Paper-Based Analytical Device for Zinc Ion Quantification in Water Samples with Power-Free Analyte Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Investigation of Reaction Time of Cu2+-Salicylaldoxime Chelation
2.3. Device Fabrication
2.4. Detection and Quantification Method
3. Results and Discussion
3.1. Roles of the 3D-PAD Components
3.1.1. Filter Paper Detection Area
3.1.2. Glass Fiber Layers
3.1.3. Absorbent Pad Layers
3.1.4. Device Holder
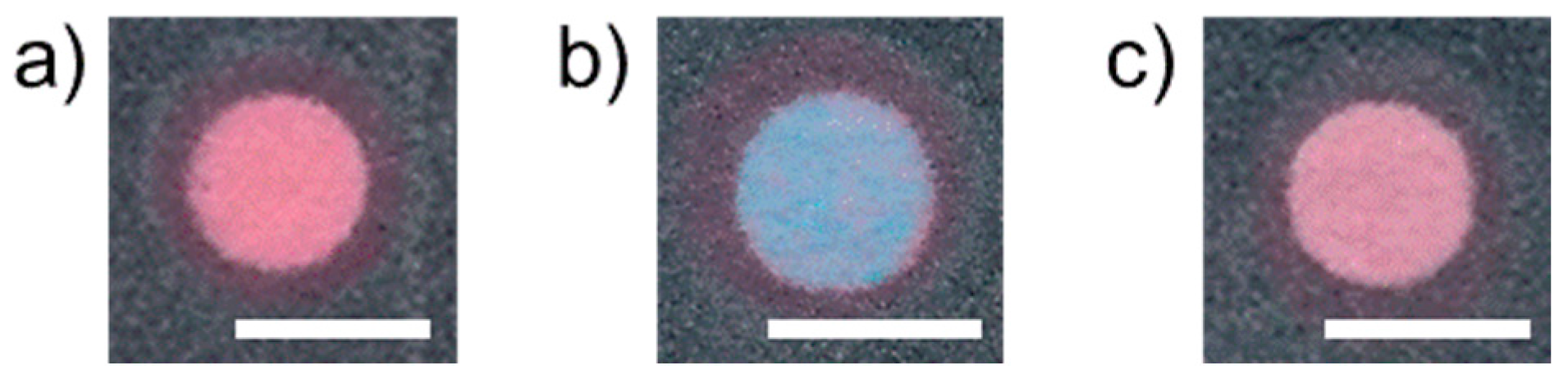
3.2. Suppression of Cu2+ Interference
3.3. Selectivity Study
3.3.1. Primary Heavy Metal Contaminants
3.3.2. Influence of Ca2+
3.4. Application in Environmental Water Sample Matrix
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- The Ministry of Health, Labour and Welfare of Japan, Drinking Water Quality Standards. Available online: http://www.mhlw.go.jp/english/policy/health/water_supply/dl/4a.pdf (accessed on 20 March 2017).
- EPA, National Recommended Water Quality Criteria—Aquatic Life Criteria Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 20 March 2017).
