Internal Laser Writing of High-Aspect-Ratio Microfluidic Structures in Silicate Glasses for Lab-on-a-Chip Applications
Abstract
:1. Introduction
2. Challenges in Real World Applications and Current Solutions
- i
- The microchannels need to be sufficiently long to ensure completion of the chemical reactions during the transportation of the liquid samples.
- ii
- The inner walls of microchannels need to be sufficiently smooth in order not to disturb the fluidic dynamics or damage the biological samples (e.g., the membranes of living cells).
- iii
- The fabrication resolution should be as high as possible to produce channels whose feature sizes can range from micrometer to nanometer scales.
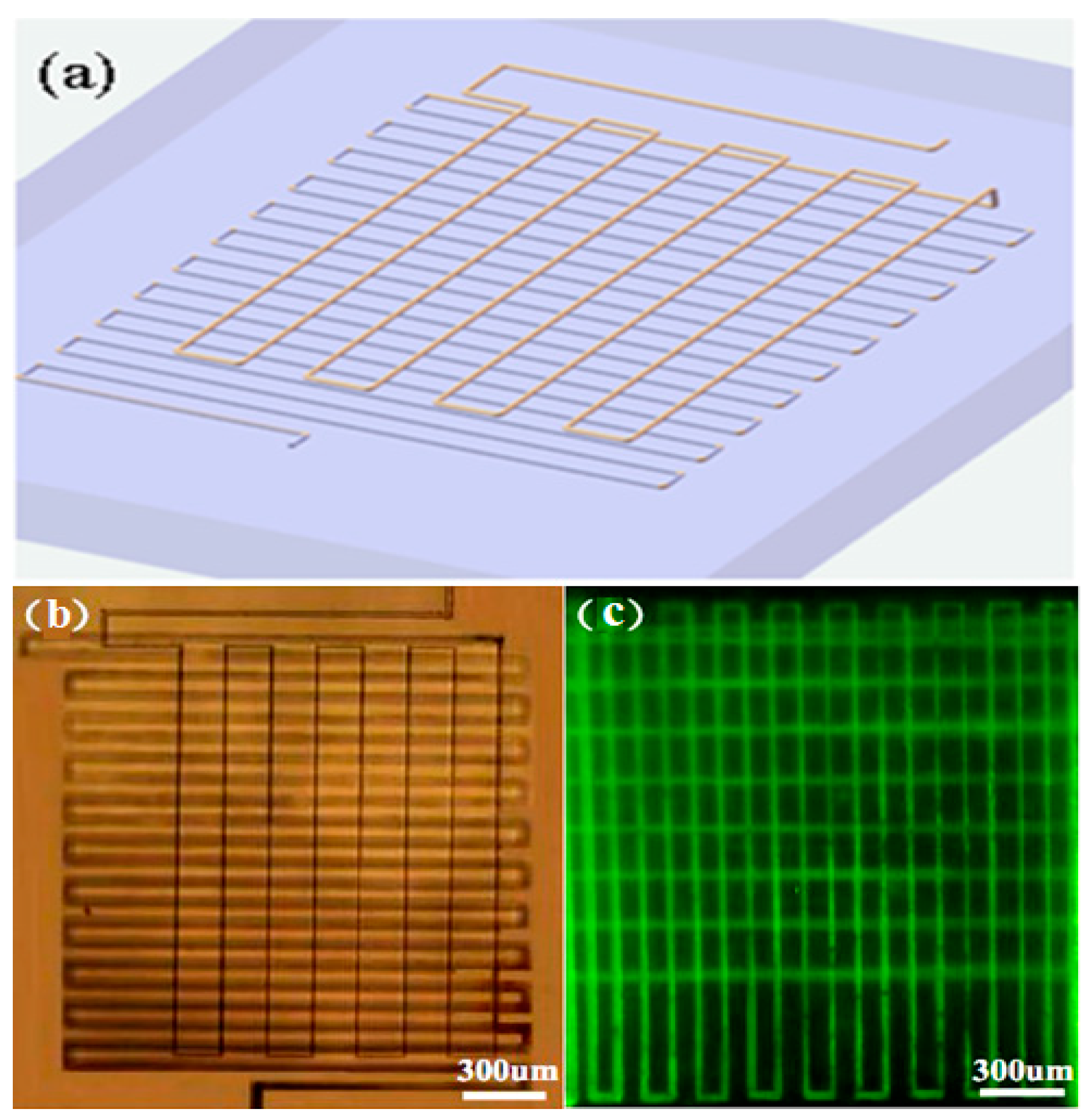
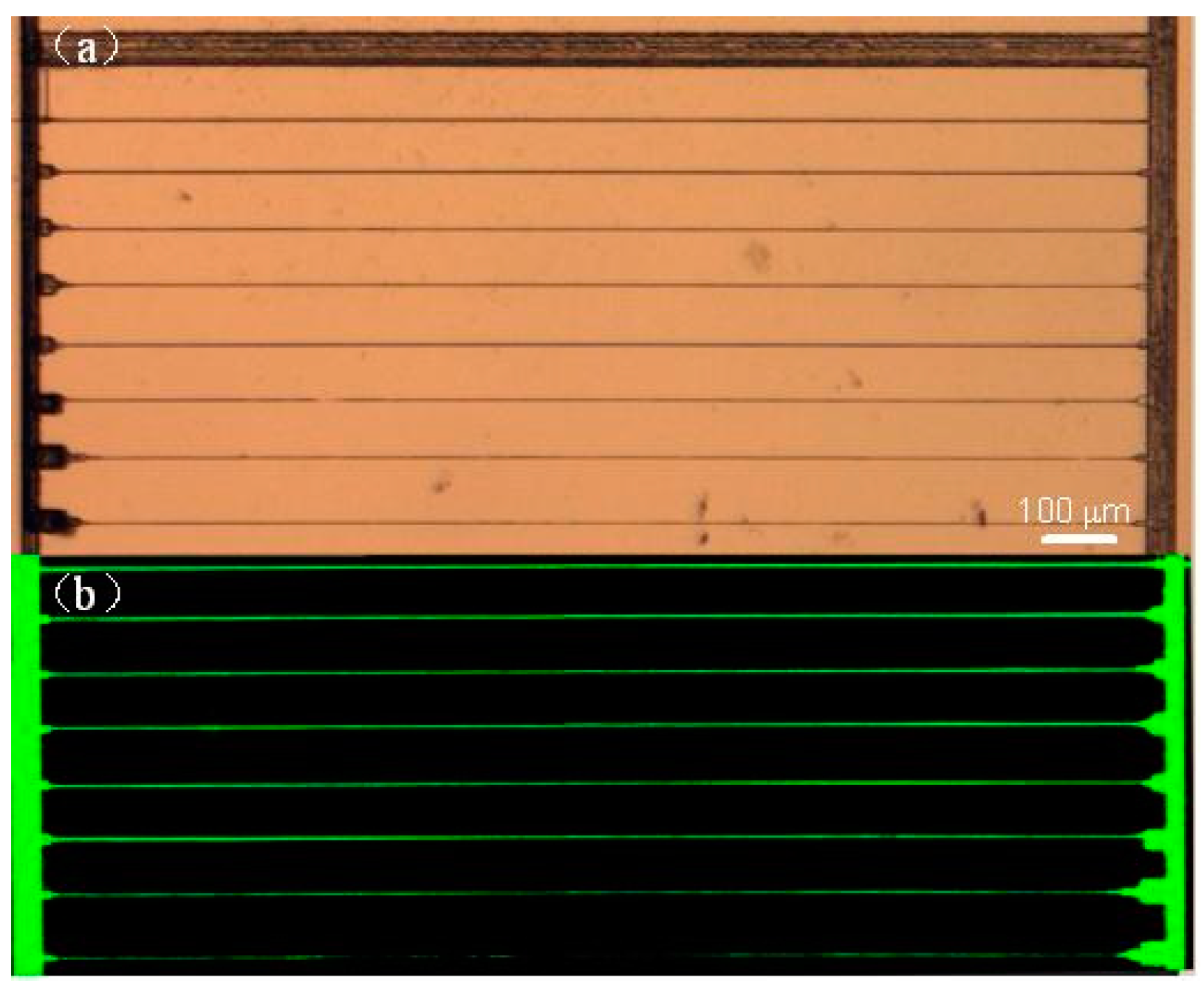
2.1. Fabrication of Homogeneous 3D Microfluidic Channels of Great Lengths
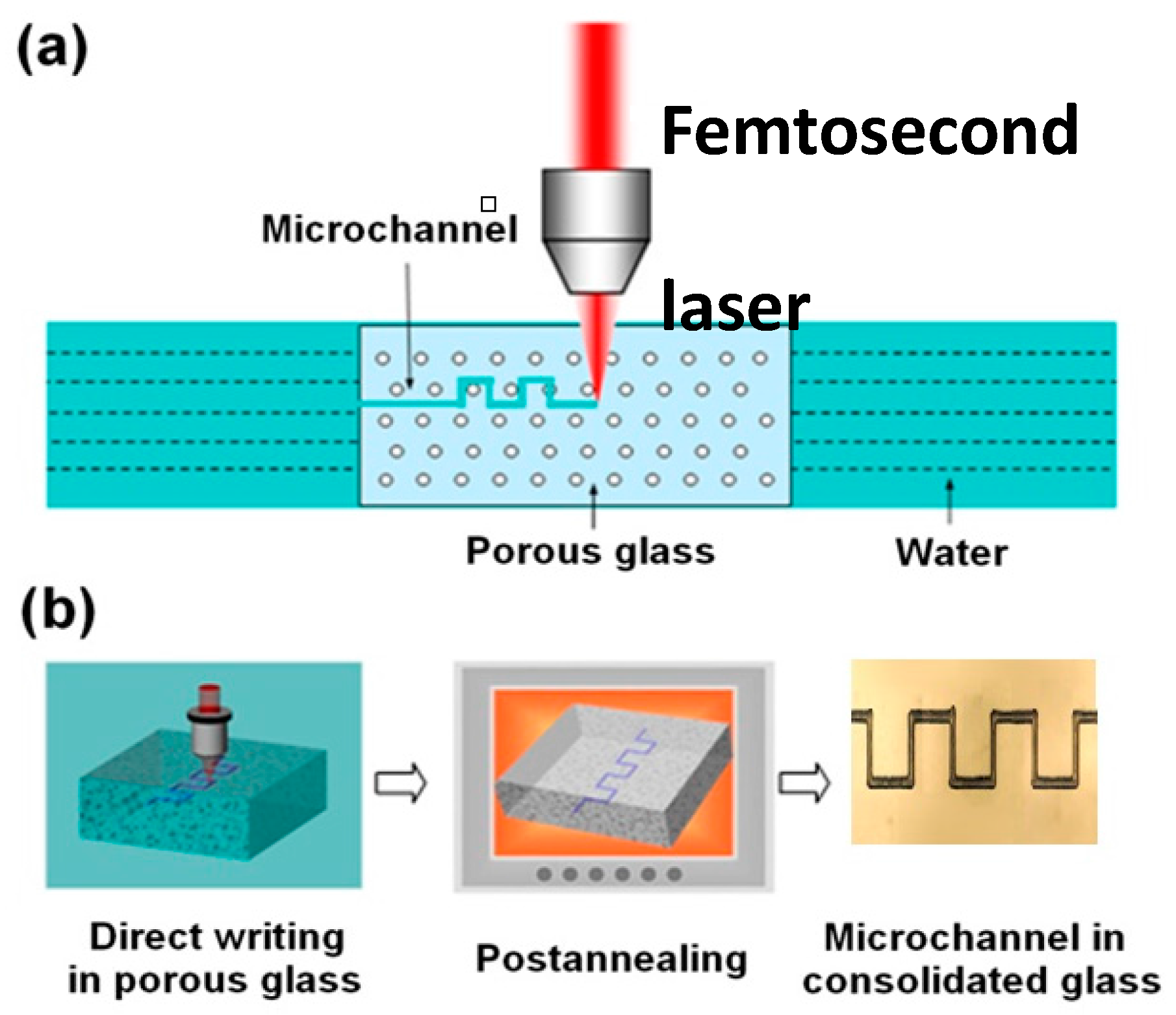
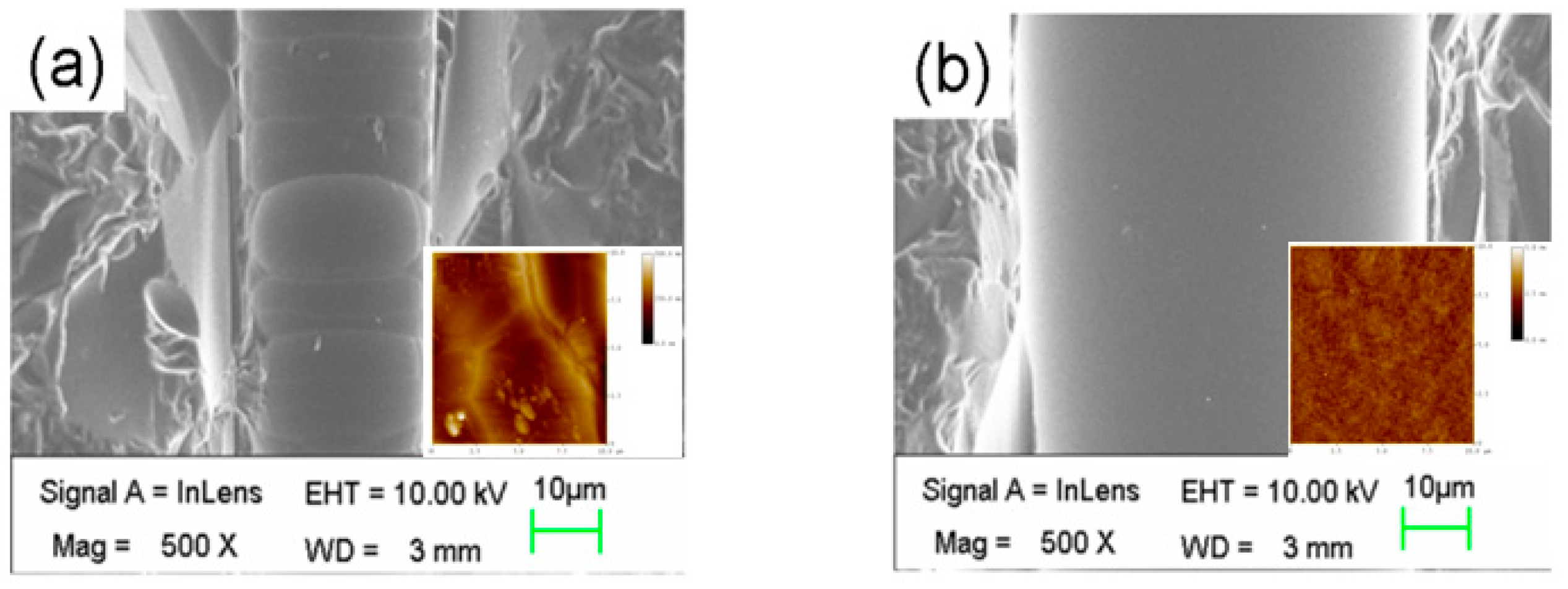
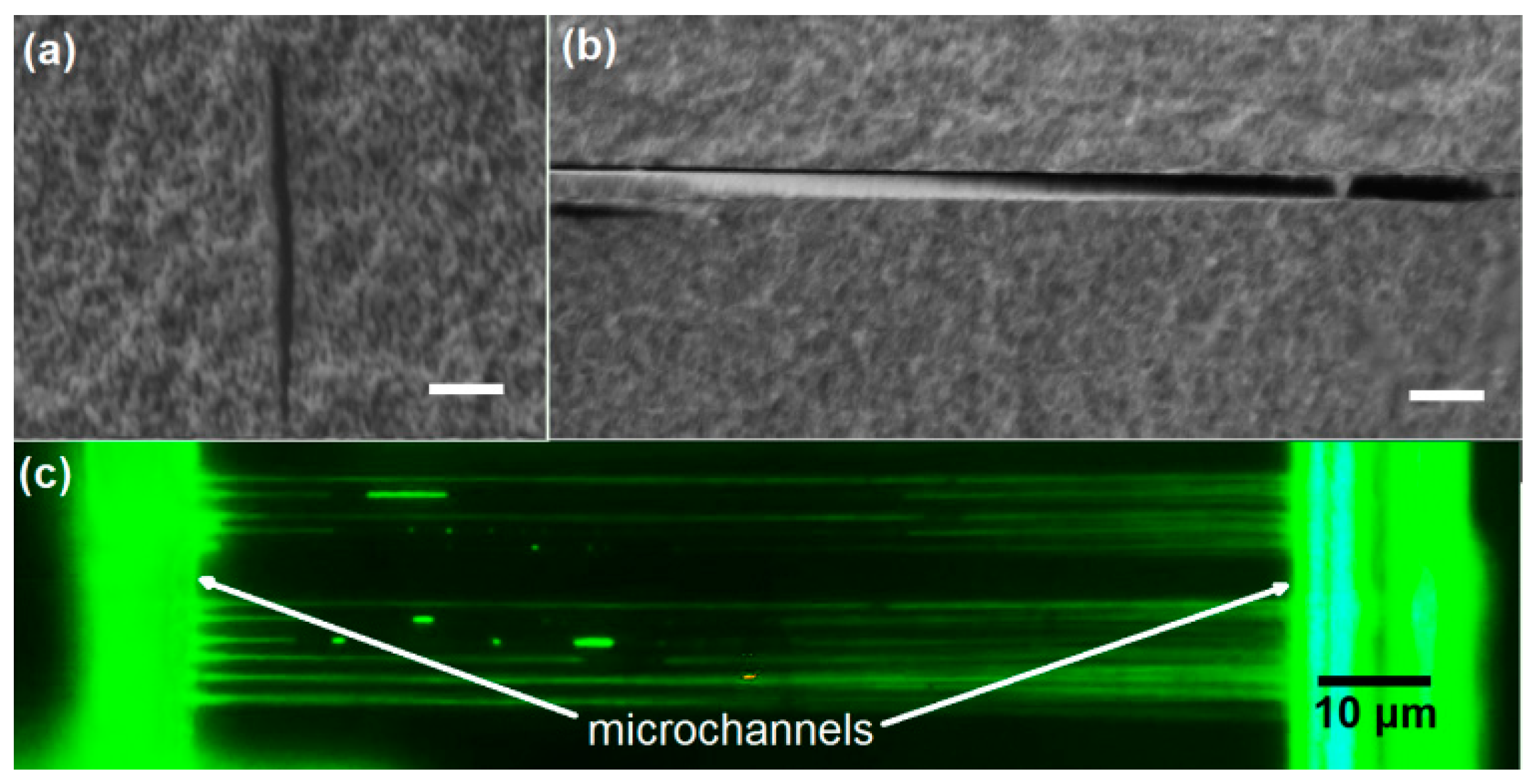
2.2. Fabrication of Microfluidic Channels of Smooth Inner Wall
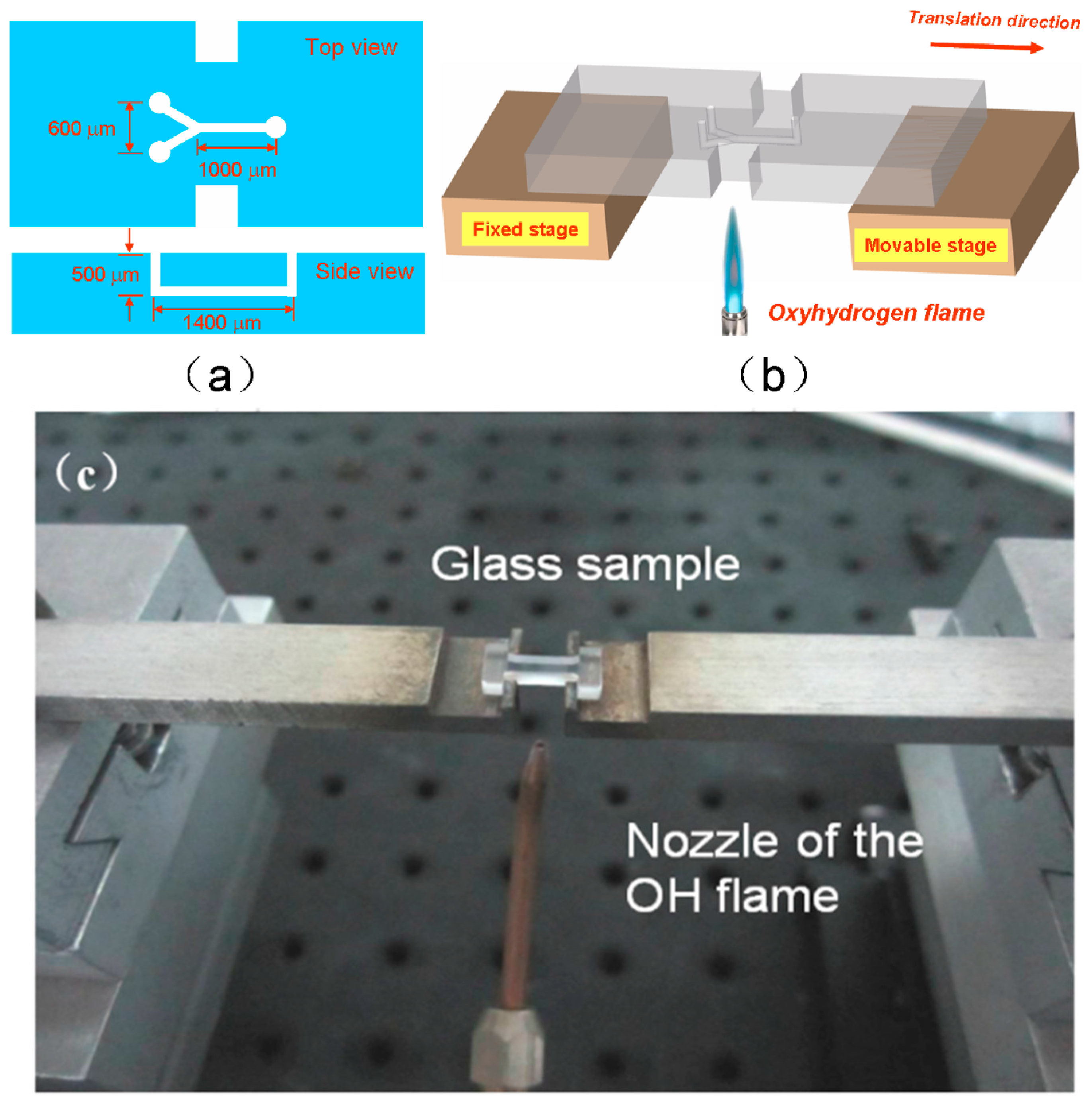
