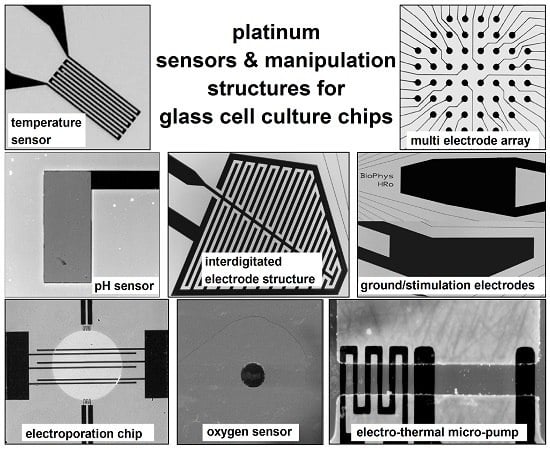
Cell Monitoring and Manipulation Systems (CMMSs) based on Glass Cell-Culture Chips (GC3s)
Abstract
:1. Introduction
2. Sensor and Manipulation Structures
2.1. Design and Fabrication of the Structures
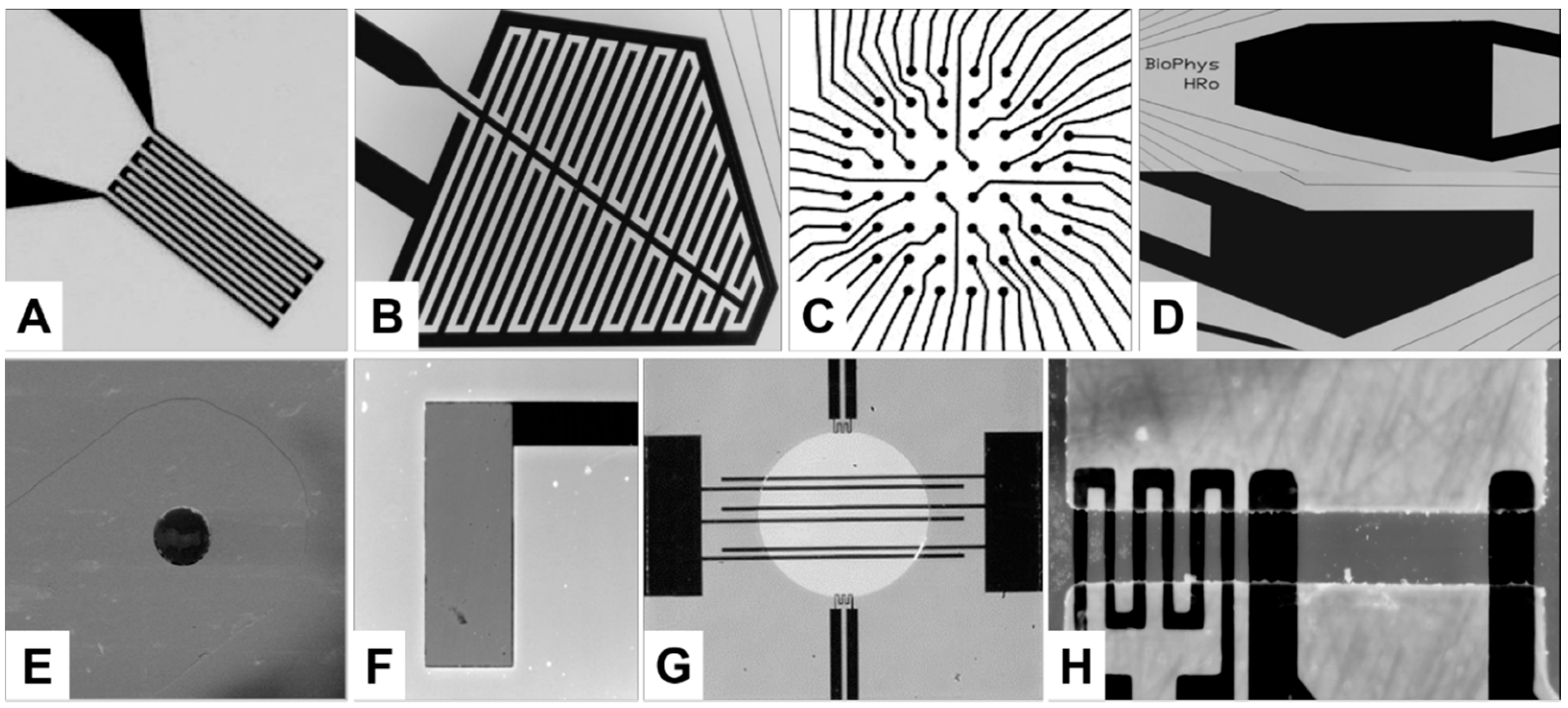
2.2. Temperature Sensors
2.3. Interdigitated Electrode Structure (IDES)
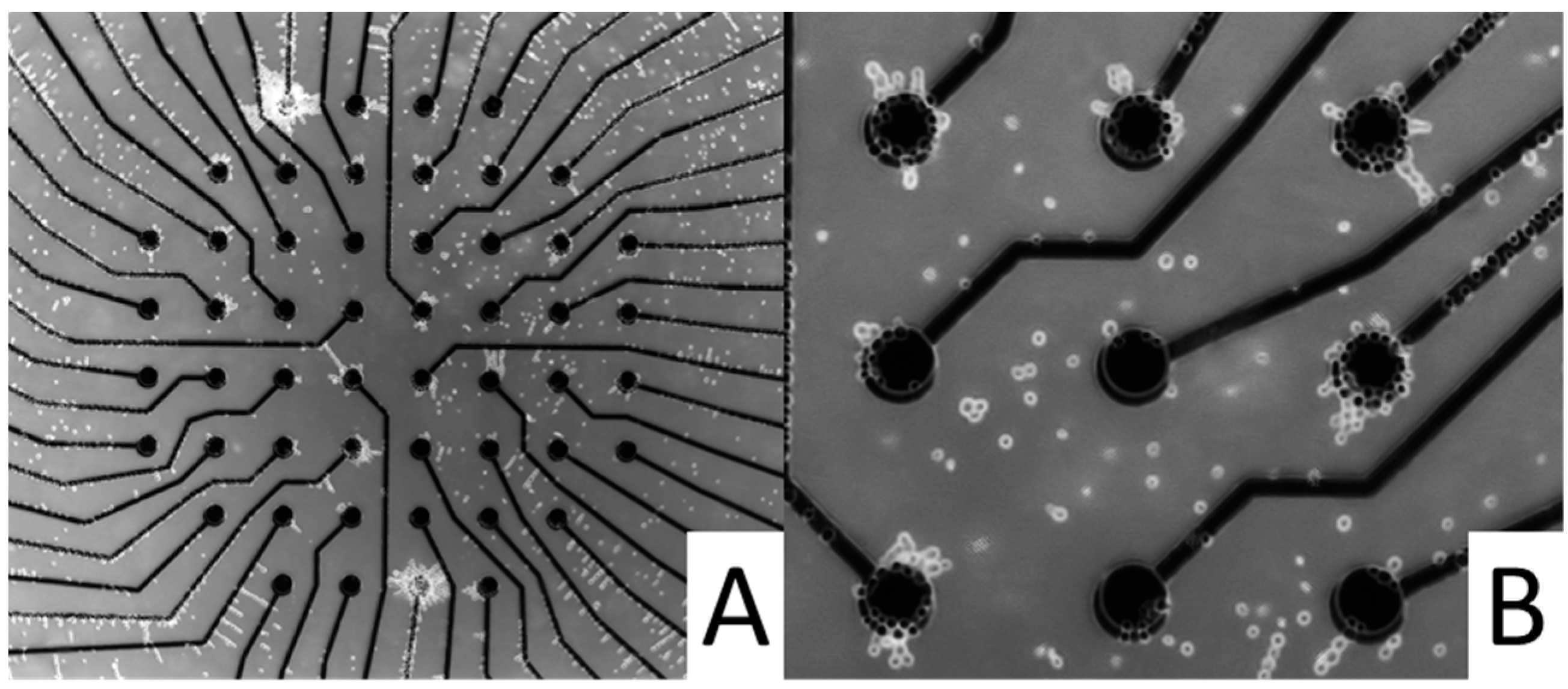
2.4. Multi-Electrode Array (MEA)
