. RSC Adv. 2016, 6, 15831–15837. [Google Scholar] [CrossRef]
- Roy, A.K.; Kim, S.M.; Paoprasert, P.; Park, S.Y.; In, I. Preparation of biocompatible and antibacterial carbon quantum dots derived from resorcinol and formaldehyde spheres. RSC Adv. 2015, 5, 31677–31682. [Google Scholar] [CrossRef]
- Gu, D.; Bongard, H.; Deng, Y.; Feng, D.; Wu, Z.; Fang, Y.; Mao, J.; Tu, B.; Schüth, F.; Zhao, D. An aqueous emulsion route to synthesize mesoporous carbon vesicles and their nanocomposites. Adv. Mater. 2010, 22, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Zeng, C.; Wang, C.; Zhang, L. Preparationof ultrafine carbon spheres bycontrolledpolymerization of furfuryl alcohol in microdroplets. Ind. Eng. Chem. Res. 2014, 53, 3084–3090. [Google Scholar] [CrossRef]
- Peer, M.; Qajar, A.; Rajagopalan, R.; Foley, H.C. On the effects of emulsion polymerization of furfuryl alcohol on the formation of carbon spheres and other structures derived by pyrolysis of polyfurfuryl alcohol. Carbon 2013, 51, 85–93. [Google Scholar] [CrossRef]
- Kim, J.W.; Utada, A.S.; Hu, Z.; Weitz, D.A. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. 2015, 119, 1851–1854. [Google Scholar] [CrossRef]
- Young, C.; Rozario, K.; Serra, C.; Poolewarren, L.; Martens, P. Poly(vinyl alcohol)-heparin biosynthetic microspheres produced by microfluidics and ultraviolet photopolymerisation. Biomicrofluidics 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; Casadevall, I.S.X.; Wootton, R.C.; Demello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, X.; Wang, J.; Tian, H.; Zhao, P.; Tian, Y.; Gu, Y.; Wang, L.; Wang, C. Droplet microfluidics for the production of microparticles and nanoparticles. Micromachine 2017, 8, 22. [Google Scholar] [CrossRef]
- Yang, P.; Xu, Y.; Chen, L.; Wang, X.; Zhang, Q. One-pot synthesis of monodisperse noble metal @ resorcinol-formaldehyde (M@RF) and M@Carbon core–shell nanostructure and their catalytic applications. Langmuir 2015, 31, 11701–11708. [Google Scholar] [CrossRef] [PubMed]
- Mitome, T.; Hirota, Y.; Uchida, Y.; Nishiyama, N. Porous structure and pore size control of mesoporous carbons using a combination of a soft-templating method and a solvent evaporation technique. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 180–185. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, H.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Scalable preparation of hollow polymer and carbon microspheres by spray drying and their application in low-density syntactic foam. Mater. Chem. Phys. 2016, 181, 150–158. [Google Scholar] [CrossRef]
- Zhang, H.; Ethan, T.; Sullan, R.M.A.; Walker, G.C.; Eugenia, K. Exploring microfluidic routes to microgels of biological polymers. Macromol. Rapid Commun. 2007, 28, 527–538. [Google Scholar] [CrossRef]
- Kong, T.; Wu, J.; To, M.; Wai, K.Y.K.; Cheung, S.H.; Wang, L. Droplet based microfluidic fabrication of designer microparticles for encapsulation applications. Biomicrofluidics 2012, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Strotos, G.; Malgarinos, I.; Nikolopoulos, N.; Gavaises, M. Aerodynamic breakup of an n -decane droplet in a high temperature gas environment. Fuel 2016, 185, 370–380. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Fox, C.A.; Worsley, M.A. On the synthesis and structure of resorcinol-formaldehyde polymeric networks—Precursors to 3D-carbon macroassemblies. Polymer 2015, 69, 45–51. [Google Scholar] [CrossRef]
- Elkhatat, A.M.; Al-Muhtaseb, S.A. Advances in tailoring resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; de Vicente, J.; Moreno-Castilla, C. Carbon xerogel microspheres and monoliths from resorcinol-formaldehyde mixtures with varying dilution ratios: Preparation, surface characteristics, and electrochemical double-layer capacitances. Langmuir 2013, 29, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Gaca, K.Z.; Parkinson, J.A.; Sefcik, J. Kinetics of early stages of resorcinol-formaldehyde polymerization investigated by solution-phase nuclear magnetic resonance spectroscopy. Polymer 2017, 110, 62–73. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Lu, C. The electrochemical performance of phenolic resin based activated carbon microbeads—I. an investigation on the preparation of the microbeads. New Carbon Mater. 2005, 20, 58–62. [Google Scholar]
- Huang, F.R.; Wan, L.Q. Phenol Formaldehyde Resin and Its Application; Chemical Industry Press: Beijing, China, 2011; pp. 16–36. [Google Scholar]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.Y.; Kim, J.W.; Fernandez-Nieves, A.; Martinez, C.J. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Zeng, X.; Lu, A.; Xu, X.; Wang, Y.; Ye, Q.; Zhang, L. Construction of blood compatible lysine-immobilized chitin/carbon nanotubes microspheres and potential applications for blood purified therapy. J. Mater. Chem. B 2017, 5, 2952–2963. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, J.; Lu, Y. Evaluation of efficacy of resin hemoperfusion in patients with acute 2,4-dinitrophenol poisoning by dynamic monitoring of plasma toxin concentration. J. Zhejiang Univ. Sci. B 2015, 16, 720–726. [Google Scholar] [CrossRef] [PubMed]
| T1 (°C) | t1 (min) | L (cm) |
| 80 | >60 | 239 |
| 90 | 20 | 79 |
| 100 | not detected | not detected |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, X.; Zhao, P.; Wang, X.; Tian, Y.; Chen, C.; Wang, J.; Li, Y.; Wan, W.; Tian, H.; et al. On-Chip Facile Preparation of Monodisperse Resorcinol Formaldehyde (RF) Resin Microspheres. Micromachines 2018, 9, 24. https://doi.org/10.3390/mi9010024
Wang J, Huang X, Zhao P, Wang X, Tian Y, Chen C, Wang J, Li Y, Wan W, Tian H, et al. On-Chip Facile Preparation of Monodisperse Resorcinol Formaldehyde (RF) Resin Microspheres. Micromachines. 2018; 9(1):24. https://doi.org/10.3390/mi9010024
Chicago/Turabian StyleWang, Jianmei, Xiaowen Huang, Pei Zhao, Xueying Wang, Ye Tian, Chengmin Chen, Jianchun Wang, Yan Li, Wei Wan, Hanmei Tian, and et al. 2018. "On-Chip Facile Preparation of Monodisperse Resorcinol Formaldehyde (RF) Resin Microspheres" Micromachines 9, no. 1: 24. https://doi.org/10.3390/mi9010024




