Investigation of Au/Si Eutectic Wafer Bonding for MEMS Accelerometers
Abstract
:1. Introduction
2. Au/Si Eutectic Bonding
3. Experimental Details
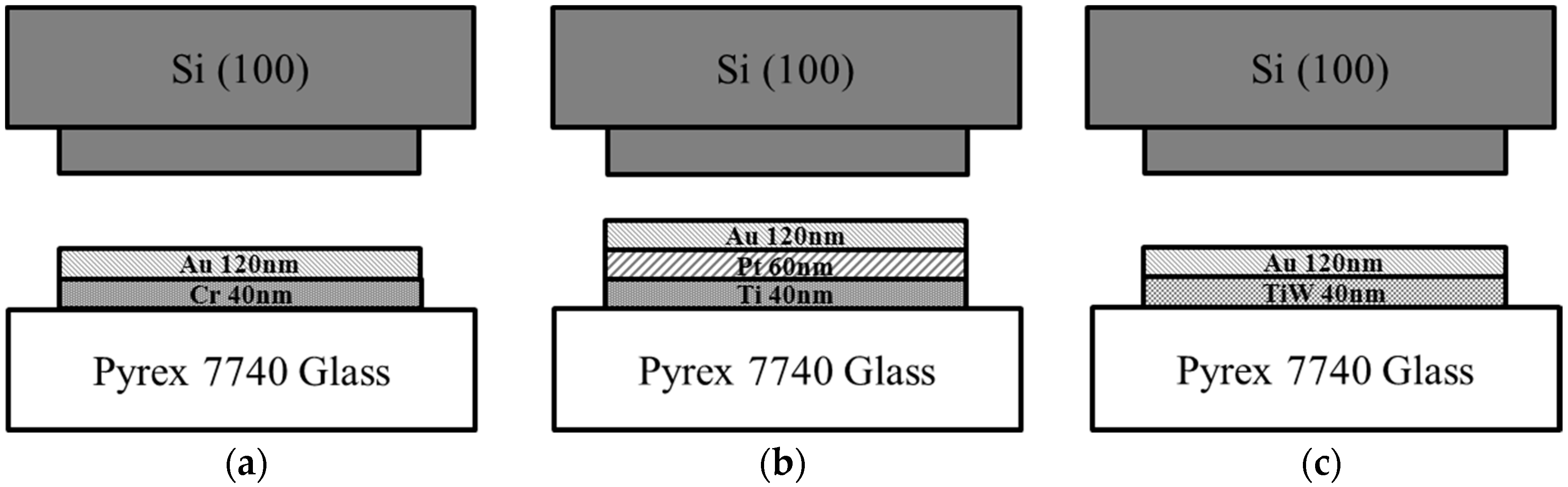
3.1. Wafer Preparation
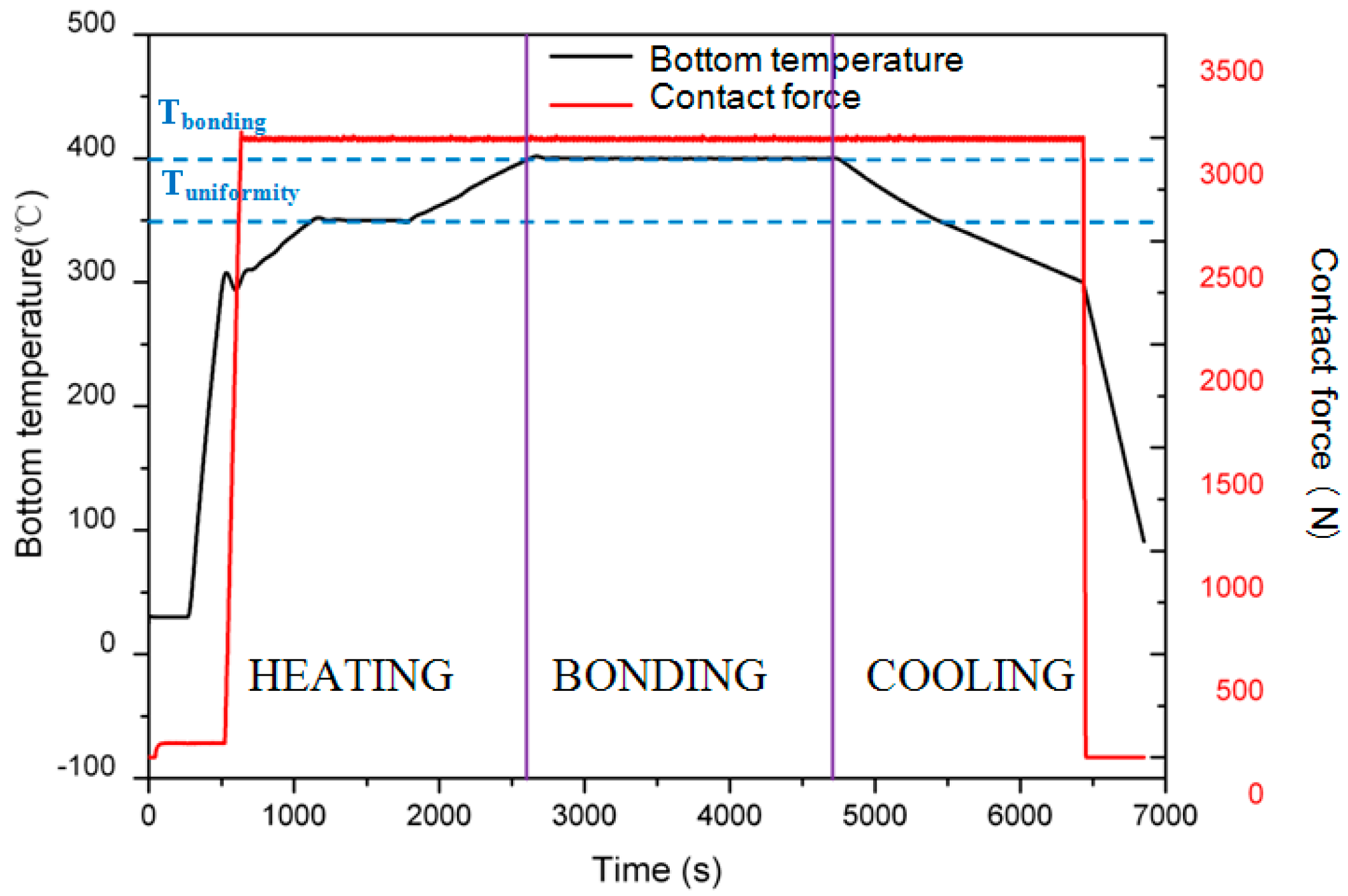
3.2. Bonding Process
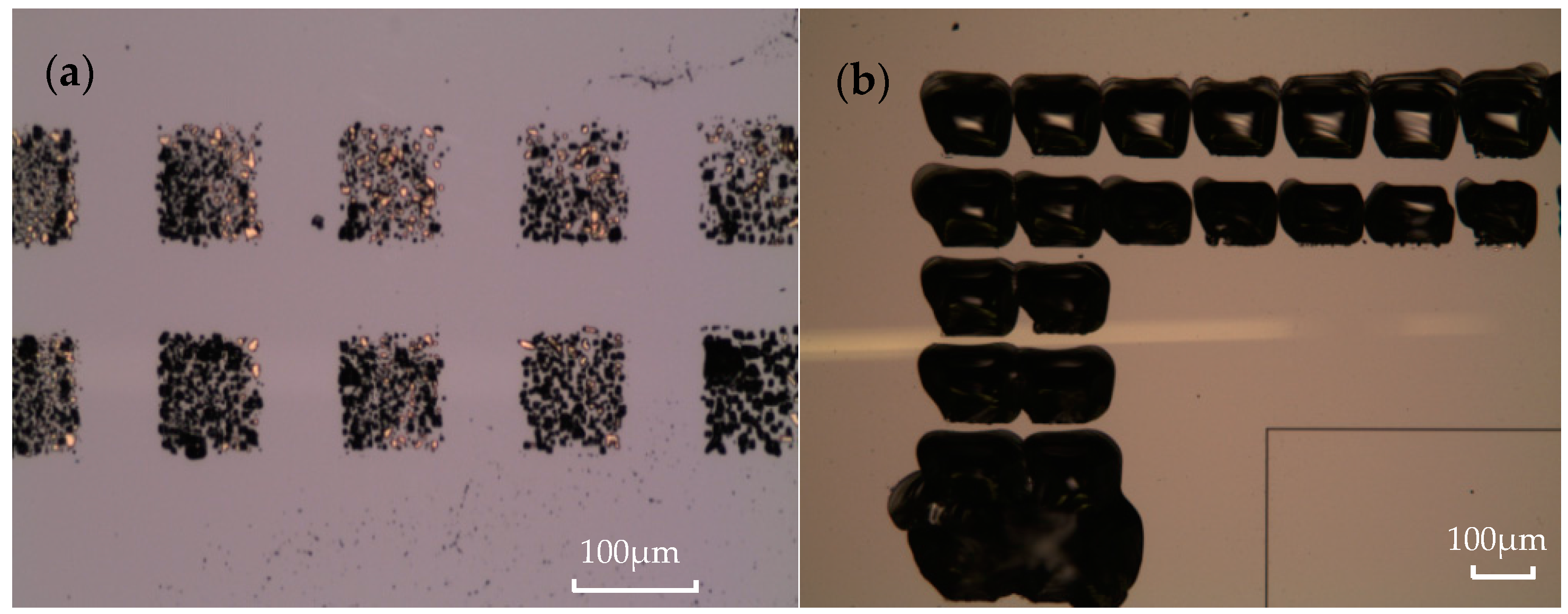
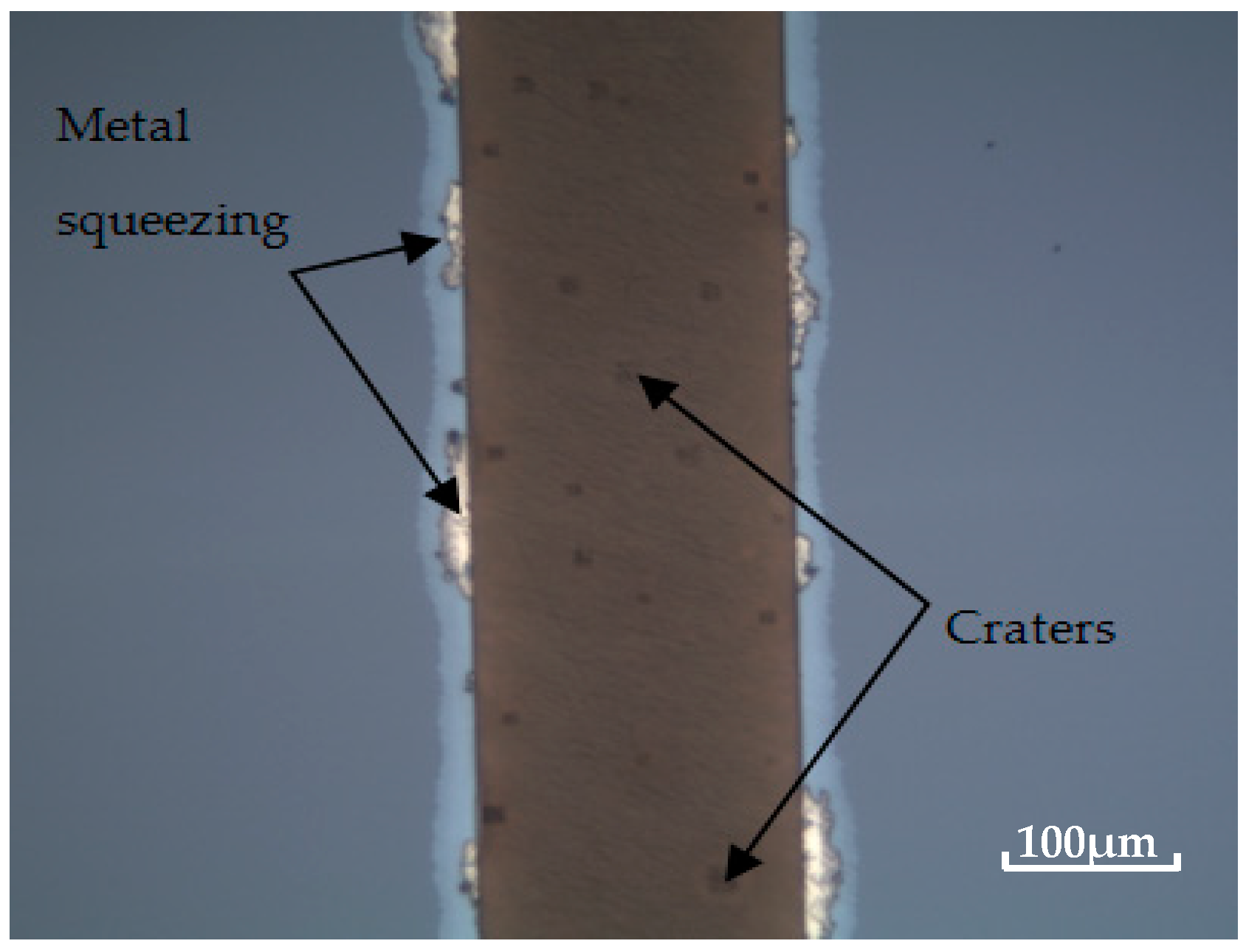
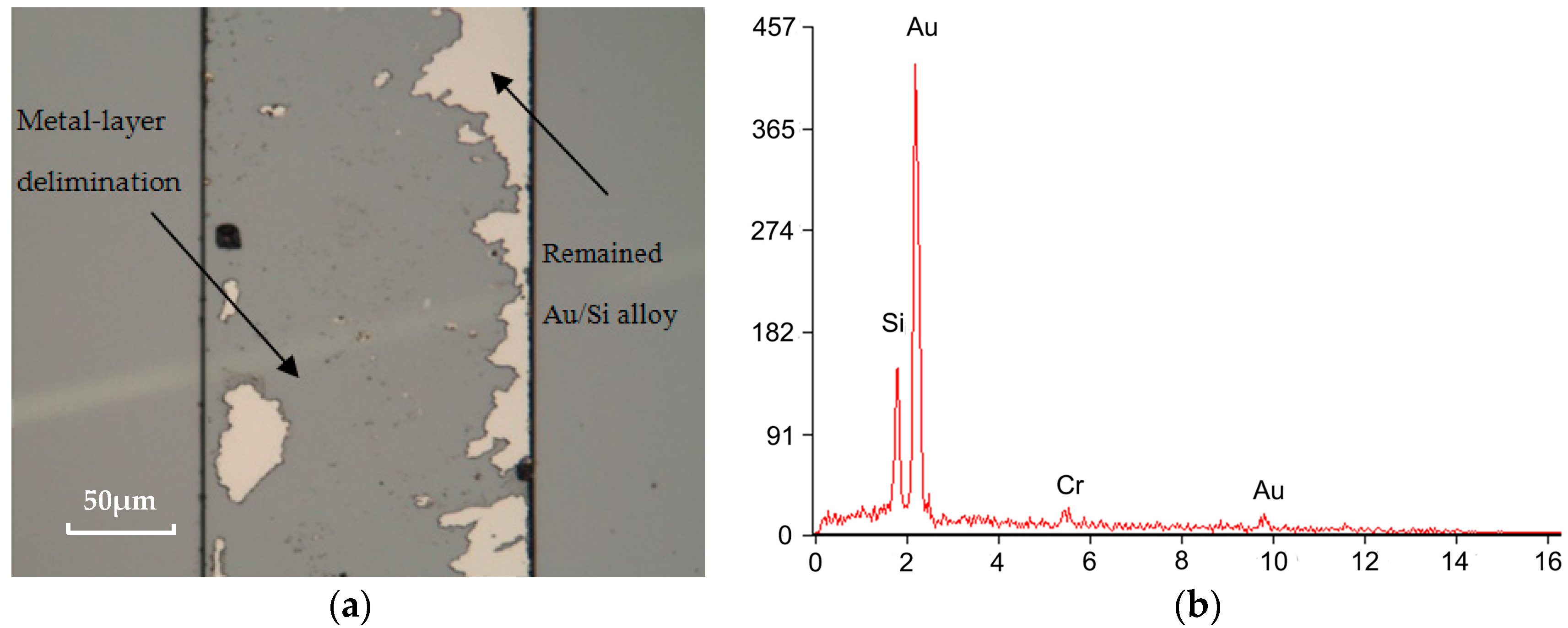
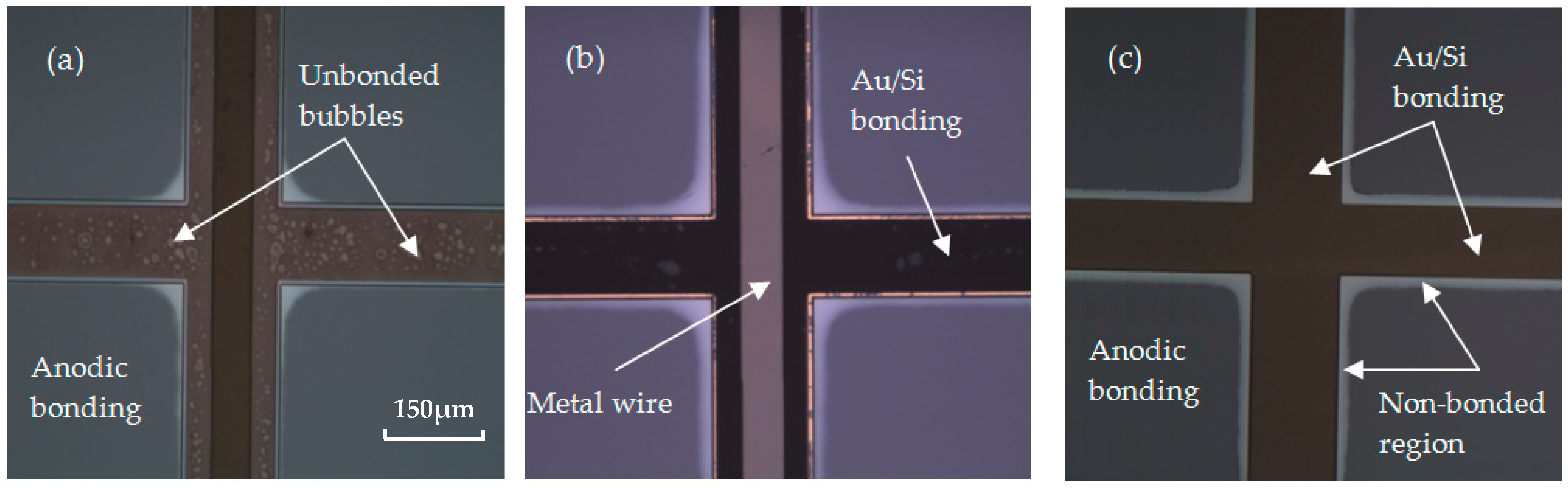
3.3. Bonding Quality Evaluation
4. Results and Discussion
4.1. Temperature
4.2. Heating/Cooling Rate
4.3. Contact Force
4.4. Adhesion Layer
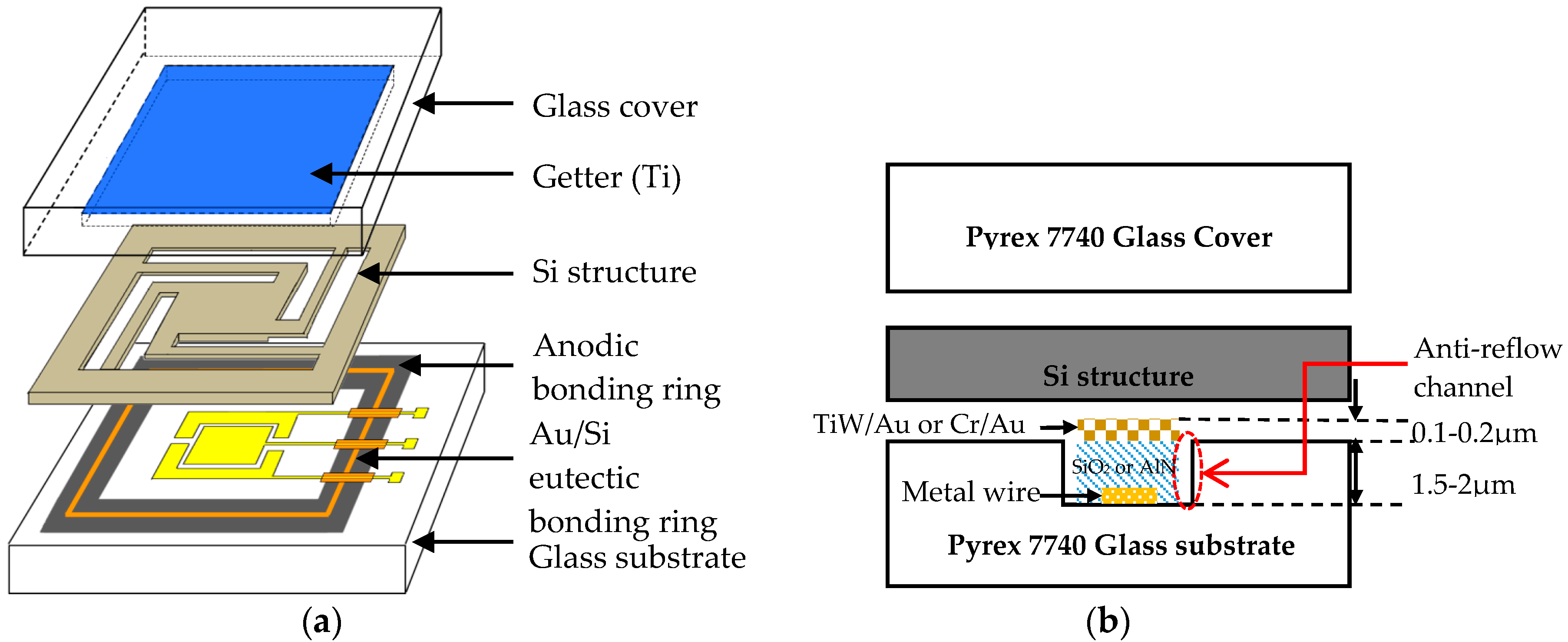
5. The Application of Au/Si Eutectic Bonding in an MEMS Accelerometer
5.1. Package Design
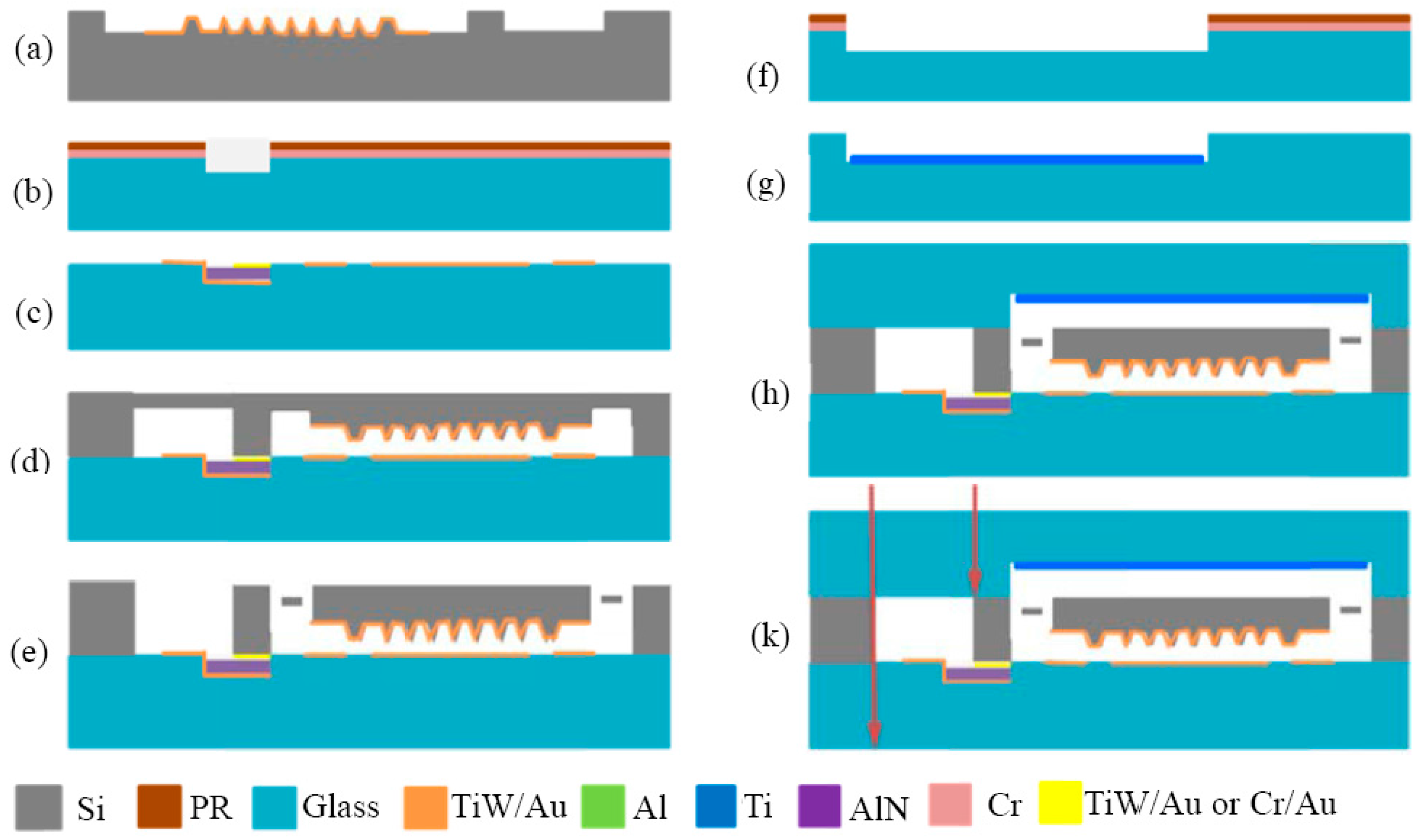
5.2. Fabrication Process