2.5. Stimulation and Ground Electrodes
2.6. Oxygen (O2) Sensor
2.7. pH Sensor
2.8. Electro-Poration Electrodes
2.9. Dielectrophoresis
2.10. Electro-Thermal Micro-Pumps (ETµPs)
3. Sensorized Glass Chips
3.1. Glass Neurochip (GNC)
3.2. Glass Metabolic Chip
4. Cell Culture
5. Results and Discussion
5.1. Temperature Sensors
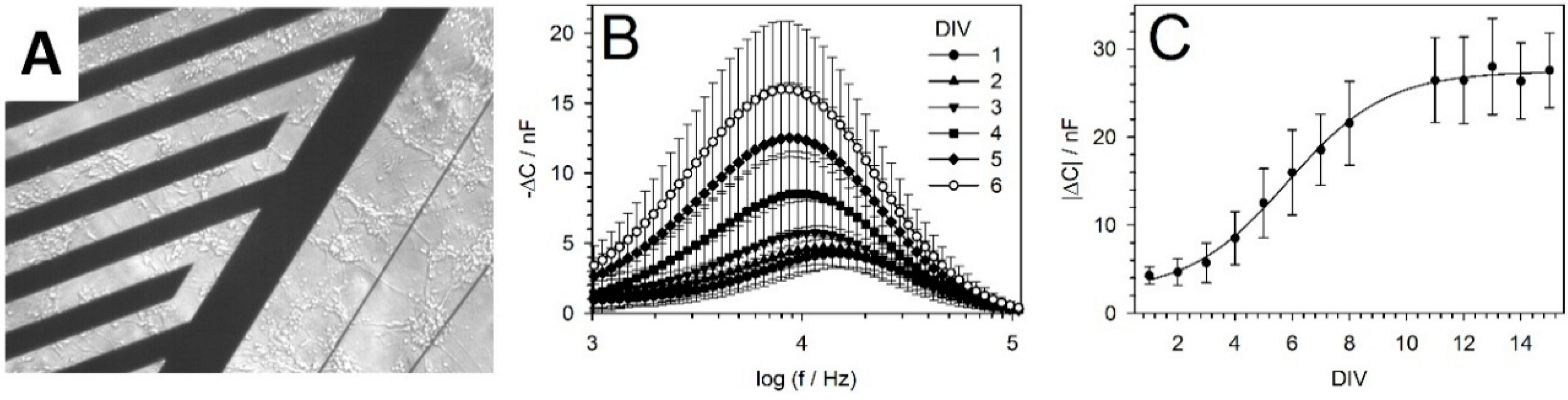
5.2. IDES Measurements
5.3. MEA: Cell Manipulation and Action-Potential Determination