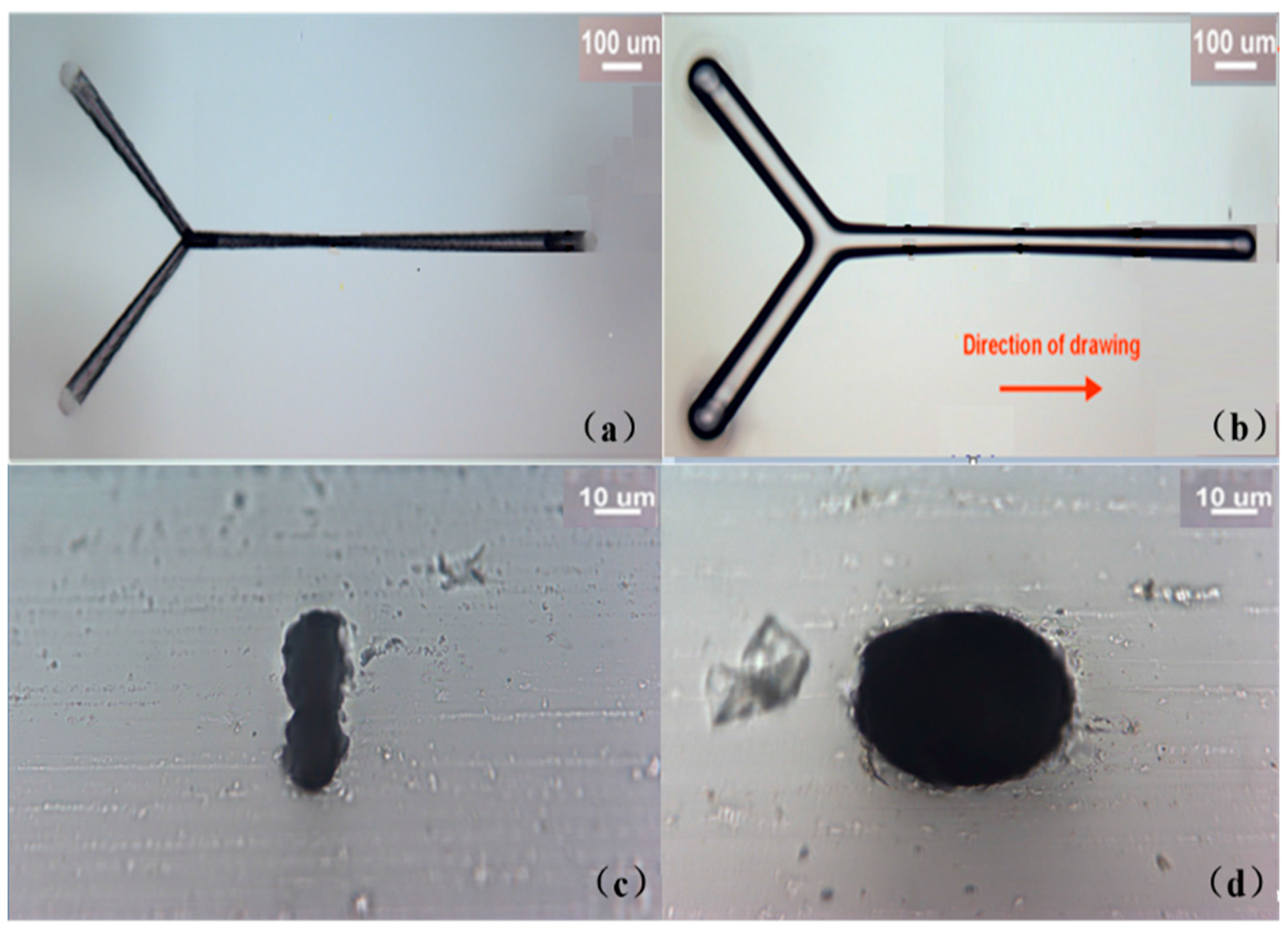
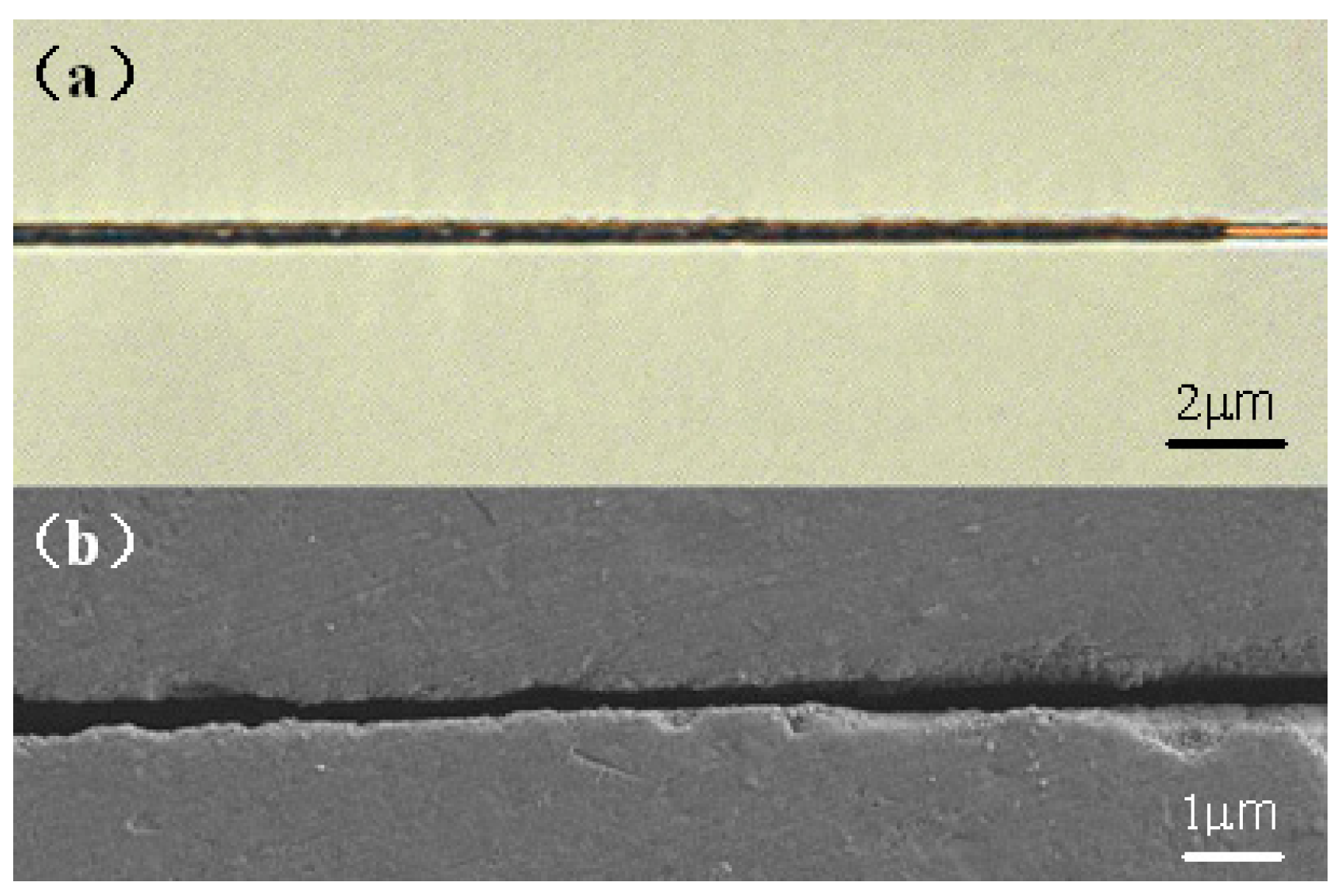
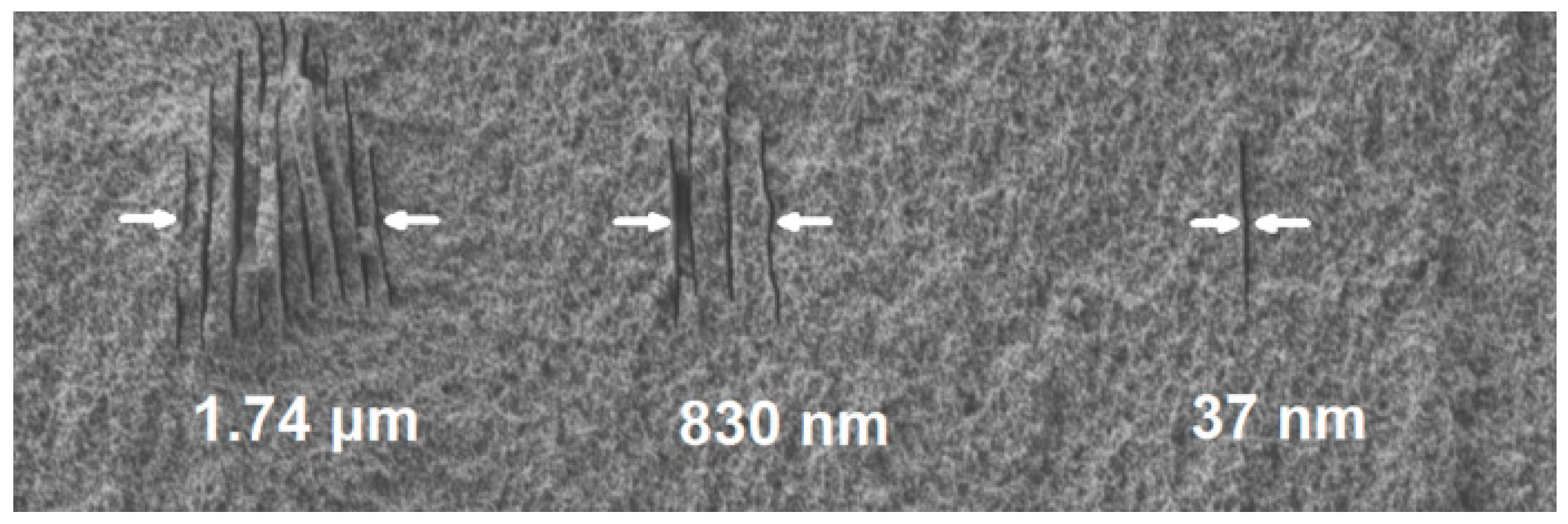
2.3. Fabrication of Nanofluidic Channels
3. Functional 3D Micro- and Nanofluidic Systems
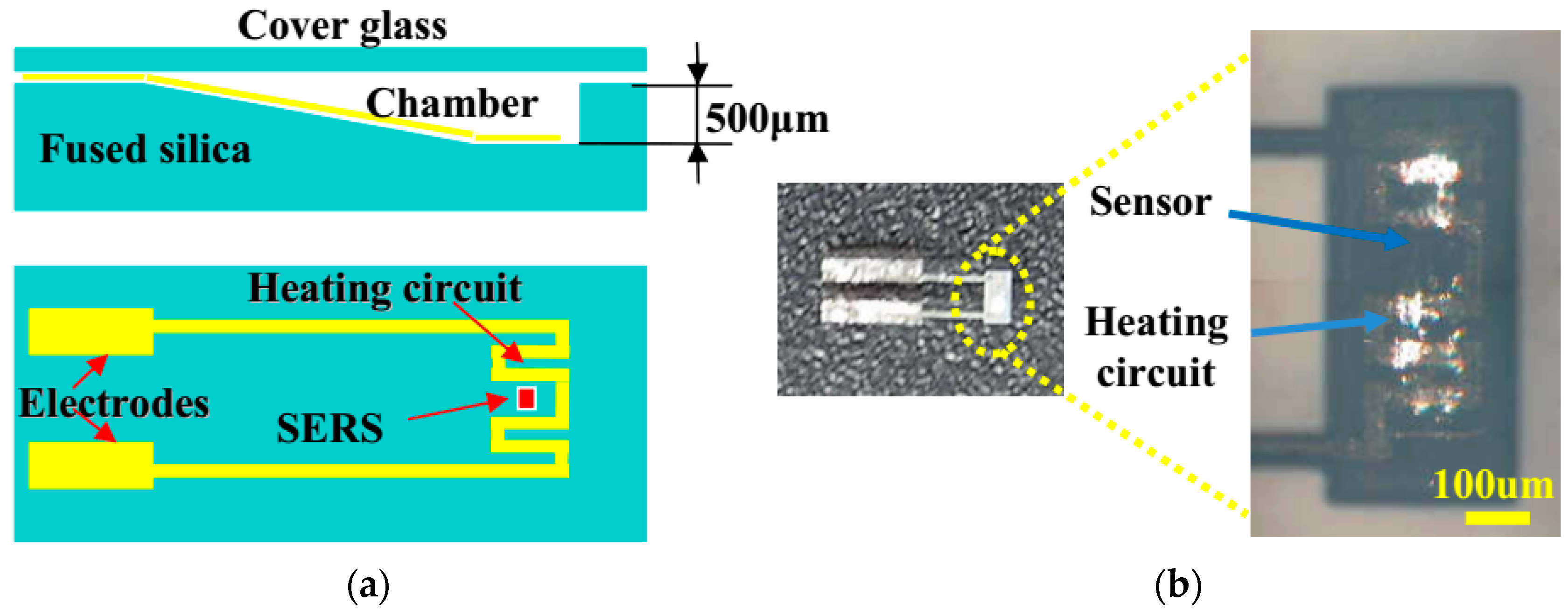
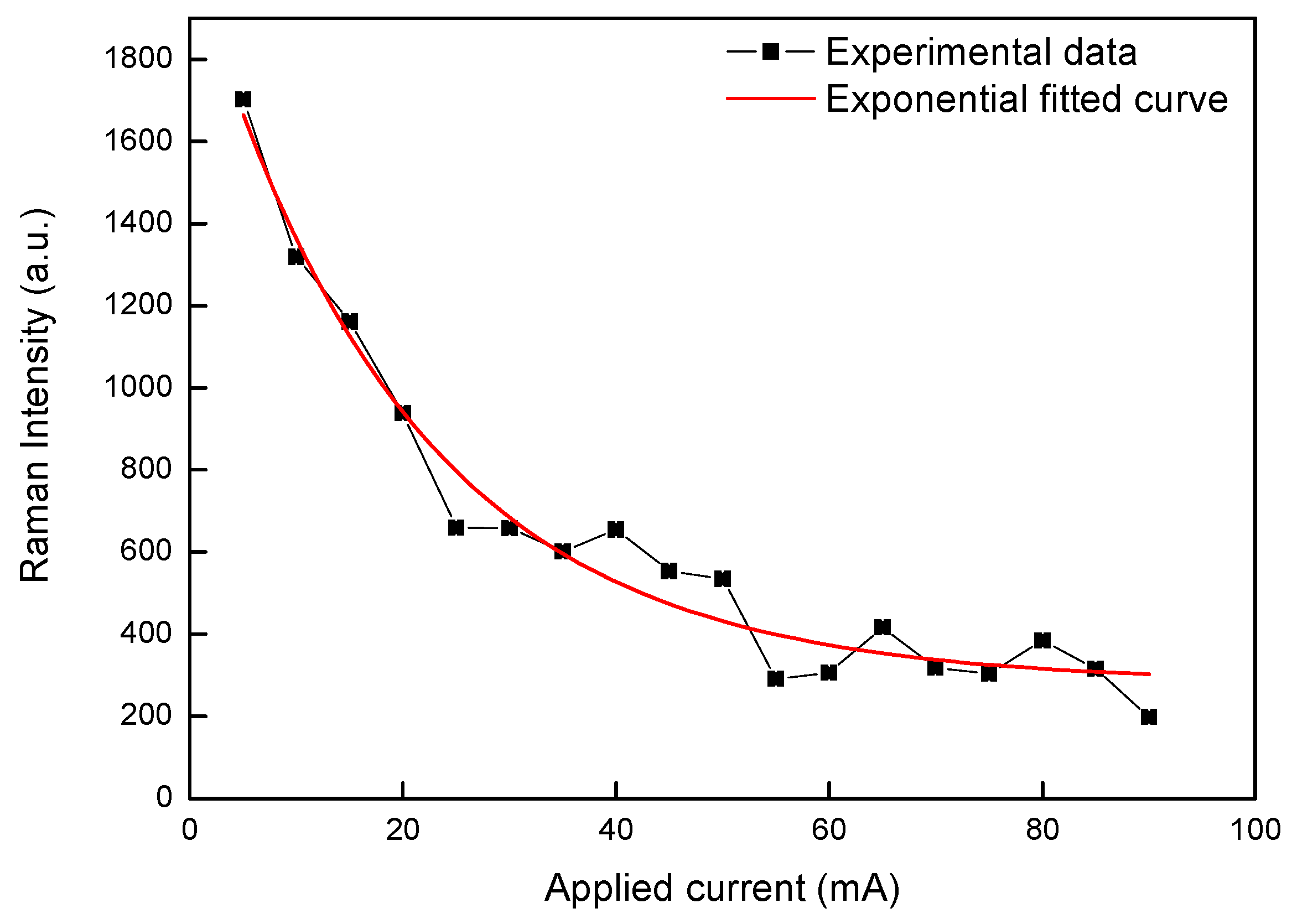
3.1. Integrated Microfluidic Surface Enhanced Raman Scattering (SERS) Sensor
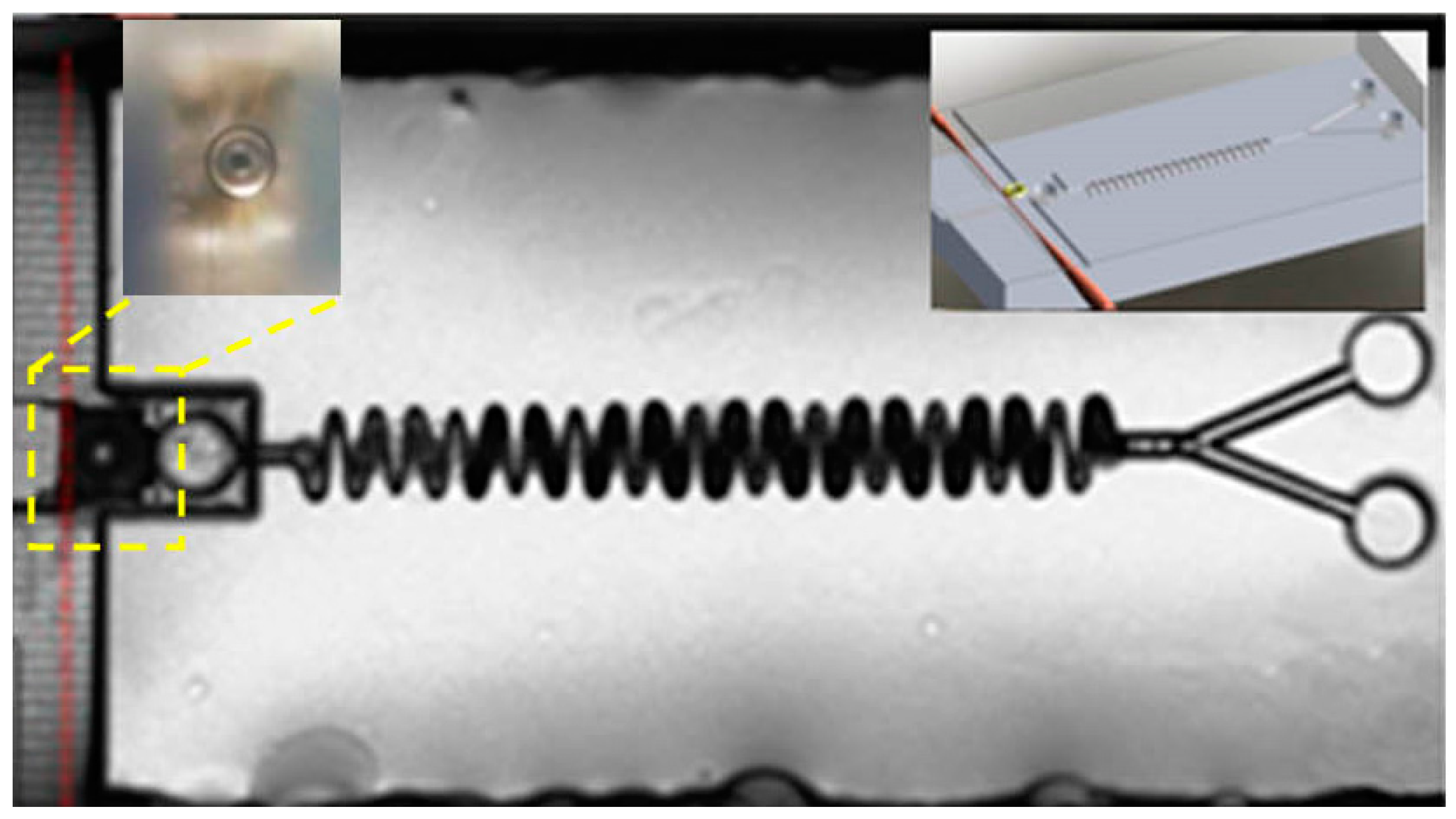
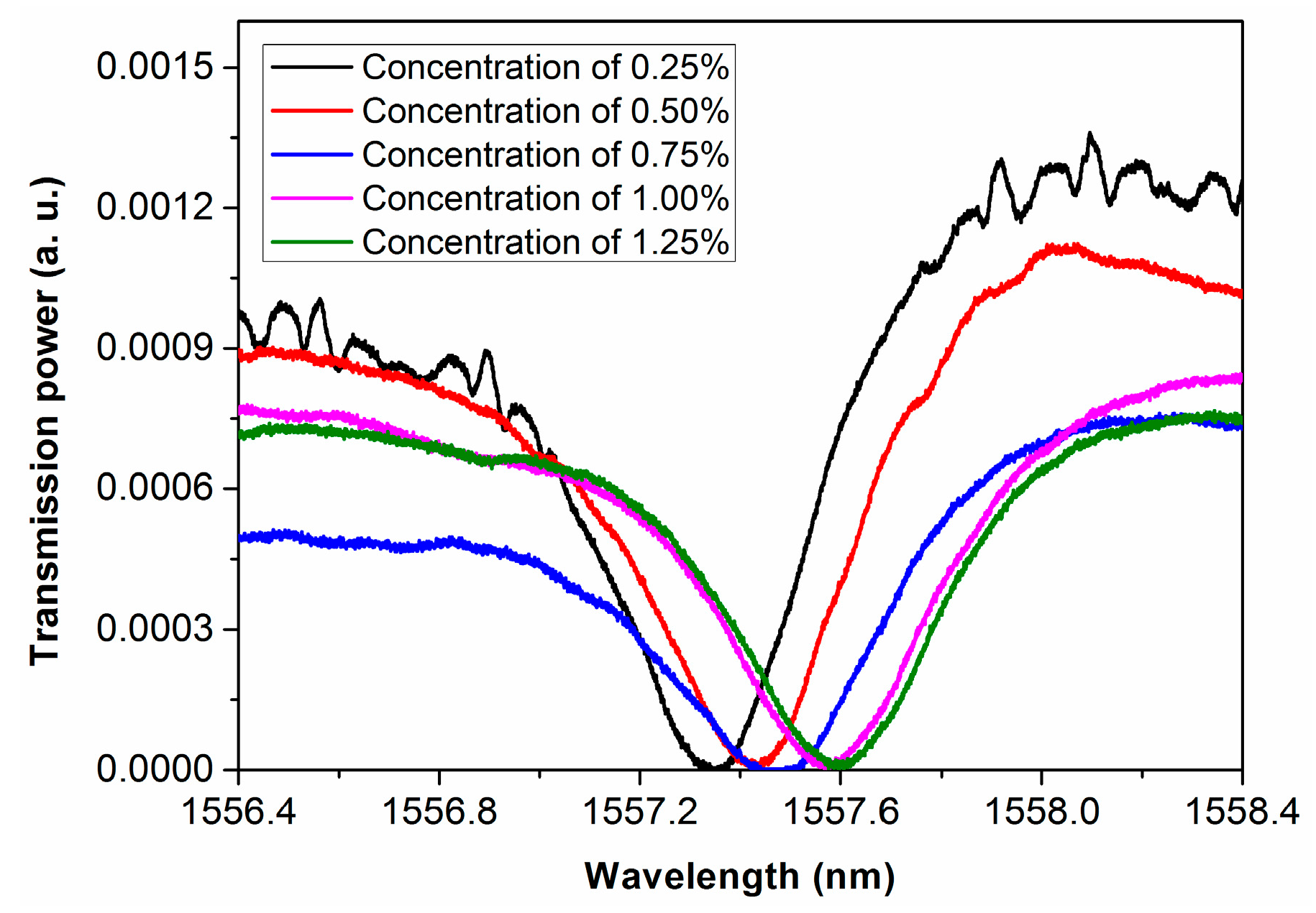
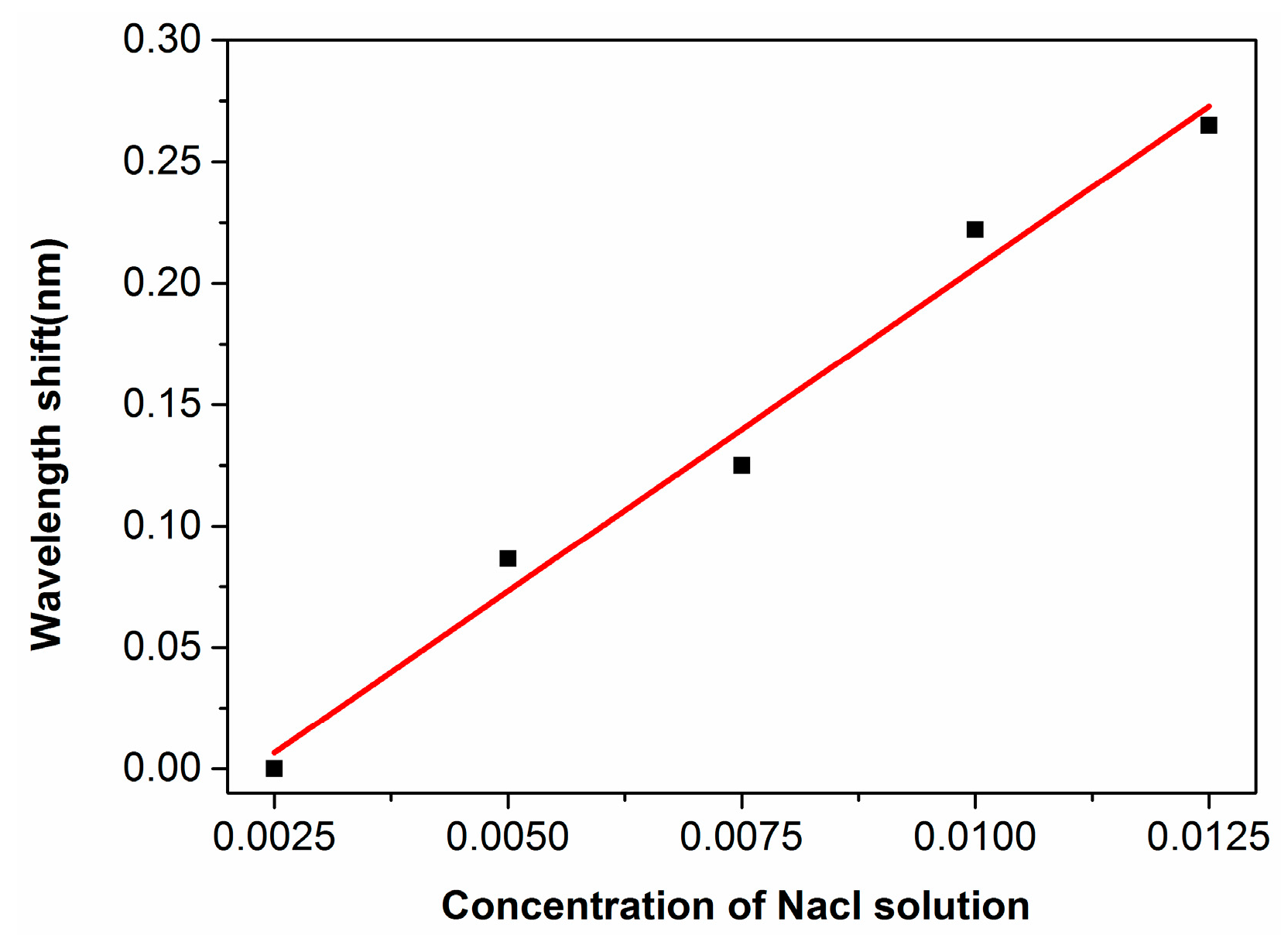
3.2. Integrated Microfluidic Whispering Gallery Mode (WGM) Microresonator (MR) Sensor
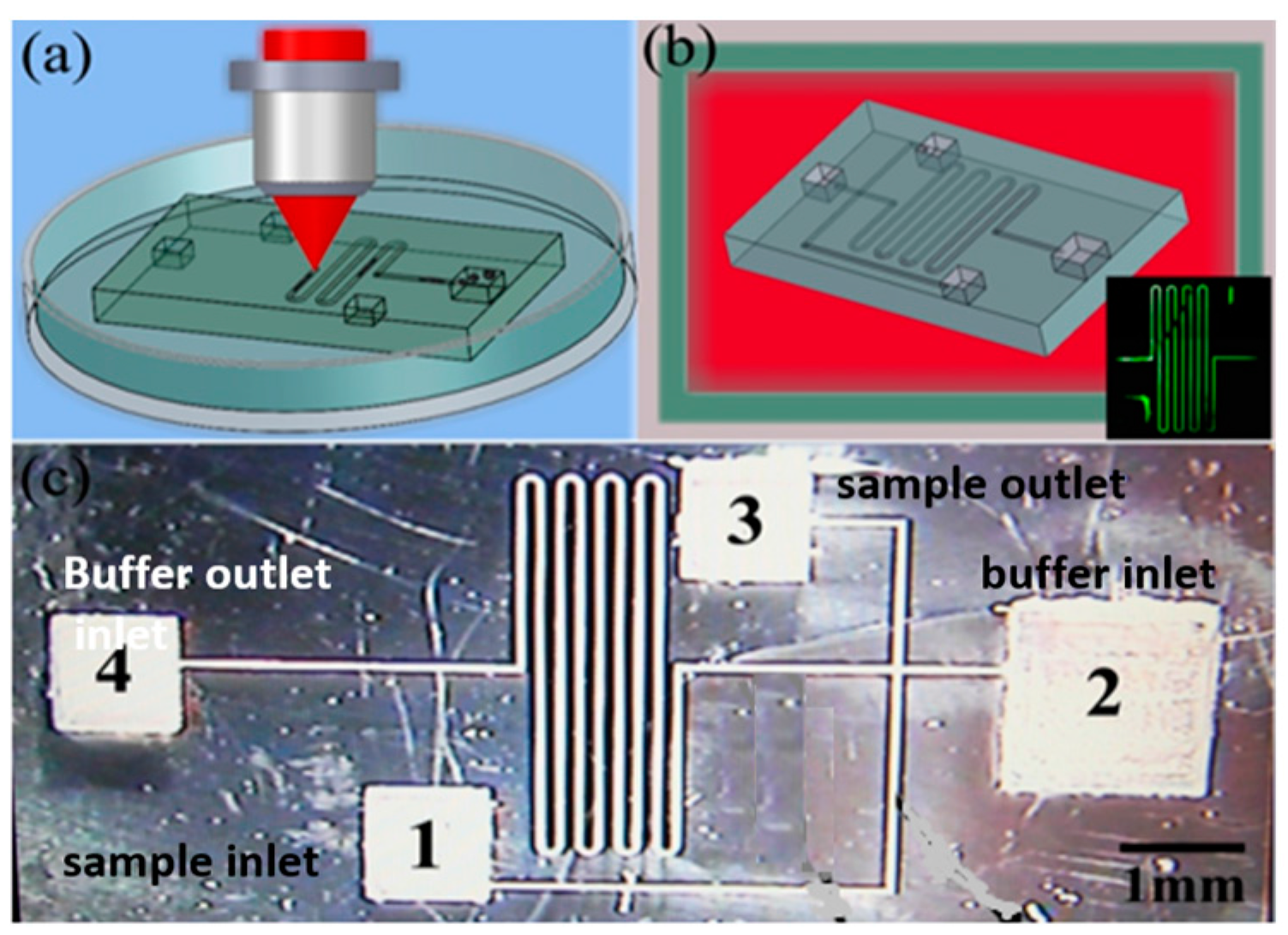
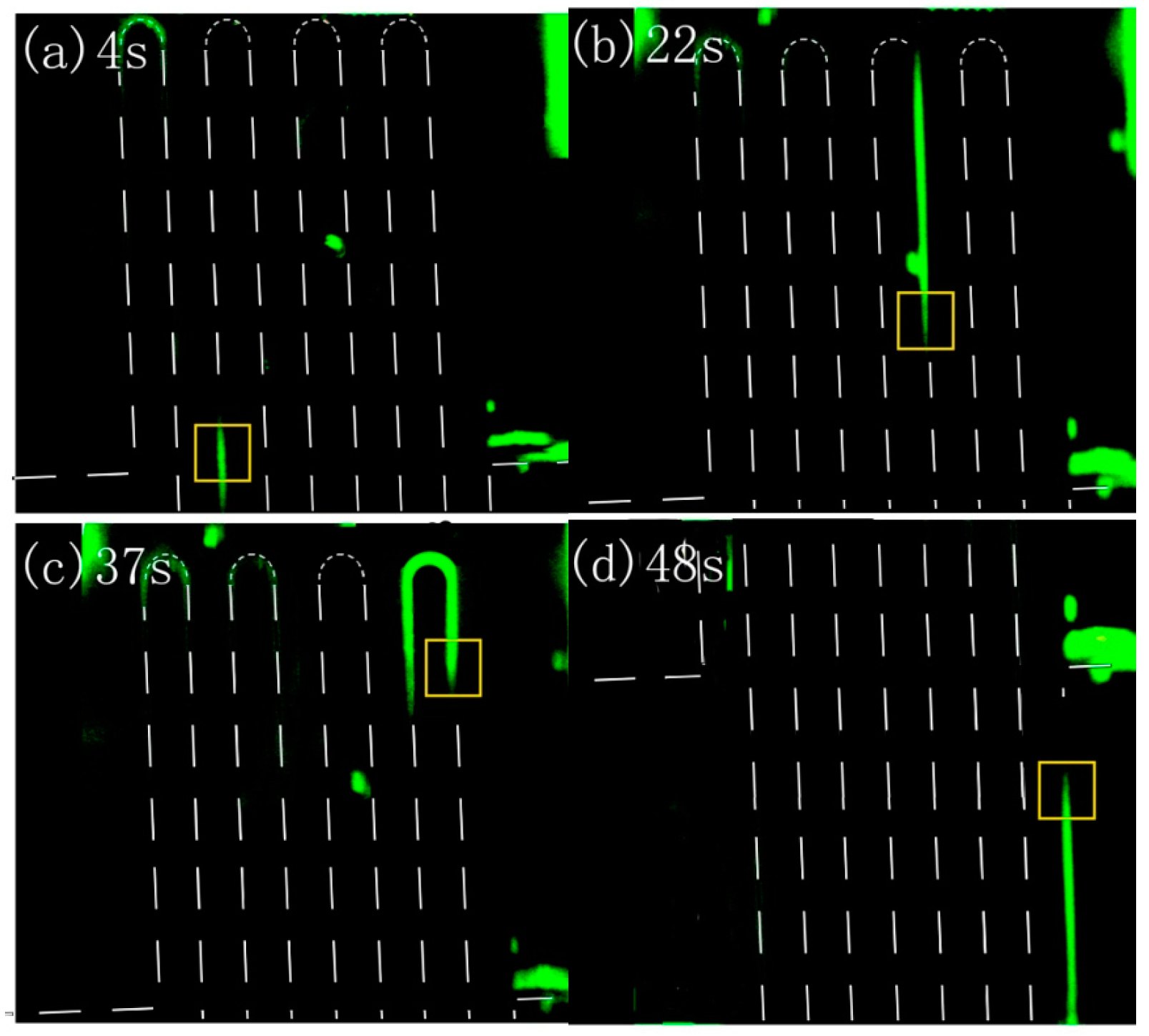
3.3. Integrated Microfluidic Chip for Electrophoresis Analysis
4. Conclusions and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Biomed. Eng. 2001, 43, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Miura, K.; Sugimoto, N.; Hirao, K. Writing waveguides in glass with a femtosecond laser. Opt. Lett. 1996, 21, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Marcinkevicius, A.; Juodkazis, S.; Watanabe, M.; Miwa, M.; Matsuo, S.; Misawa, H.; Nishii, J. Femtosecond laser-assisted three-dimensional microfabrication in silica. Opt. Lett. 2001, 26, 277–279. [Google Scholar] [CrossRef]
