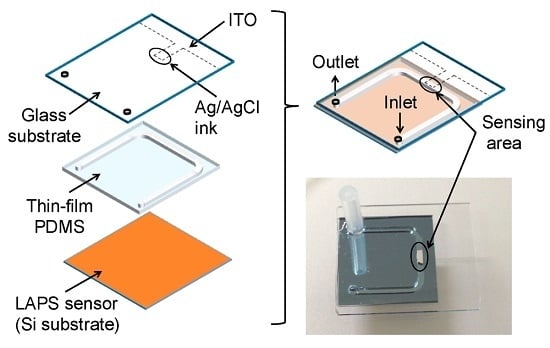
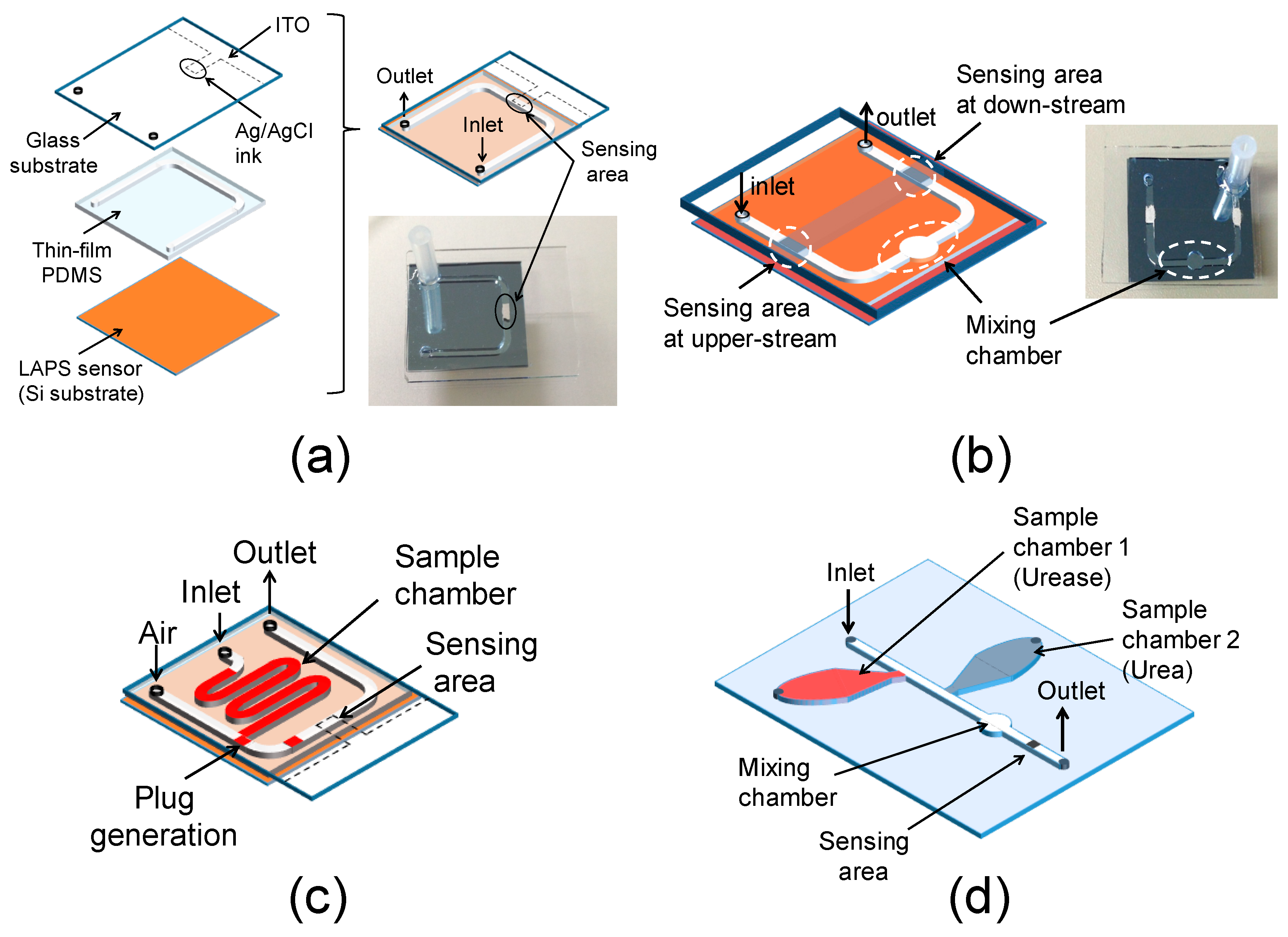
Light-Addressable Potentiometric Sensor as a Sensing Element in Plug-Based Microfluidic Devices
Abstract
:1. Introduction
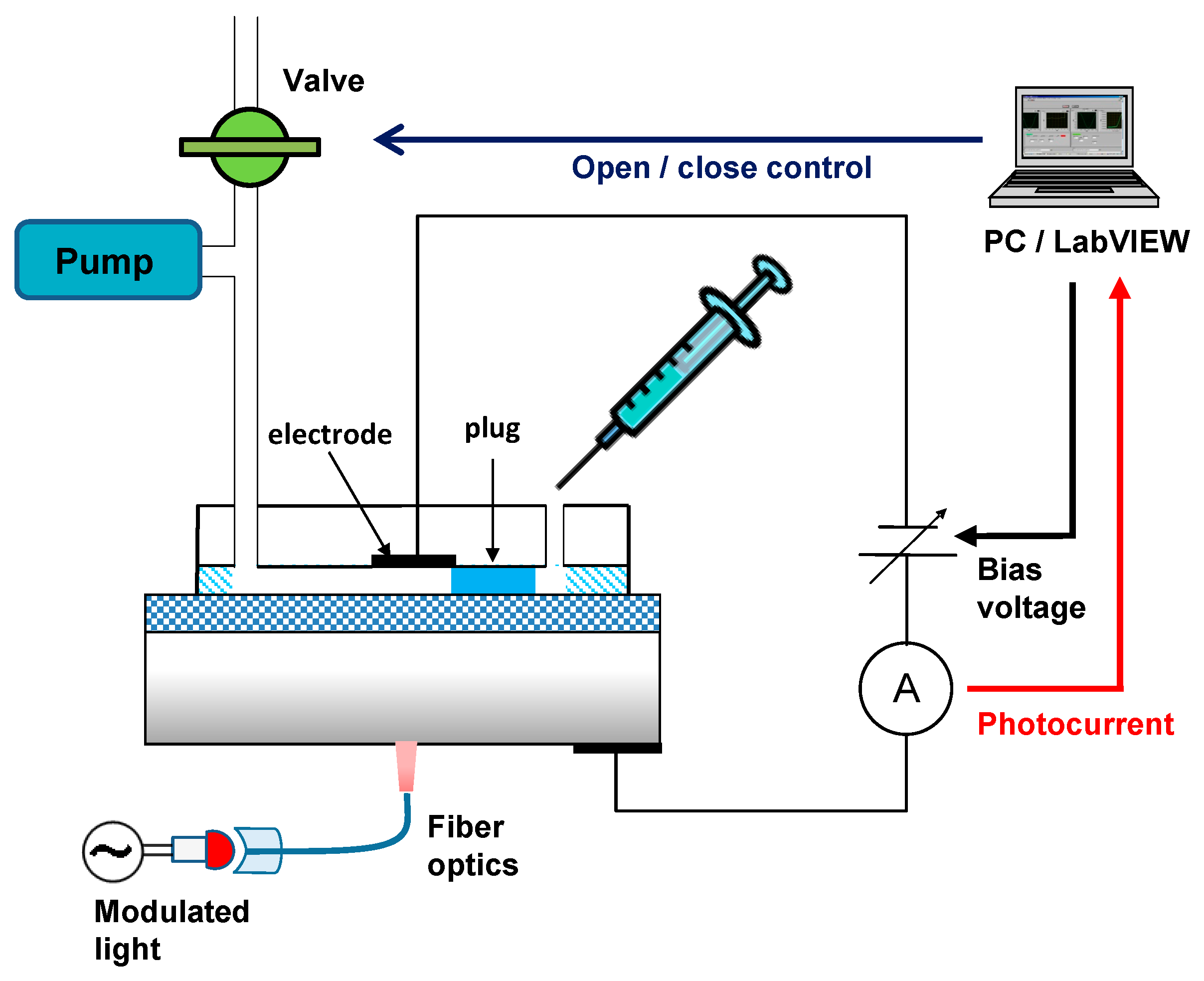
2. Experiments
3. Results and Discussion
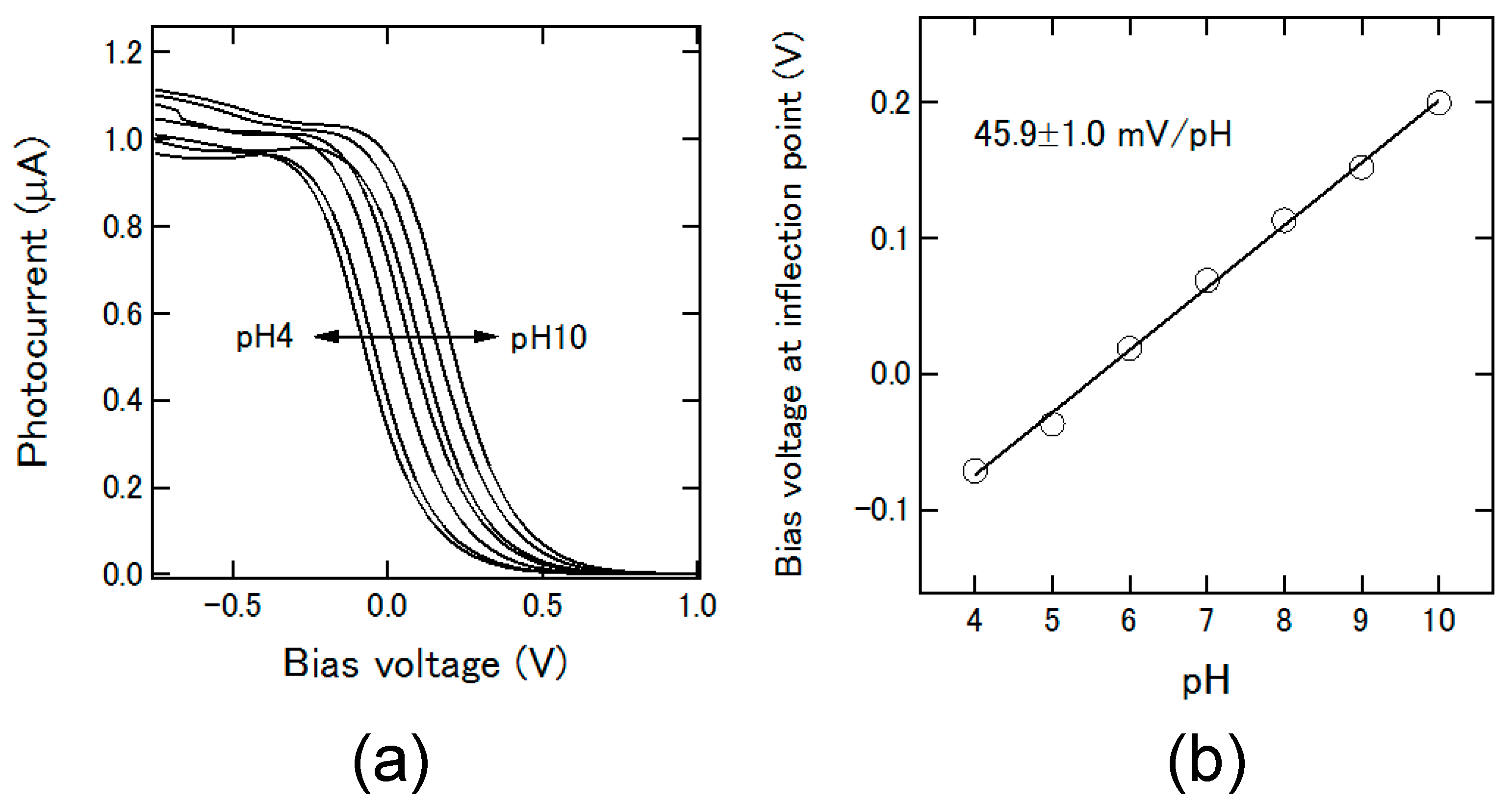
3.1. Control of the Plug Motion and LAPS Measurement
3.2. Merging Plugs and Differential Measurement
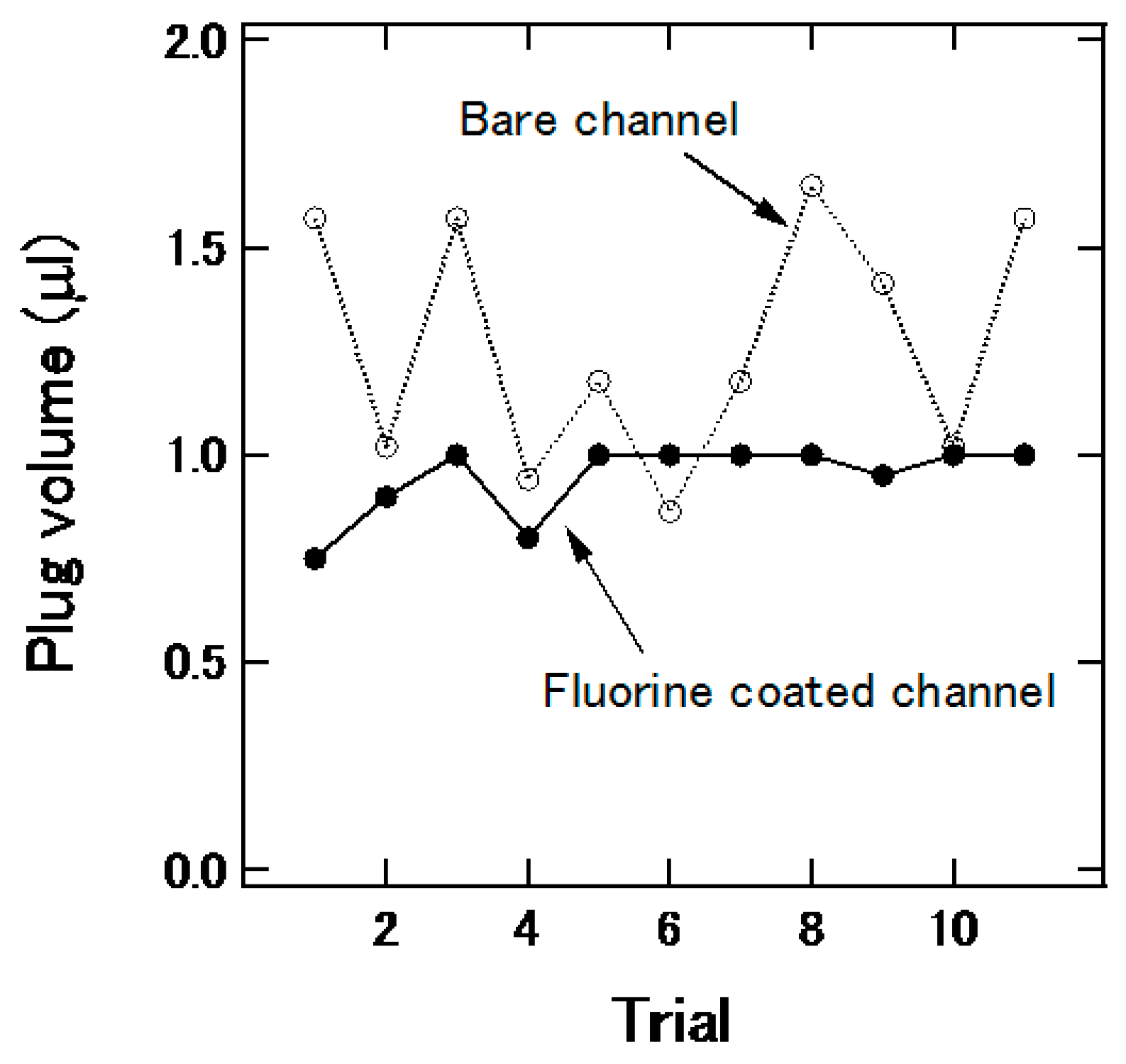
3.3. Plug Generation in the Channel
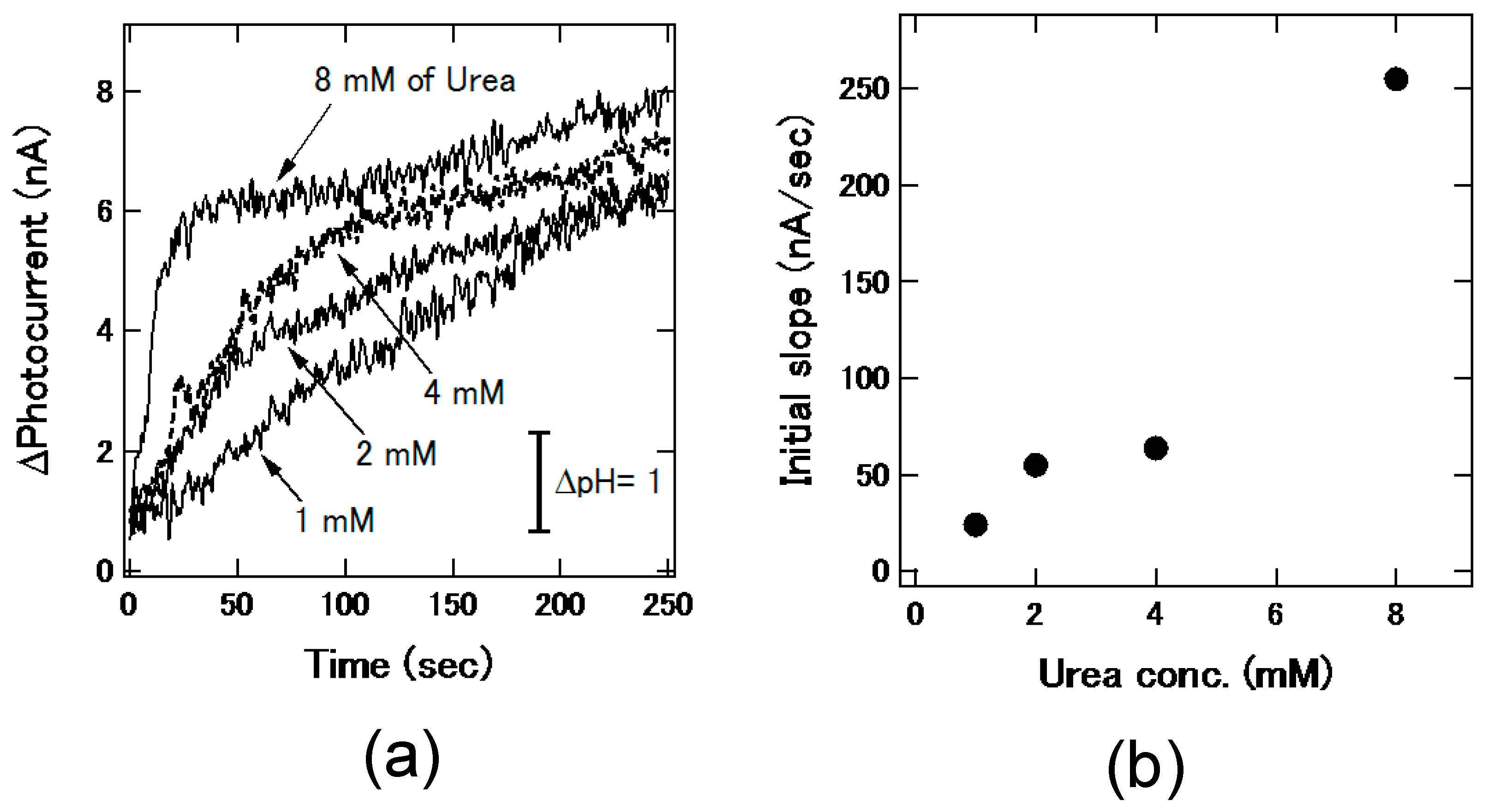