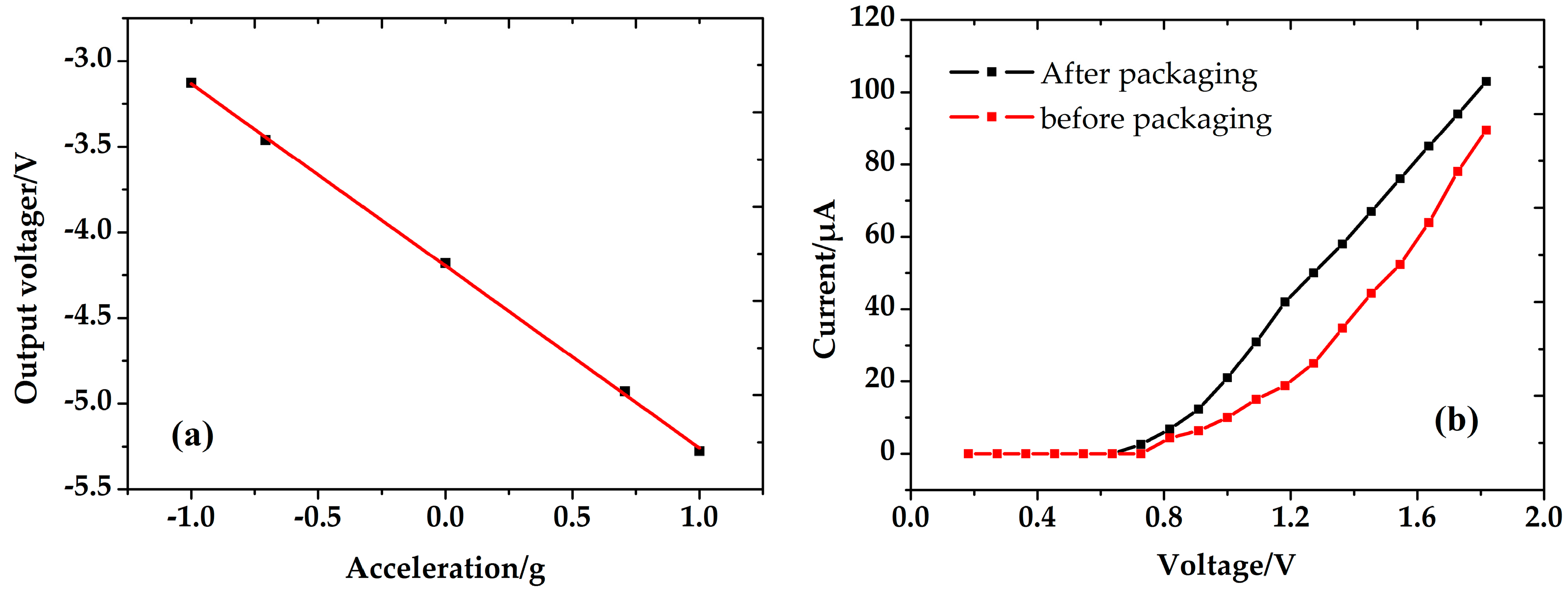
5.3. Performance of the Accelerometer
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; He, Y.; Zhan, Z.; Yu, L.; Wang, H.; Chen, D. A novel sacrificial-layer process based on anodic bonding and its application in an accelerometer. AIP Adv. 2015, 5, 041323. [Google Scholar] [CrossRef]
- Li, D.L.; Shang, Z.G.; Wang, S.Q.; Wen, Z.Y. Low temperature Si/Si wafer direct bonding using a plasma activated method. J. Zhejiang Univ. Sci. C 2013, 14, 244–251. [Google Scholar] [CrossRef]
- Shinohara, H.; Mizuno, J.; Shoji, S. Studies on low-temperature direct bonding of VUV, VUV/O3 and O2 plasma pretreated cyclo-olefin polymer. Sens. Actuators A Phys. 2011, 165, 124–131. [Google Scholar] [CrossRef]