- Masuda, M.; Sugioka, K.; Cheng, Y.; Aoki, N.; Kawachi, M.; Shihoyama, K.; Toyoda, K.; Helvajian, H.; Midorikawa, K. 3-D microstructuring inside photosensitive glass by femtosecond laser excitation. Appl. Phys. A 2003, 76, 857–860. [Google Scholar] [CrossRef]
- Li, Y.; Itoh, K.; Watanabe, W.; Yamada, K.; Kuroda, D.; Nishii, J.; Jiang, Y.Y. Three-dimensional hole drilling of silica glass from the rear surface with femtosecond laser pulses. Opt. Lett. 2001, 26, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Hasselbrink, E.F.; Hunt, A.J. Rapidly prototyped three-dimensional nanofluidic channel networks in glass substrates. Anal. Chem. 2005, 77, 5083–5088. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Cheng, Y. Femtosecond laser processing for optofluidic fabrication. Lab Chip 2012, 12, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Schaap, A.; Rohrlack, T.; Bellouard, Y. Lab on a chip technologies for algae detection: A review. J. Biophotonics 2012, 5, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Osellame, R.; Hoekstra, H.J.W.M.; Cerullo, G.; Pollnau, M. Femtosecond laser microstructuring: An enabling tool for optofluidic lab-on-chips. Laser Photonics Rev. 2011, 5, 442–463. [Google Scholar] [CrossRef]
- Liao, Y.; Ju, Y.; Zhang, L.; He, F.; Zhang, Q.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K.; et al. Three-dimensional microfluidic channel with arbitrary length and configuration fabricated inside glass by femtosecond laser direct writing. Opt. Lett. 2010, 35, 3225–3227. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Miyoshi, H.; Akai, T.; Yazawa, T. Colorless transparent fluorescence material: Sintered porous glass containing rare-earth and transition-metal ions. Appl. Phys. Lett. 2005, 86, 231908. [Google Scholar] [CrossRef]
- Ju, Y.; Liao, Y.; Zhang, L.; Zhang, Q.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Fabrication of large-volume microfluidic chamber embedded in glass using three-dimensional femtosecond laser micromachining. Microfluid. Nanofluid. 2011, 11, 111–117. [Google Scholar] [CrossRef]
