Rapid Capture and Analysis of Airborne Staphylococcus aureus in the Hospital Using a Microfluidic Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Reagents
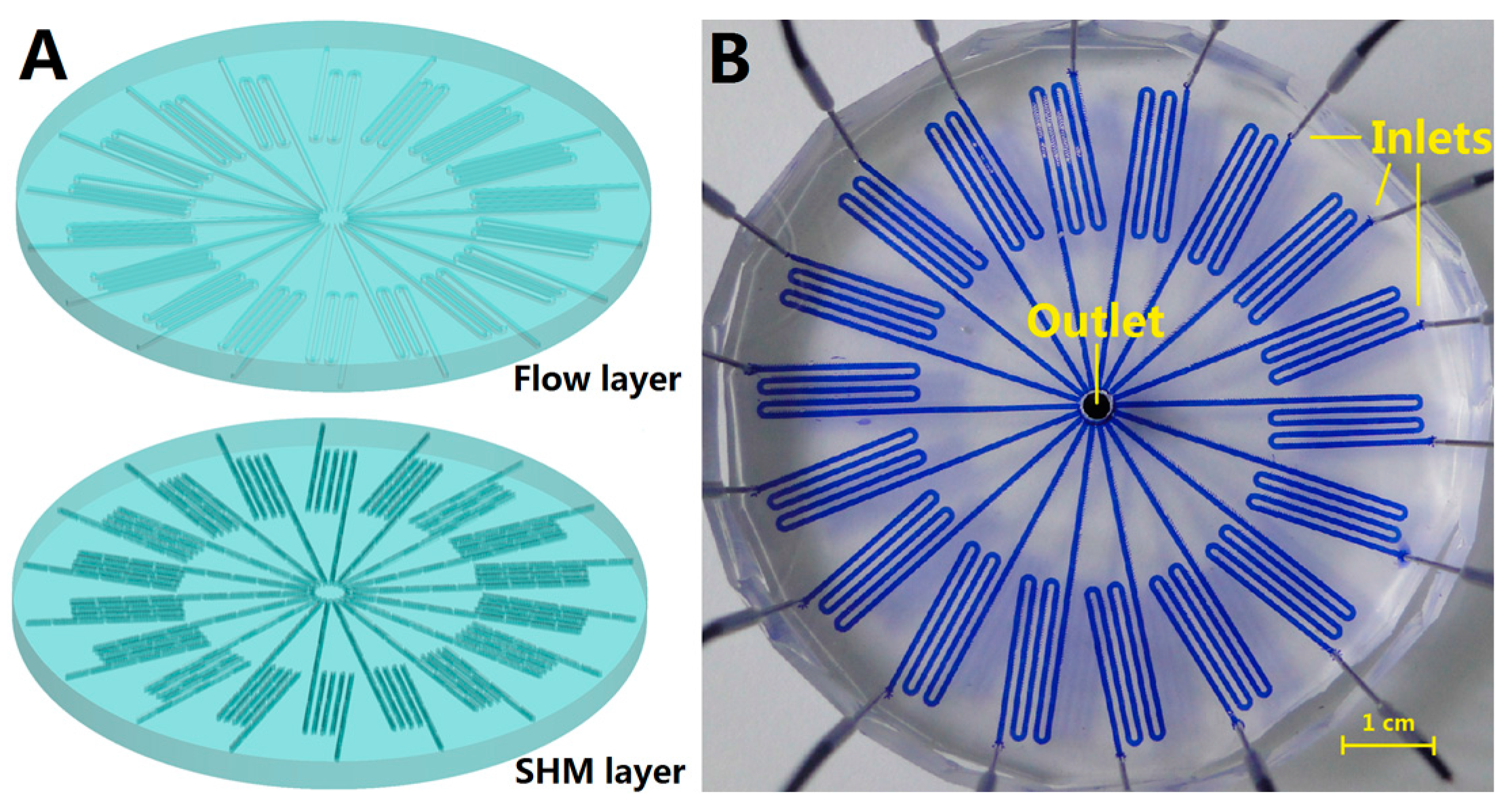
2.2. Chip Fabrication
2.3. Airborne Staphylococcus aureus Capture and Enrichment
2.4. LAMP Reaction System for Nuc Gene Detection
2.5. Clinical Airborne Staphylococcus aureus Analysis
3. Results and Discussion
3.1. Chip Design
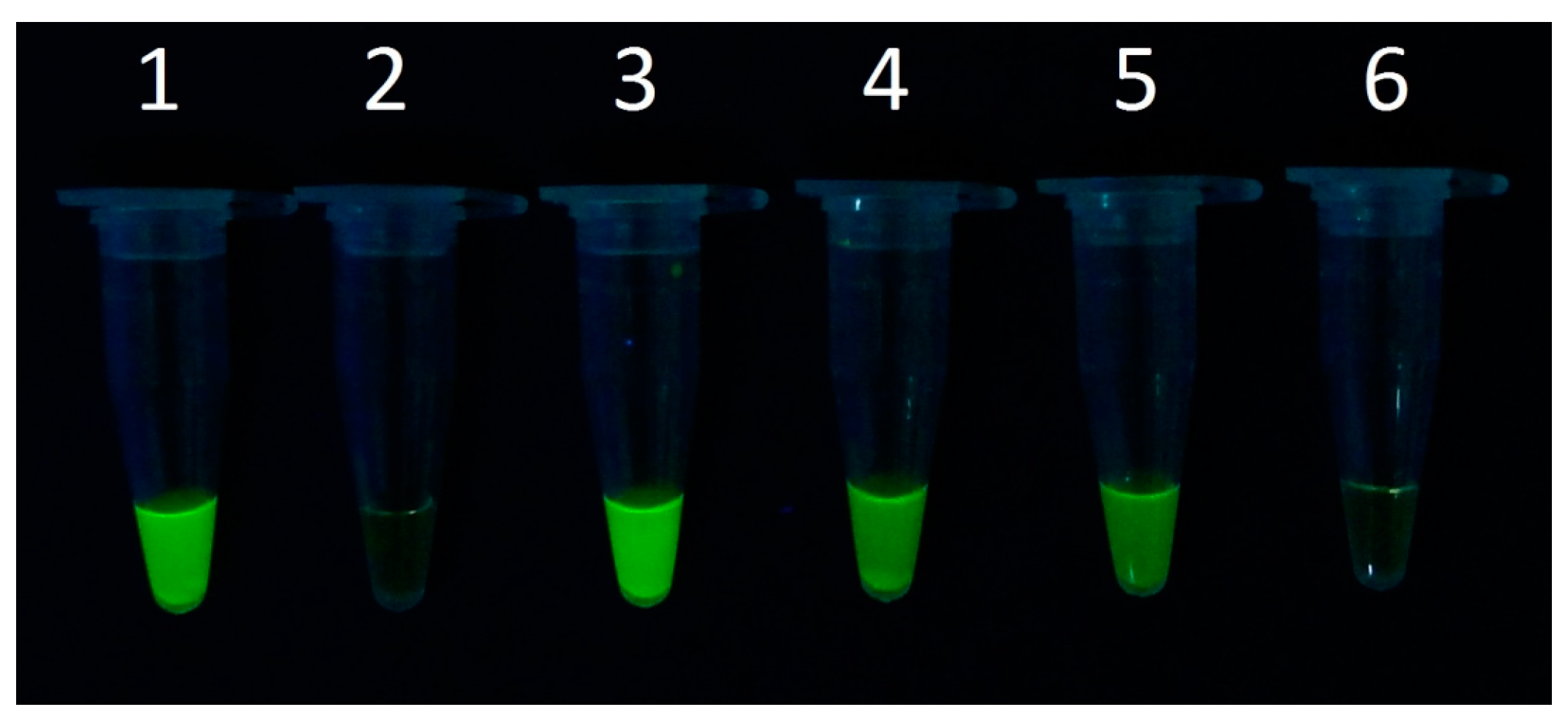
3.2. Detection Limit of Staphylococcus aureus in the Microfluidic Chip
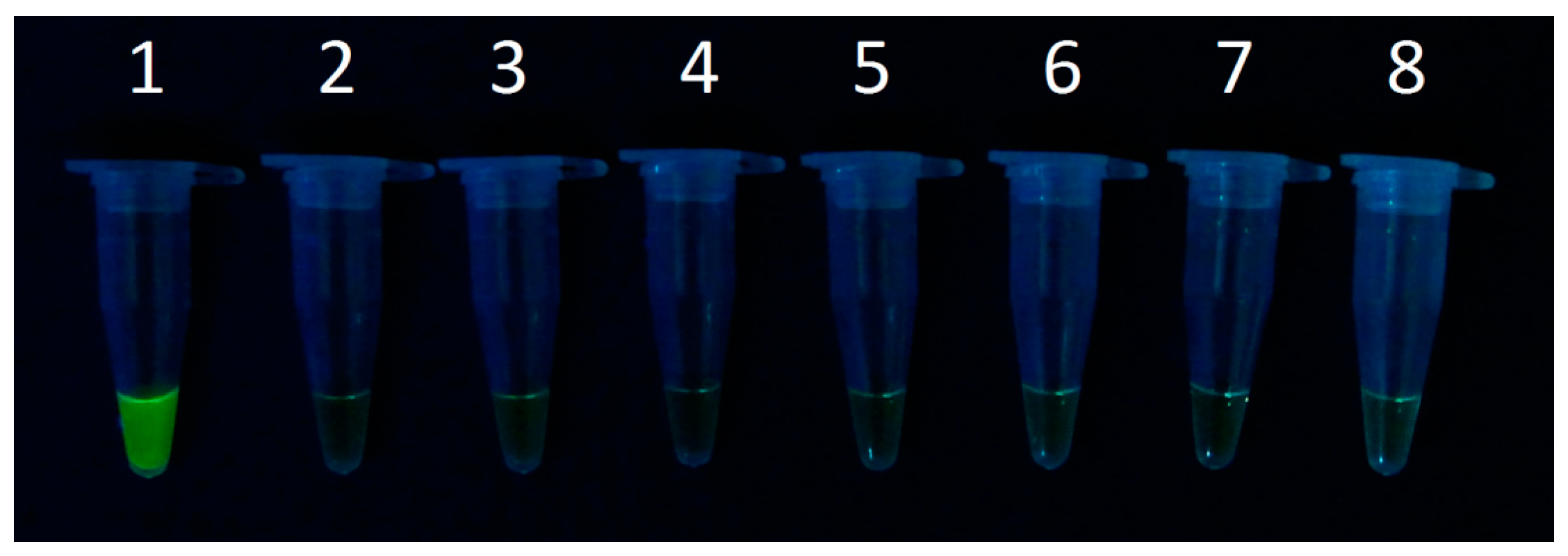
3.3. Clinical Airborne Staphylococcus aureus Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aires, S.M.; Lencastre, H. Bridges from hospitals to the laboratory: Genetic portraits of methicillin-resistant Staphylococcus aureus clones. FEMS Immunol. Med. Microbiol. 2004, 40, 101–111. [Google Scholar] [CrossRef]
- Rodriguez, E.A.; Correa, M.M.; Ospina, S.; Atehortua, S.L.; Jimenez, J.N. Differences in Epidemiological and Molecular Characteristics of Nasal Colonization with Staphylococcus aureus (MSSA-MRSA) in Children from a University Hospital and Day Care Centers. PLoS ONE 2014, 9, e101417. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, H.B.; Burkholder, J.M.; Schmechel, D.E.; Tester, P.A.; Rublee, P.A. Insidious effects of a toxic estuarine dinoflagellate on fish survival and human health. Toxicol. Environ. Health 1995, 46, 501–522. [Google Scholar] [CrossRef] [PubMed]