- Torunbalci, M.M.; Alper, S.E.; Akin, T. Advanced MEMS Process for Wafer Level Hermetic Encapsulation of MEMS Devices Using SOI Cap Wafers With Vertical Feedthroughs. J. Microelectromech. Syst. 2015, 24, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, D.Q.; He, J.; Fan, X.J.; Yang, F.; Zhang, D.C. Electrical interconnect in MEMS/NEMS devices by Au/a-Si eutectic reaction. In Proceedings of the 2013 IEEE 13th International Conference on Nanotechnology (IEEE-NANO), Beijing, China, 5–8 August 2013; pp. 971–974. [Google Scholar]
- Tez, S.; Akin, T. Fabrication of a sandwich type three axis capacitive MEMS accelerometer. In Proceedings of the IEEE SENSORS, Baltimore, MD, USA, 4–6 November 2013; pp. 1–4. [Google Scholar]
- Abouie, M.; Liu, Q.; Ivey, D.G. Eutectic and solid-state wafer bonding of silicon with gold. Mater. Sci. Eng. B Solid State Adv. Technol. 2012, 177, 1748–1758. [Google Scholar] [CrossRef]
- Henry, M.D.; Ahlers, C.R. Platinum Diffusion Barrier Breakdown in a-Si/Au Eutectic Wafer Bonding. IEEE Trans. Compon. Packag. Manufact. Technol. 2013, 3, 899–903. [Google Scholar] [CrossRef]
- Jing, E.; Xiong, B.; Wang, Y. The Bond Strength of Au/Si Eutectic Bonding Studied by IR Microscope. IEEE Trans. Electron. Packag. Manuf. 2010, 33, 31–37. [Google Scholar] [CrossRef]
- Chang, L.B.; Yen, C.I.; You, T.W.; Jeng, M.J.; Wu, C.T.; Hu, S.C.; Kuo, Y.K. Improving the reliability of eutectic bonding vertical power light-emitting diodes by a Mo buffer layer. Thin Solid Films 2014, 570, 500–503. [Google Scholar] [CrossRef]