- Liu, C.N.; Liao, Y.; He, F.; Lin, D.; Song, J.X.; Lin, J.T.; Cheng, Y.; Sugioka, K.; Midorikawa, K. Compact 3D microfluidic channel structures embedded in glass fabricated by femtosecond laser direct writing. J. Laser Micro/Nanoeng. 2013, 8, 170–174. [Google Scholar] [CrossRef]
- He, F.; Lin, J.; Cheng, Y. Fabrication of hollow optical waveguides in fused silica by three-dimensional femtosecond laser micromachining. Appl. Phys. B 2011, 105, 379–384. [Google Scholar] [CrossRef]
- He, F.; Cheng, Y.; Xu, Z.; Liao, Y.; Xu, J.; Sun, H. Direct fabrication of homogeneous microfluidic channels embedded in fused silica using a femtosecond laser. Opt. Lett. 2010, 35, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, A.P.; Liu, H.; Meyhofer, E.; Mourou, G.; Hunt, A.J. Optics at critical intensity: Applications to nanomorphing. Proc. Natl. Acad. Sci. USA 2004, 101, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cheng, Y.; Liu, C.N.; Song, J.X.; He, F.; Shen, Y.L.; Chen, D.P.; Xu, Z.Z.; Fan, Z.C.; Wei, X.B.; et al. Direct laser writing of sub-50 