Human Liver Sinusoid on a Chip for Hepatitis B Virus Replication Study
Abstract
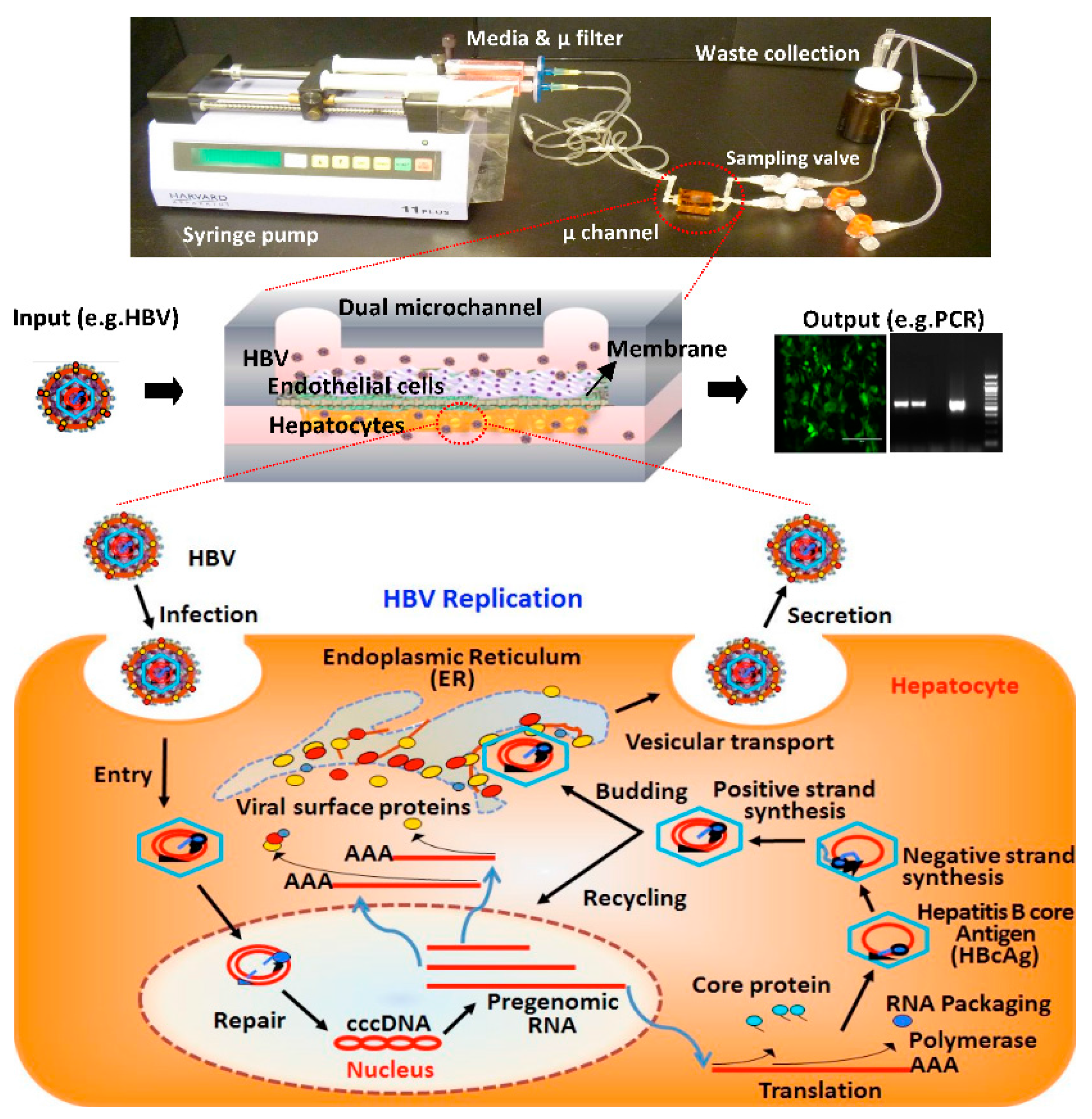
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Microfluidic Platforms
2.2. Layered Co-Culture of Primary Human Hepatocytes and Endothelial Cells
2.3. Live-Dead Staining and Imaging