- Liu, Q.; Du, L.; Zhao, Z.; Xiao, L.; Sun, X. Localized Si-Au eutectic bonding around sunken pad for fabrication of a capacitive absolute pressure sensor. Sens. Actuators A Phys. 2013, 201, 241–245. [Google Scholar] [CrossRef]
- Baum, M.; Jia, C.; Haubold, M.; Wiemer, M. Eutectic wafer bonding for 3-D integration. In Proceedings of the 3rd Electronics System Integration Technology Conference (ESTC 2010), Berlin, Germany, 13–16 September 2010; pp. 1–6. [Google Scholar]
- Iliescu, C.; Miao, J.; Poenar, D.P.; Sun, T. Thick and thin diaphragms fabrication using gold-silicon eutectic. Int. Semicond. Conf. 2002, 1, 189–192. [Google Scholar]
- Lani, S.; Bosseboeuf, A.; Belier, B.; Clerc, C.; Gousset, C.; Aubert, J. Gold metallizations for eutectic bonding of silicon wafers. Microsyst. Technol. 2006, 12, 1021–1025. [Google Scholar] [CrossRef]
- Lin, B.W.; Wu, N.J.; Wu, Y.C.S.; Hsu, S.C. A Stress Analysis of Transferred Thin-GaN Light-Emitting Diodes Fabricated by Au-Si Wafer Bonding. IEEE/OSA J. Disp. Technol. 2013, 9, 371–376. [Google Scholar] [CrossRef]
- Bokhonov, B.; Korchagin, M. In situ investigation of stage of the formation of eutectic alloys in Si-Au and Si-Al systems. J. Alloys Compd. 2000, 312, 238–250. [Google Scholar] [CrossRef]
- Jing, E.; Xiong, B.; Wang, Y. Low-temperature Au-Si wafer bonding. J. Micromech. Microeng. 2010, 20, 1143–1144. [Google Scholar] [CrossRef]
- Jing, E.; Xiong, B.; Wang, Y. Low-Temperature Wafer Bonding Based on Gold-Induced Crystallization of Amorphous Silicon. IEEE Electron Device Lett. 2010, 31, 1011–1013. [Google Scholar] [CrossRef]