- Goullé, J.-P.; Mahieu, L.; Castermant, J.; Neveu, N.; Bonneau, L.; Lainé, G.; Bouige, D.; Lacroix, C. Metal and metalloid Multi-Elementary ICP-MS validation in whole blood, plasma, urine and hair: Reference values. Forensic Sci. Int. 2005, 153, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen Cassella, R.; Teixeira Bitencourt, D.; Garcia Branco, A.; Luis Costa Ferreira, S.; Santiago de Jesus, D.; Souza de Carvalho, M.; Erthal Santelli, R. On-line preconcentration system for flame atomic absorption spectrometry using unloaded polyurethane foam: Determination of zinc in waters and biological materials. J. Anal. At. Spectrom. 1999, 14, 1749–1753. [Google Scholar] [CrossRef]
- Taylor, A.; Branch, S.; Halls, D.J.; Owen, L.M.W.; White, M. Atomic Spectrometry Update: Clinical and biological materials, foods and beverages. J. Anal. At. Spectrom. 2000, 15, 451–487. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Marzo, A.M.; Merkoci, A. Paper-based sensors and assays: A success of the engineering design and the convergence of knowledge areas. Lab Chip 2016, 16, 3150–3176. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-paper Micro- and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim. Acta 2016, 183, 1521–1542. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-based analytical devices for environmental analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef] [PubMed]
- López Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoçi, A. All-integrated and highly sensitive paper based device with sample treatment platform for Cd2+ immunodetection in drinking/tap waters. Anal. Chem. 2013, 85, 3532–3538. [Google Scholar] [CrossRef] [PubMed]
- Ruecha, N.; Rodthongkum, N.; Cate, D.M.; Volckens, J.; Chailapakul, O.; Henry, C.S. Sensitive electrochemical sensor using a graphene-polyaniline nanocomposite for simultaneous detection of Zn (II), Cd (II), and Pb (II). Anal. Chim. Acta 2015, 874, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, X.; Li, H.; Yang, W.; Chen, L.; Guan, Y. Enhancement of sensitivity of paper-based sensor array for the identification of heavy-metal ions. Anal. Chim. Acta 2013, 780, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Cabodi, M.; Rolland, J.; Klapperich, C.M. Evaporative concentration on a paper-based device to concentrate analytes in a biological fluid. Anal. Chem. 2014, 86, 11981–11985. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-H.; Chou, K.-H.; Yang, R.-J. Sample pre-concentration with high enrichment factors at a fixed location in paper-based microfluidic devices. Lab Chip 2016, 16, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Satarpai, T.; Shiowatana, J.; Siripinyanond, A. Paper-based analytical device for sampling, on-site preconcentration and detection of ppb lead in water. Talanta 2016, 154, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Tenda, K.; Ota, R.; Yamada, K.; Henares, T.; Suzuki, K.; Citterio, D. High-resolution microfluidic paper-based analytical devices for sub-microliter sample analysis. Micromachines 2016, 7, 80. [Google Scholar] [CrossRef]
- Henares, T.G.; Yamada, K.; Takaki, S.; Suzuki, K.; Citterio, D. “Drop-Slip” bulk sample flow on fully inkjet-printed microfluidic paper-based analytical device. Sens. Actuators B Chem. 2017, 244, 1129–1137. [Google Scholar] [CrossRef]
- Rattanarat, P.; Dungchai, W.; Cate, D.M.; Siangproh, W.; Volckens, J.; Chailapakul, O.; Henry, C.S. A Microfluidic paper-based analytical device for rapid quantification of particulate chromium. Anal. Chim. Acta 2013, 800, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Distance-based tear lactoferrin assay on microfluidic paper device using interfacial interactions on surface-modified cellulose. ACS Appl. Mater. Interfaces 2015, 7, 24864–24875. [Google Scholar] [CrossRef] [PubMed]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A Spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Lucia, M.; Campos, A.M.; van den Berg, C.M.G. Determination of copper complexation in sea water by cathodic stripping voltammetry and ligand competition with salicylaldoxime. Anal. Chim. Acta 1994, 284, 481–496. [Google Scholar] [CrossRef]
- Shibukawa, M.; Shirota, D.; Saito, S.; Nagasawa, S.; Saitoh, K.; Minamisawa, H. Simple spectrophotometric determination of trace amounts of zinc in environmental water samples using aqueous biphasic extraction. Bunseki Kagaku 2010, 59, 847–854. [Google Scholar] [CrossRef]