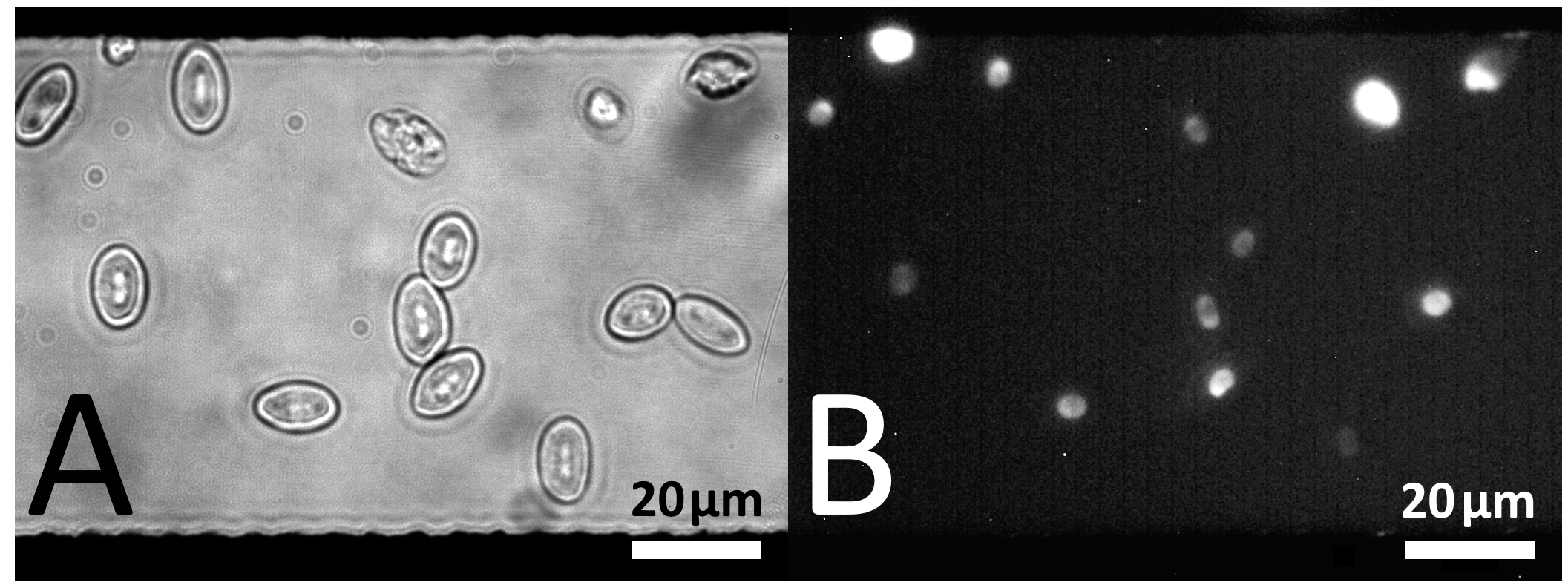
5.3.1. Dielectrophoretic Cell Positioning
5.3.2. Detection of Action Potentials
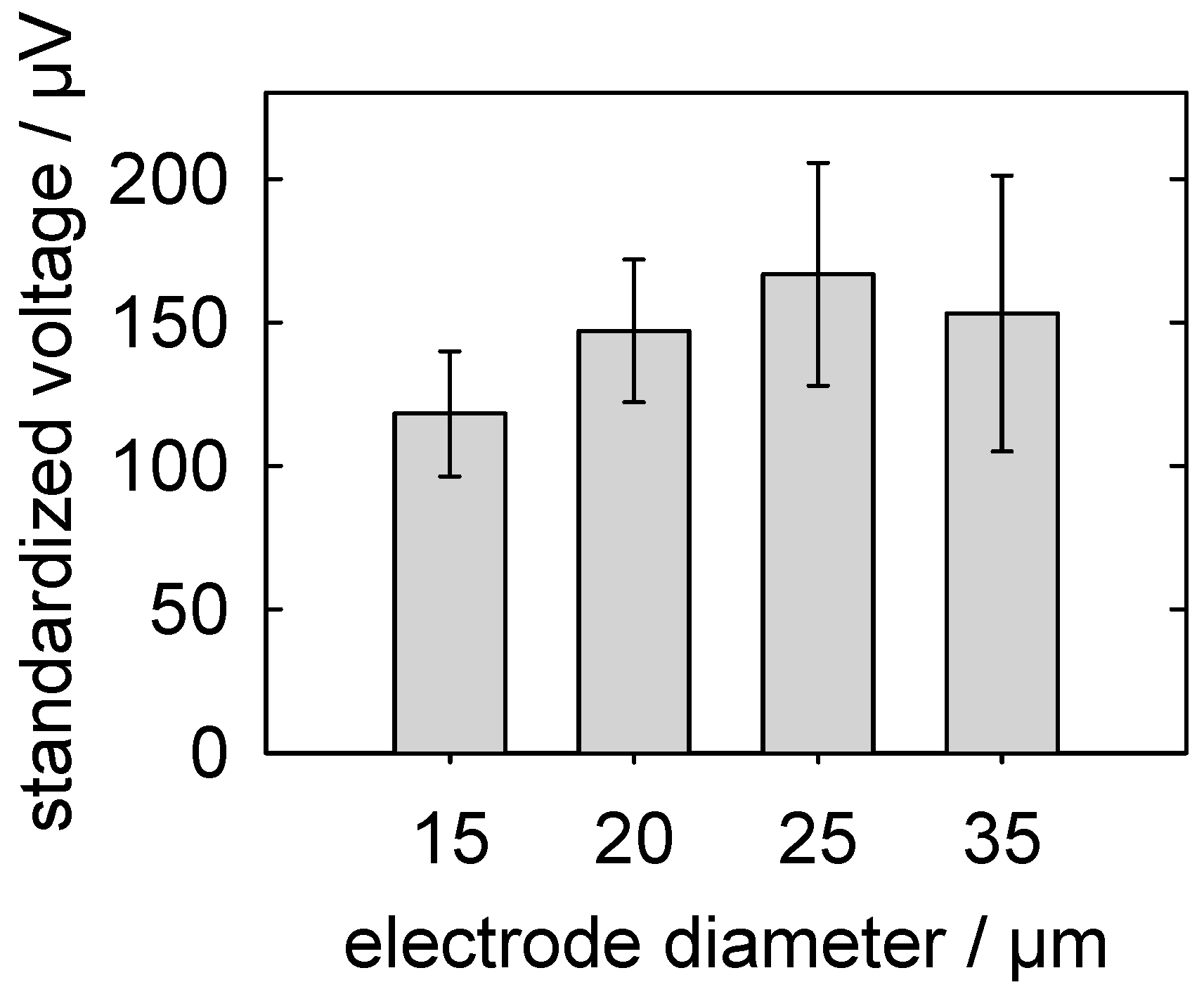
5.3.3. Detection of Action Potentials: Relationship