2.4. Source, Purification, and Quantification of Infectious HBV
2.5. Infection of Primary Human Hepatocytes with HBV or Recombinant Adenoviruses
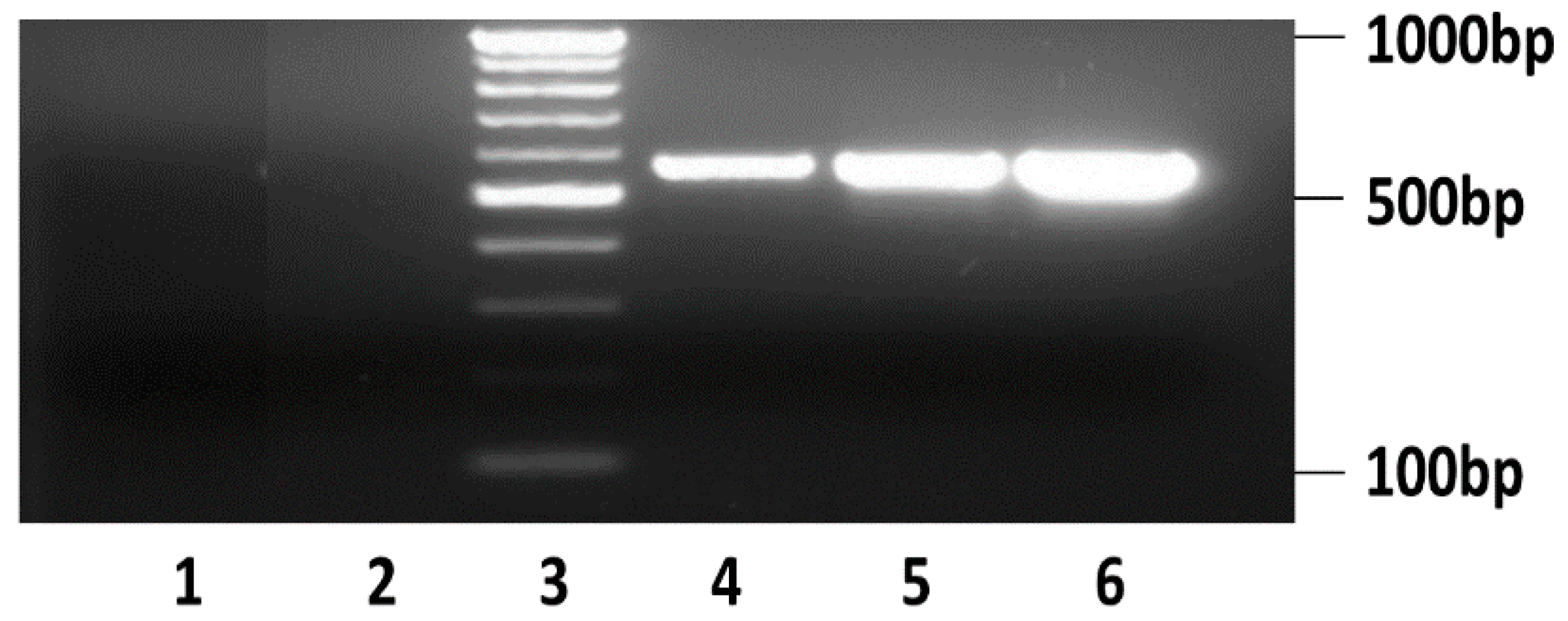
2.6. Analysis of Secreted HBV DNA
2.7. Immunofluorescence Assay
2.8. Image Assay
3. Results and Discussion
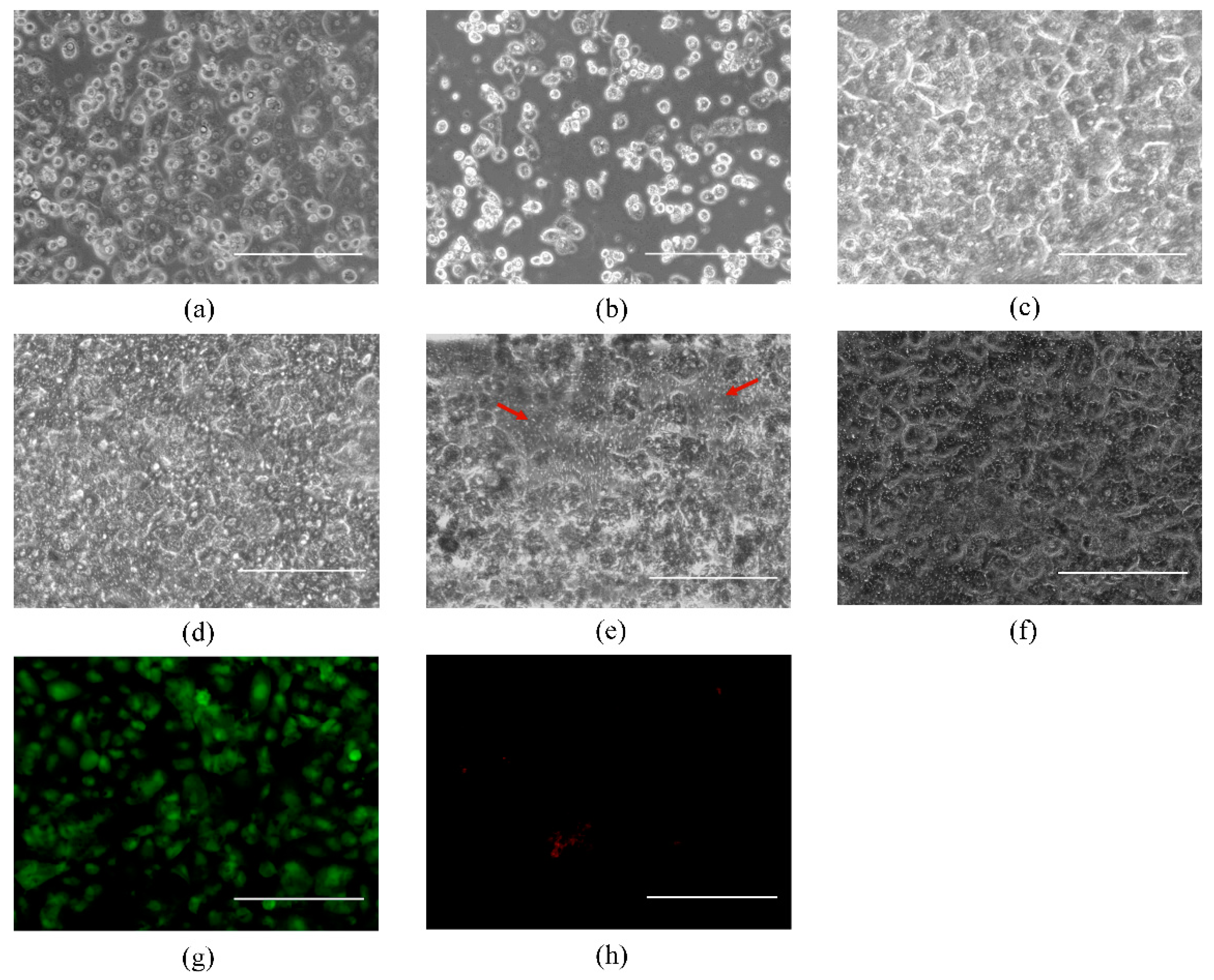