- Pan, J.; Pafchek, R.M.; Judd, F.F.; Baxter, J. Effect of Chromium-Gold and Titanium-Titanium Nitride-Platinum-Gold Metallization on Wire/Ribbon Bondability. IEEE Trans. Adv. Packag. 2006, 29, 707–713. [Google Scholar] [CrossRef]
- Wolffenbuttel, R.F. Low-temperature intermediate Au-Si wafer bonding: Eutectic or silicide bond. Sens Actuators A Phys. 1997, 62, 680–686. [Google Scholar] [CrossRef]



| No. | Temperature (°C) | Contact Force (N) | Pressure (MPa) | Time (min) | Bonding Strength (MPa) 1 | Bonding Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 380 | 3000 | 3.5 | 20 | 6.7 ± 1.2 (18%) | 23 |
| 2 | 400 | 2000 | 2.3 | 20 | 37.5 ± 3.8 (11%) | 67 |
| 3 | 400 | 3000 | 3.5 | 20 | 66.8 ± 4.6 (7%) | 91 |
| 4 | 400 | 3000 | 3.5 | 20 | 57.6 ± 5.6 (9%) | 92 |
| 5 | 400 | 3000 | 3.5 | 20 | 62.4 ± 6.2 (10%) | 90 |
| 6 | 400 | 5000 | 5.8 | 20 | 41.4 ± 2.1 (5%) | 85 |
| 7 | 400 | 3000 | 3.5 | 40 | 74.3 ± 4.5 (6%) | 93 |
| 8 | 420 | 3000 | 3.5 | 20 | 84 ± 6.8 (8%) | 88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Shang, Z.; She, Y.; Wen, Z. Investigation of Au/Si Eutectic Wafer Bonding for MEMS Accelerometers. Micromachines 2017, 8, 158. https://doi.org/10.3390/mi8050158
Li D, Shang Z, She Y, Wen Z. Investigation of Au/Si Eutectic Wafer Bonding for MEMS Accelerometers. Micromachines. 2017; 8(5):158. https://doi.org/10.3390/mi8050158
Chicago/Turabian StyleLi, Dongling, Zhengguo Shang, Yin She, and Zhiyu Wen. 2017. "Investigation of Au/Si Eutectic Wafer Bonding for MEMS Accelerometers" Micromachines 8, no. 5: 158. https://doi.org/10.3390/mi8050158