- Mehio, N.; Ivanov, A.S.; Williams, N.J.; Mayes, R.T.; Bryantsev, V.S.; Hancock, R.D.; Dai, S. Quantifying the binding strength of salicylaldoxime-uranyl complexes relative to competing salicylaldoxime-transition metal ion complexes in aqueous solution: A combined experimental and computational study. Dalton Trans. 2016, 45, 9051–9064. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.; Egyed, I. Some theoretical and practical problems in the use of organic reagents in chemical analysis—V: Effect of electrophilic and nucleophilic substituents on the stability of salicylaldoxime complexes of transition metals. J. Inorg. Nucl. Chem. 1965, 27, 2361–2370. [Google Scholar] [CrossRef]
- Thorpe, J.M.; Beddoes, R.L.; Collison, D.; Garner, C.D.; Helliwell, M.; Holmes, J.M.; Tasker, P.A. Surface coordination chemistry: Corrosion inhibition by tetranuclear cluster formation of iron with salicylaldoxime. Angew. Chem. Int. Ed. 1999, 38, 1119–1121. [Google Scholar] [CrossRef]
- Smith, A.G.; Tasker, P.A.; White, D.J. The structures of phenolic oximes and their complexes. Coord. Chem. Rev. 2003, 241, 61–85. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Crooks, R.M. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem. Soc. 2011, 133, 17564–17566. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.G.; DiTucci, M.J.; Baker, M.S.; Phillips, S.T. High throughput method for prototyping Three-dimensional, paper-based microfluidic devices. Lab Chip 2012, 12, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Jauregui, D.; Martinez, A.W. Paper and toner three-dimensional fluidic devices: Programming fluid flow to improve point-of-care diagnostics. Lab Chip 2013, 13, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K. Astatistical aspects of the stabilities of ternary complexes of cobalt(II), nickel(II), copper(II) and zinc(II) involving aminopolycarboxylic acids as primary ligands and salicylaldoxime as a secondary ligand. Trans. Metal Chem. 1990, 15, 75–77. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Fernández-Ramos, M.D.; Capitán-Vallvey, L.F. Fluorescence resonance energy transfer disposable sensor for copper (II). Anal. Chim. Acta 2006, 555, 299–307. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, L.; Qin, J.; Li, Z. An alternative approach to develop a highly sensitive and selective chemosensor for the colorimetric sensing of cyanide in water. Chem. Commun. 2008, 44, 5848–5850. [Google Scholar] [CrossRef] [PubMed]
- Hilario, E.; Romero, I.; Celis, H. Determination of the physicochemical constants and spectrophotometric characteristics of the metallochromic Zincon and its potential use in biological systems. J. Biochem. Biophys. Methods 1990, 21, 197–207. [Google Scholar] [CrossRef]
- The Ministry of Health, Labour and Welfare of Japan. Revision of Drinking Water Quality in Japan. Available online: http://www.mhlw.go.jp/topics/bukyoku/kenkou/suido/kijun/dl/k34.pdf (accessed on 20 March 2017).






| Glass Fiber Disk Diameter | Added Reagent | Pipetting Amount |
|---|---|---|
| 5 mm | 14.3 mM of salicylaldoxime in buffer solution (TAPS/TMAOH, pH 8.5, 400 mM) | 10.0 μL |
| 6 mm | 14.4 μL | |
| 8 mm | 25.6 μL | |
| 11 mm | 50.0 μL | |
| 14 mm | 80.0 μL | |
| 17 mm | 116.0 μL | |
| 20 mm | 100 μM of aqueous CuCl2 solution | 160.0 μL |
| Sample | Added Zn/μM | Found Zn/μM | Recovery/% |
|---|---|---|---|
| Tap water | 0 | Not detectable | - |
| 5 | 5.76 ± 1.04 | 115 | |
| 10 | 8.93 ± 1.08 | 89 | |
| River water | 0 | Not detectable | - |
| 5 | 6.48 ± 0.72 | 129 | |
| 10 | 10.6 ± 0.47 | 106 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, H.; Yamada, K.; Watanabe, D.; Suzuki, K.; Citterio, D. Paper-Based Analytical Device for Zinc Ion Quantification in Water Samples with Power-Free Analyte Concentration. Micromachines 2017, 8, 127. https://doi.org/10.3390/mi8040127
Kudo H, Yamada K, Watanabe D, Suzuki K, Citterio D. Paper-Based Analytical Device for Zinc Ion Quantification in Water Samples with Power-Free Analyte Concentration. Micromachines. 2017; 8(4):127. https://doi.org/10.3390/mi8040127
Chicago/Turabian StyleKudo, Hiroko, Kentaro Yamada, Daiki Watanabe, Koji Suzuki, and Daniel Citterio. 2017. "Paper-Based Analytical Device for Zinc Ion Quantification in Water Samples with Power-Free Analyte Concentration" Micromachines 8, no. 4: 127. https://doi.org/10.3390/mi8040127