3.1. Primary Human Hepatocyte-Only Culture and Co-Culture of Hepatocytes and BAECs
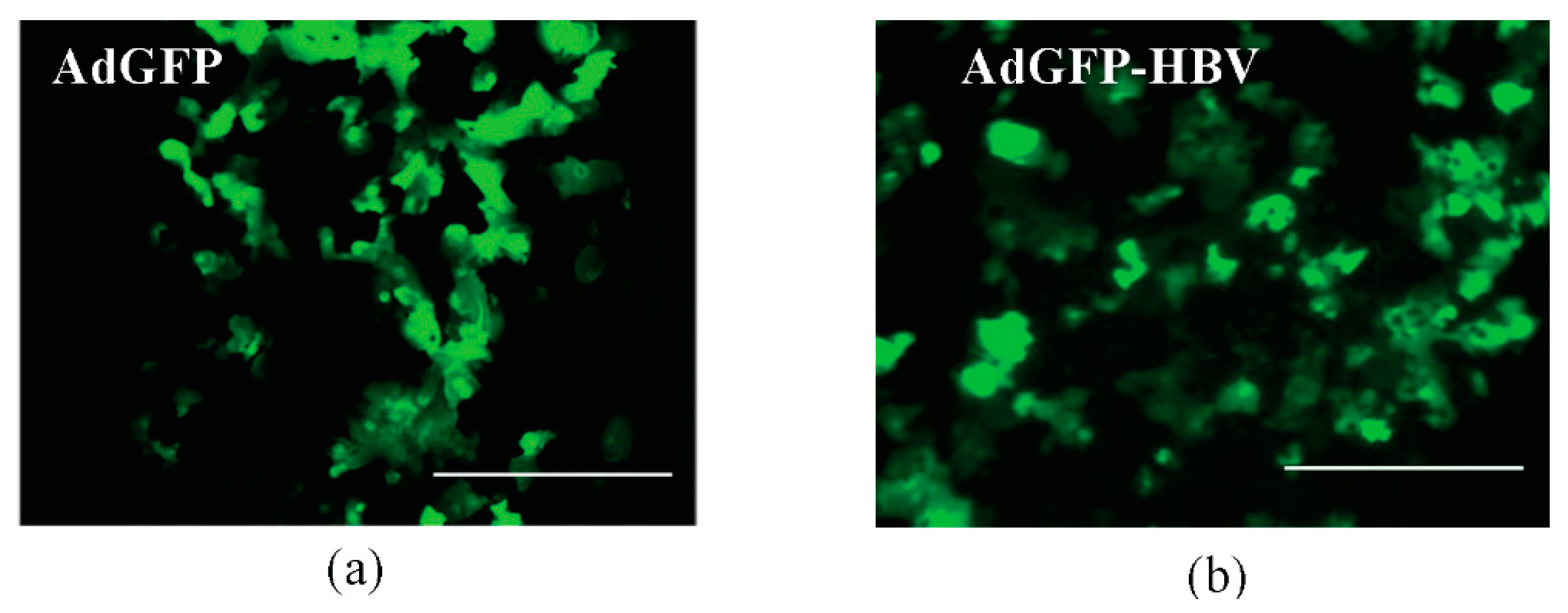
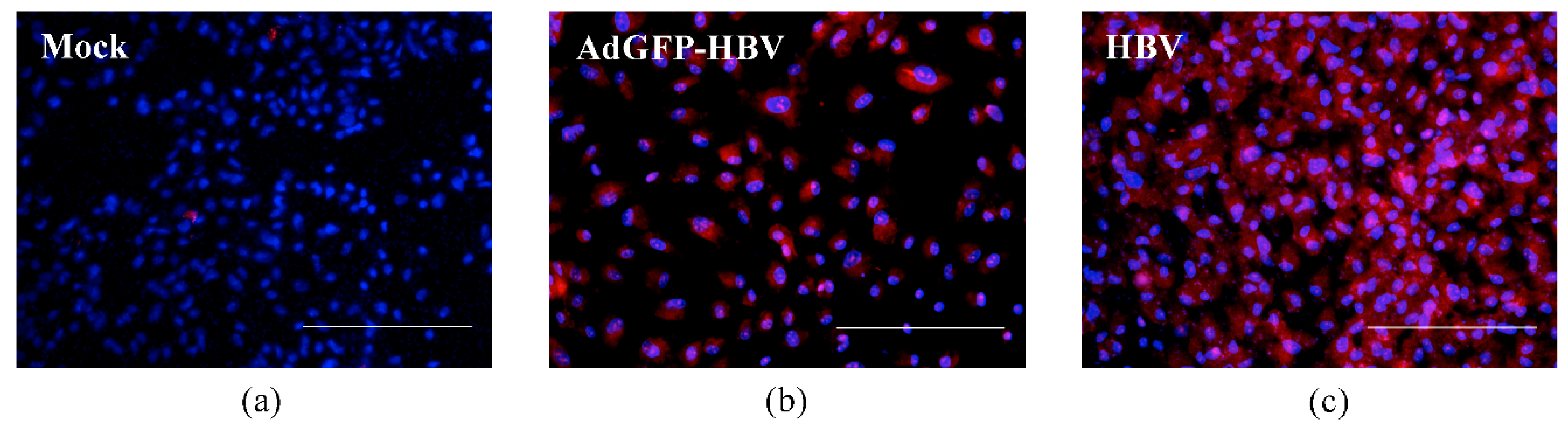
3.2. Infection of Primary Human Hepatocytes with Recombinant Adenoviruses and Expression of HBV Core Antigen
3.3. HBV Infection and Replication in Primary Human Hepatocytes in Microchannels
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seeger, C.; Zoulin, F.; Mason, W.S. Fields Virology: Hepadnaviruses, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- World Health Organization (WHO). Hepatitis B. Available online: http://www.who.int/mediacentre/factsheets/fs204_Jul2014/en/ (accessed on 1 July 2015).