nm nanofluidic channels buried in glass for three dimensional micro-nanofluidic integration. Lab Chip 2013, 13, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Shimotsuma, Y.; Kazansky, P.; Qiu, J.; Hirao, K. Self-organized nanogratings in glass irradiated by ultrashort light pulses. Phys. Rev. Lett. 2003, 91, 247405. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Ni, J.; Qiao, L.; Huang, M.; Bellouard, Y.; Sugioka, K.; Cheng, Y. High-fidelity visualization of formation of volume nanogratings in porous glass by femtosecond laser irradiation. Optica 2015, 2, 329–334. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Liao, Y.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Fabrication of an integrated Raman sensor by selective surface metallization using a femtosecond laser oscillator. Opt. Commun. 2009, 282, 1370–1373. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Surface-enhanced Raman scattering substrate fabricated by femtosecond laser direct writing. Jpn. J. Appl. Phys. 2008, 47, 189. [Google Scholar] [CrossRef]
- Albano, E.V.; Daiser, S.; Ertl, G.; Miranda, R.; Wandelt, K.; Garcia, N. Nature of surface-enhanced-raman-scattering active sites on coldly condensed AG films. Phys. Rev. Lett. 1983, 51, 2314–2317. [Google Scholar] [CrossRef]
- Lin, J.; Yu, S.; Ma, Y.; Fang, W.; He, F.; Qiao, L.; Tong, L.; Cheng, Y.; Xu, Z. On-chip three-dimensional high-Q microcavities fabricated by femtosecond laser direct writing. Opt. Express 2012, 20, 10212–10217. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lin, J.; Tang, J.; Liao, Y.; He, F.; Wang, Z.; Qiao, L.; Sugiokac, K.; Cheng, Y. Fabrication of an integrated high-quality-factor (high-Q) optofluidic sensor by femtosecond laser micromachining. Opt. Express 2014, 22, 14792–14802. [Google Scholar] [CrossRef] [PubMed]
- Crespi, A.; Gu, Y.; Ngamsom, B.; Hoekstra, H.J.W.M.; Dongre, C.; Pollnau, M.; Ramponi, R.; van den Vlekkert, H.H.; Watts, P.; Cerullo, G.; et al. Three-dimensional Mach-Zehnder interferometer in a microfluidic chip for spatially-resolved label-free detection. Lab Chip 2010, 10, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Liu, C.; Liao, Y.; Liu, Y.; Zhang, L.; Shen, Y.; Chen, D.; Cheng, Y. Direct fabrication of a microfluidic chip for electrophoresis analysis by water-assisted femtosecond laser writing in porous glass. Chin. Opt. Lett. 2013, 11, 072201. [Google Scholar]
- Tan, Y.; Wang, Z.; Chu, W.; Liao, Y.; Qiao, L.; Cheng, Y. High-throughput in-volume processing in glass with isotropic spatial resolutions in three dimensions. Opt. Mater. Express 2016, 6, 3787–3793. [Google Scholar] [CrossRef]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y. Internal Laser Writing of High-Aspect-Ratio Microfluidic Structures in Silicate Glasses for Lab-on-a-Chip Applications. Micromachines 2017, 8, 59. https://doi.org/10.3390/mi8020059
Cheng Y. Internal Laser Writing of High-Aspect-Ratio Microfluidic Structures in Silicate Glasses for Lab-on-a-Chip Applications. Micromachines. 2017; 8(2):59. https://doi.org/10.3390/mi8020059
Chicago/Turabian StyleCheng, Ya. 2017. "Internal Laser Writing of High-Aspect-Ratio Microfluidic Structures in Silicate Glasses for Lab-on-a-Chip Applications" Micromachines 8, no. 2: 59. https://doi.org/10.3390/mi8020059