3.4. Mixing of Two Plugs for Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; deMello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, D.G.; Parce, J.W.; McConnell, H.M. Light-addressable potentiometric sensor for biochemical systems. Science 1988, 240, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Yoshida, M.; Sakai, T.; Matsuzaka, A.; Wagner, T.; Kanoh, S.; Yoshinobu, T.; Schöning, M.J. Differential setup of light-addressable potentiometric sensor with an enzyme reactor in a flow channel. Jpn. J. Appl. Phys. 2011, 50, 04dl08. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hirayama, Y.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Visualization of enzymatic reaction in a microfluidic channel using chemical imaging sensor. Electrochim. Acta 2013, 113, 768–772. [Google Scholar] [CrossRef]
- Miyamoto, K.; Itabashi, A.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. High-speed chemical imaging inside a microfluidic channel. Sens. Actuators B Chem. 2014, 194, 521–527. [Google Scholar] [CrossRef]
- Itoh, D.; Sassa, F.; Nishi, T.; Kani, Y.; Murata, M.; Suzuki, H. Droplet-based microfluidic sensing system for rapid fish freshness determination. Sens. Actuators B Chem. 2012, 171–172, 619–626. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.; Parak, W.J.; George, M.; Zhang, G. A novel design of multi-light LAPS based on digital compensation of frequency domain. Sens. Actuators B Chem. 2001, 73, 152–156. [Google Scholar]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, K.-I.; Sato, T.; Abe, M.; Wagner, T.; Schöning, M.J.; Yoshinobu, T. Light-Addressable Potentiometric Sensor as a Sensing Element in Plug-Based Microfluidic Devices. Micromachines 2016, 7, 111. https://doi.org/10.3390/mi7070111
Miyamoto K-I, Sato T, Abe M, Wagner T, Schöning MJ, Yoshinobu T. Light-Addressable Potentiometric Sensor as a Sensing Element in Plug-Based Microfluidic Devices. Micromachines. 2016; 7(7):111. https://doi.org/10.3390/mi7070111
Chicago/Turabian StyleMiyamoto, Ko-Ichiro, Takuya Sato, Minami Abe, Torsten Wagner, Michael J. Schöning, and Tatsuo Yoshinobu. 2016. "Light-Addressable Potentiometric Sensor as a Sensing Element in Plug-Based Microfluidic Devices" Micromachines 7, no. 7: 111. https://doi.org/10.3390/mi7070111