- Rawat, S.; Clippinger, A.J.; Bouchard, M.J. Modulation of apoptotic signaling by the Hepatitis B virus X protein. Viruses 2012, 4, 2945–2972. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnot, P.; Kew, M. Hepatitis B virus and hepatocellular carcinoma. Int. J. Exp. Pathol. 2001, 82, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49 (Suppl. 5), S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Underhill, G.H.; Zaret, K.S.; Fox, I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.; Gong, Z.J.; Suwandhi, W.; van Pelt, J.; Soumillion, A.; Yap, S.H. Organ and species specificity of hepatitis B virus (HBV) infection: A review of literature with a special reference to preferential attachment of HBV to human hepatocytes. J. Viral Hepat. 1997, 4, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, M.F.; Oberwinkler, H.; Schaller, H.; Protzer, U. Transfer of Hepatitis B virus genome by adenovirus vectors into cultured cells and mice: crossing the species barrier. J. Virol. 2001, 75, 5108–5118. [Google Scholar] [CrossRef] [PubMed]
- Watashi, K.; Urban, S.; Li, W.; Wakita, T. NTCP and beyond: Opening the door to unveil Hepatitis B virus entry. Int. J. Mol. Sci. 2014, 15, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dial, S.; Shi, L.; Branham, W.; Liu, J.; Fang, J.L.; Green, B.; Deng, H.; Kaput, J.; Ning, B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab. Dispos. 2011, 39, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Nahmias, Y.; Berthiaume, F.; Yarmush, M.L. Integration of technologies for hepatic tissue engineering. Adv. Biochem. Eng. Biotechnol. 2007, 103, 309–329. [Google Scholar] [PubMed]
- Guillouzo, A. Liver cell models in in vitro toxicology. Environ. Health Perspect. 1998, 106 (Suppl. 2), 511–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Hung, P.J.; Lee, L.P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 2007, 97, 1340–1346. [Google Scholar] [CrossRef]
- Goral, V.N.; Hsieh, Y.C.; Petzold, O.N.; Clark, J.S.; Yuen, P.K.; Faris, R.A. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip 2010, 10, 3380–3386. [Google Scholar] [CrossRef]
- Prodanov, L.; Jindal, R.; Bale, S.S.; Hegde, M.; McCarty, W.J.; Golberg, I.; Bhushan, A.; Yarmush, M.L.; Usta, O.B. Long term maintenance of a microfluidic 3-D human liver sinusoid. Biotechnol. Bioeng. 2016, 113, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.S.; Golberg, I.; Jindal, R.; McCarty, W.J.; Luitje, M.; Hegde, M.; Bhushan, A.; Usta, O.B.; Yarmush, M.L. Long-term coculture strategies for primary hepatocytes and liver sinusoidal endothelial cells. Tissue Eng. Part C Methods 2015, 21, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.S.; Vernetti, L.; Senutovitch, N.; Jindal, R.; Hegde, M.; Gough, A.; McCarty, W.J.; Bakan, A.; Bhushan, A.; Shun, T.Y.; et al. In vitro platforms for evaluating liver toxicity. Exp. Biol. Med. 2014, 239, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Sodunke, T.R.; Bouchard, M.J.; Noh, H.M. Microfluidic platform for Hepatitis B viral replication study. Biomed. Microdevices 2008, 10, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.B.; Sodunke, T.R.; Lamontagne, J.; Cirillo, J.; Rajiv, C.; Bouchard, M.J.; Noh, M. Liver sinusoid on a chip: Long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol. Bioeng. 2015, 112, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chen, C.C.; Chang, W.C.; Tao, M.H.; Huang, C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J. Virol. 2012, 86, 9443–9453. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.; Mason, W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Ganem, D.; Schnider, R.J. The Molecular Biology of the Hepatitis B Viruses, 4th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; Volume 2. [Google Scholar]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection—Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sorensen, E.M.; Naito, A.; Schott, M.; Kim, S.; Ahlquist, P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc. Natl. Acad. Sci. USA 2007, 104, 10205–10210. [Google Scholar] [CrossRef] [PubMed]
- Loggi, E.; Vitale, G.; Conti, F.; Bernardi, M.; Andreone, P. Chronic hepatitis B: Are we close to a cure? Dig. Liver Dis. 2015, 47, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. New horizon for radical cure of chronic Hepatitis B virus infection. World J. Hepatol. 2016, 8, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B.; Ferrari, C.; Pasquinelli, C.; Chisari, F.V. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 1996, 2, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.B.; Sodunke, T.R.; Cirillo, J.; Bouchard, M.J.; Noh, H. Liver on a chip: Engineering the liver sinusoid. In Proceedings of the 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013.