- Heidelberg, J.F.; Eisen, J.A.; Nelson, W.C.; Clayton, R.A.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Umayam, L.; et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 2000, 406, 477–483. [Google Scholar] [PubMed]
- Jiang, X.R.; Jing, W.W.; Zheng, L.L.; Liu, S.X.; Wu, W.J.; Sui, G.D. A Continuous-flow high-throughput microfluidic device for airborne bacteria PCR detection. Lab Chip 2014, 14, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.D.; Cheng, X.J. Microfluidics for detection of airborne pathogens: What challenges remain. Bioanalysis 2014, 6, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.W.; Zhao, W.; Liu, S.X.; Li, L.; Tsai, C.T.; Fan, X.Y.; Wu, W.J.; Li, J.Y.; Yang, X.; Sui, G.D. Microfluidic Device for efficient airborne bacteria capture and enrichment. Anal. Chem. 2013, 85, 5255–5262. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.Y.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Rapid fabrication of microfluidic chip with three-dimensional structures using natural lotus leaf template. Microfluid. Nanofluid. 2010, 9, 923–931. [Google Scholar] [CrossRef]
- Jing, W.W.; Jiang, X.R.; Zhao, W.; Liu, S.X.; Cheng, X.J.; Sui, G.D. Microfluidic platform for direct capture and analysis of airborne Mycobacterium tuberculosis. Anal. Chem. 2014, 86, 5815–5821. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Y.C.; Jiang, X.R.; Xu, S.C.; Sui, G.D. Microfluidic chip for rapid analysis of cerebrospinal fluid infected with Staphylococcus aureus. Anal. Methods 2014, 6, 2015–2019. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by Polymerase Chain Reaction Amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [PubMed]
- Lin, A.H.; Zhang, S.H. Analysis of airborne bacteria concentration and distribution in hospital wards. Hubei J. Prev. Med. 2002, 4, 29–36. [Google Scholar]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Liu, Y.; Liu, Q.; Jing, W.; Qin, K.; Sui, G. Rapid Capture and Analysis of Airborne Staphylococcus aureus in the Hospital Using a Microfluidic Chip. Micromachines 2016, 7, 169. https://doi.org/10.3390/mi7090169
Jiang X, Liu Y, Liu Q, Jing W, Qin K, Sui G. Rapid Capture and Analysis of Airborne Staphylococcus aureus in the Hospital Using a Microfluidic Chip. Micromachines. 2016; 7(9):169. https://doi.org/10.3390/mi7090169
Chicago/Turabian StyleJiang, Xiran, Yingchao Liu, Qi Liu, Wenwen Jing, Kairong Qin, and Guodong Sui. 2016. "Rapid Capture and Analysis of Airborne Staphylococcus aureus in the Hospital Using a Microfluidic Chip" Micromachines 7, no. 9: 169. https://doi.org/10.3390/mi7090169