- Kang, Y.B.; Rawat, S.; Cirillo, J.; Bouchard, M.; Noh, H.M. Layered long-term co-culture of hepatocytes and endothelial cells on a transwell membrane: Toward engineering the liver sinusoid. Biofabrication 2013, 5, 045008. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M. Selection and characterization of bovine aortic endothelial cells. In Vitro 1978, 14, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, A.M.; Guo, H.; Westby, G.; Liu, Y.; Simsek, E.; Guo, J.T.; Mehta, A.; Norton, P.; Gu, B.; Block, T.; et al. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob. Agents Chemother. 2007, 51, 4427–4437. [Google Scholar] [CrossRef] [PubMed]
- Sells, M.A.; Chen, M.L.; Acs, G. Production of hepatitis B virus particles in HepG2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 1987, 84, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Gripon, P.; Rumin, S.; Urban, S.; Le Seyec, J.; Glaise, D.; Cannie, I.; Guyomard, C.; Lucas, J.; Trepo, C.; Guguen-Guillouzo, C. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 2002, 99, 15655–15660. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.; Sohn, J.A. Targeting hepatitis B virus with CRISPR/Cas9. Mol. Ther. Nucleic Acids 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Clippinger, A.J.; Gearhart, T.L.; Bouchard, M.J. Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-κB and the mitochondrial permeability transition pore. J. Virol. 2009, 83, 4718–4731. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.N.; Riccalton, L.A.; Lewis, A.L.; Fry, J.R.; Hammond, A.H.; Tendler, S.J.; Shakesheff, K.M. Liver tissue engineering: A role for co-culture systems in modifying hepatocyte function and viability. Tissue Eng. 2001, 7, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Balis, U.J.; Yarmush, M.L.; Toner, M. Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999, 13, 1883–1900. [Google Scholar] [PubMed]
- Rawat, S. Regulation of Hepatitis B Virus Replication by AKT and NF-κB Signaling Pathways in Primary Hepatocytes in Biochemistry and Molecular Biology; Drexel University: Philadelphia, PA, USA, 2014. [Google Scholar]
- Gearhart, T.L.; Bouchard, M.J. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology 2010, 407, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.F.; Murray, R. Relationship of virus dose to incubation time of clinical hepatitis and time of appearance of hepatitis—Associated antigen. Am. J. Med. Sci. 1972, 263, 27–33. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.B.; Rawat, S.; Duchemin, N.; Bouchard, M.; Noh, M. Human Liver Sinusoid on a Chip for Hepatitis B Virus Replication Study. Micromachines 2017, 8, 27. https://doi.org/10.3390/mi8010027
Kang YB, Rawat S, Duchemin N, Bouchard M, Noh M. Human Liver Sinusoid on a Chip for Hepatitis B Virus Replication Study. Micromachines. 2017; 8(1):27. https://doi.org/10.3390/mi8010027
Chicago/Turabian StyleKang, Young Bok (Abraham), Siddhartha Rawat, Nicholas Duchemin, Michael Bouchard, and Moses Noh. 2017. "Human Liver Sinusoid on a Chip for Hepatitis B Virus Replication Study" Micromachines 8, no. 1: 27. https://doi.org/10.3390/mi8010027