between the MEA-Pad Diameter and the Signal Voltage
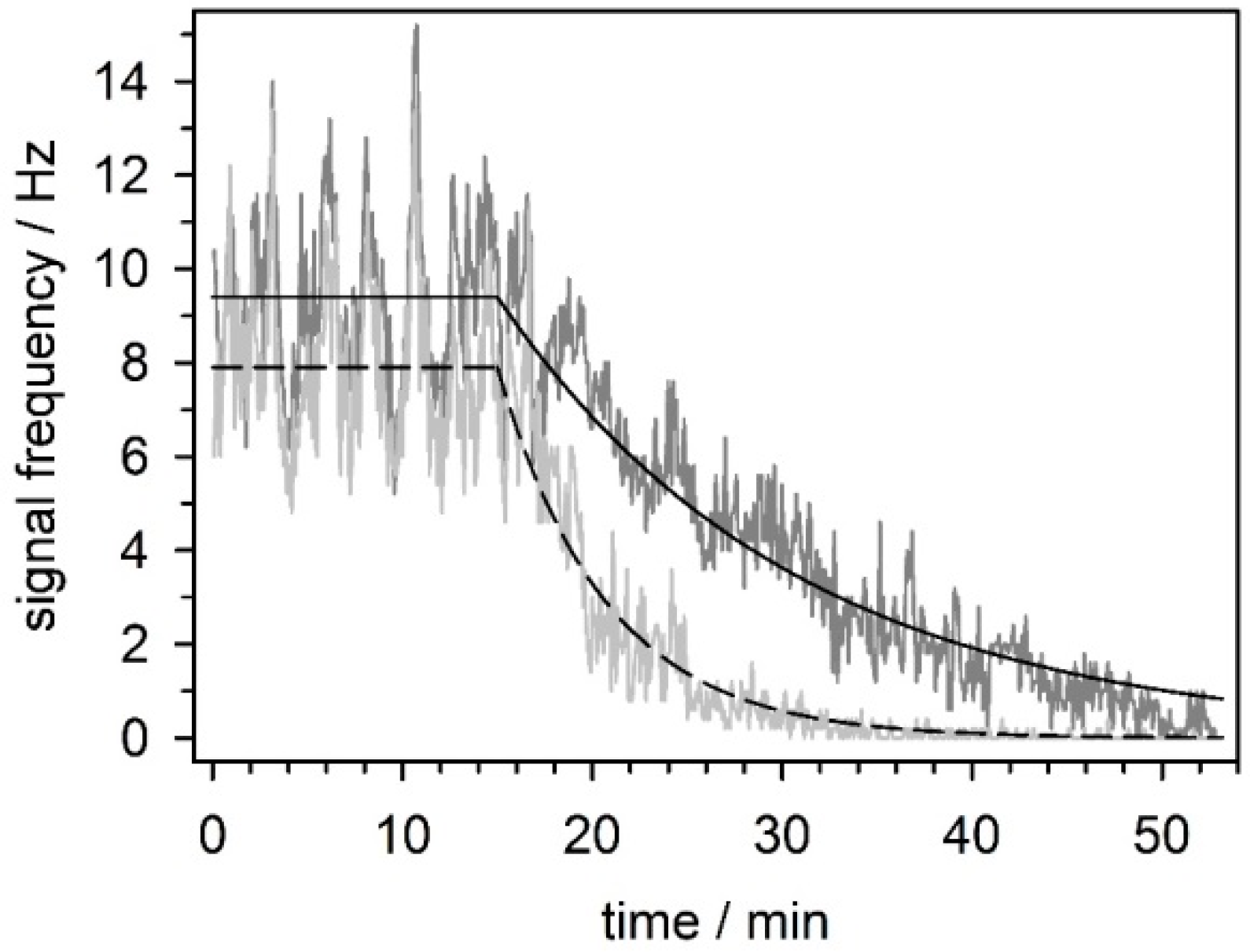
5.3.4. Effect of Mefloquine on Neuronal Network Activity
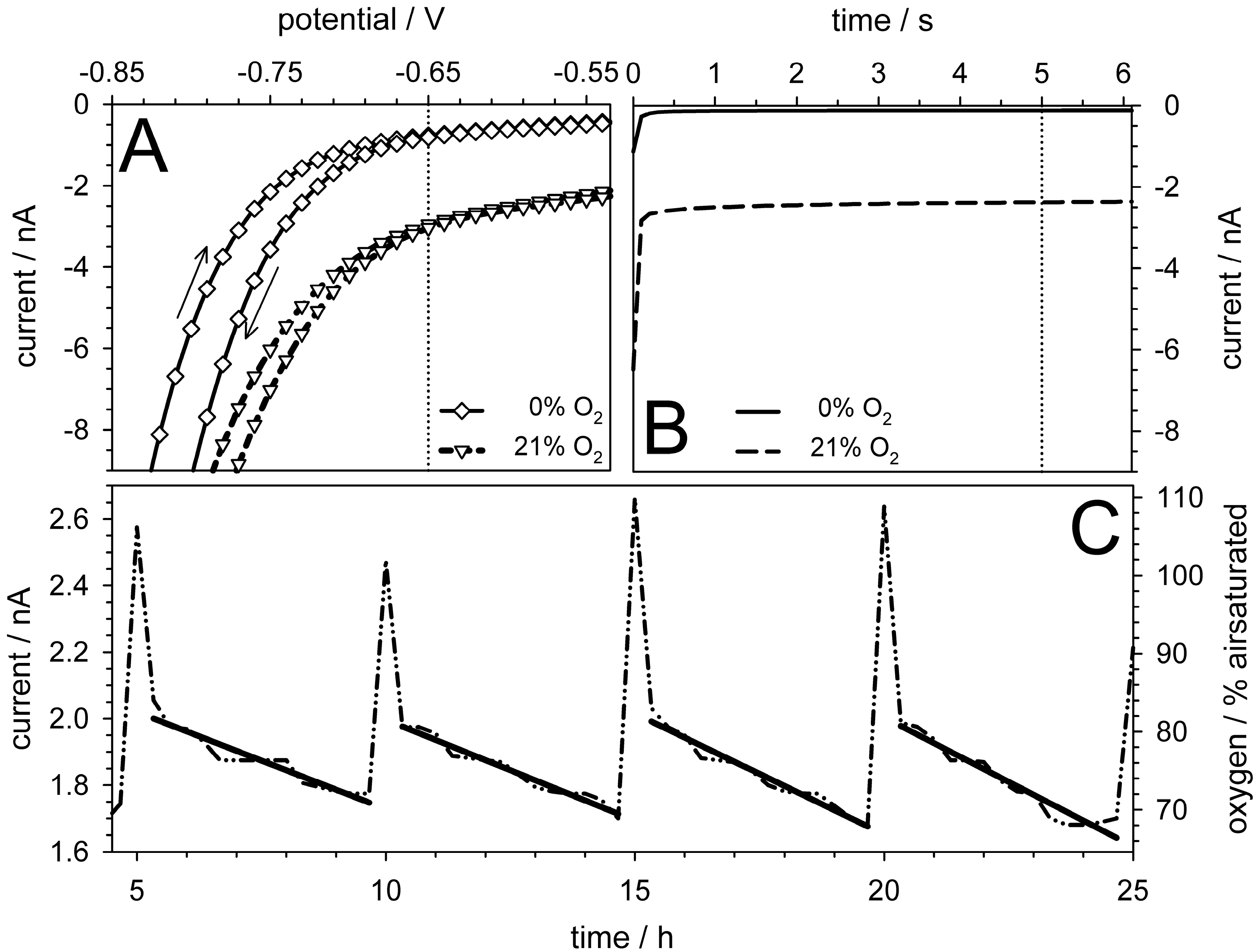
5.4. Oxygen Measurements
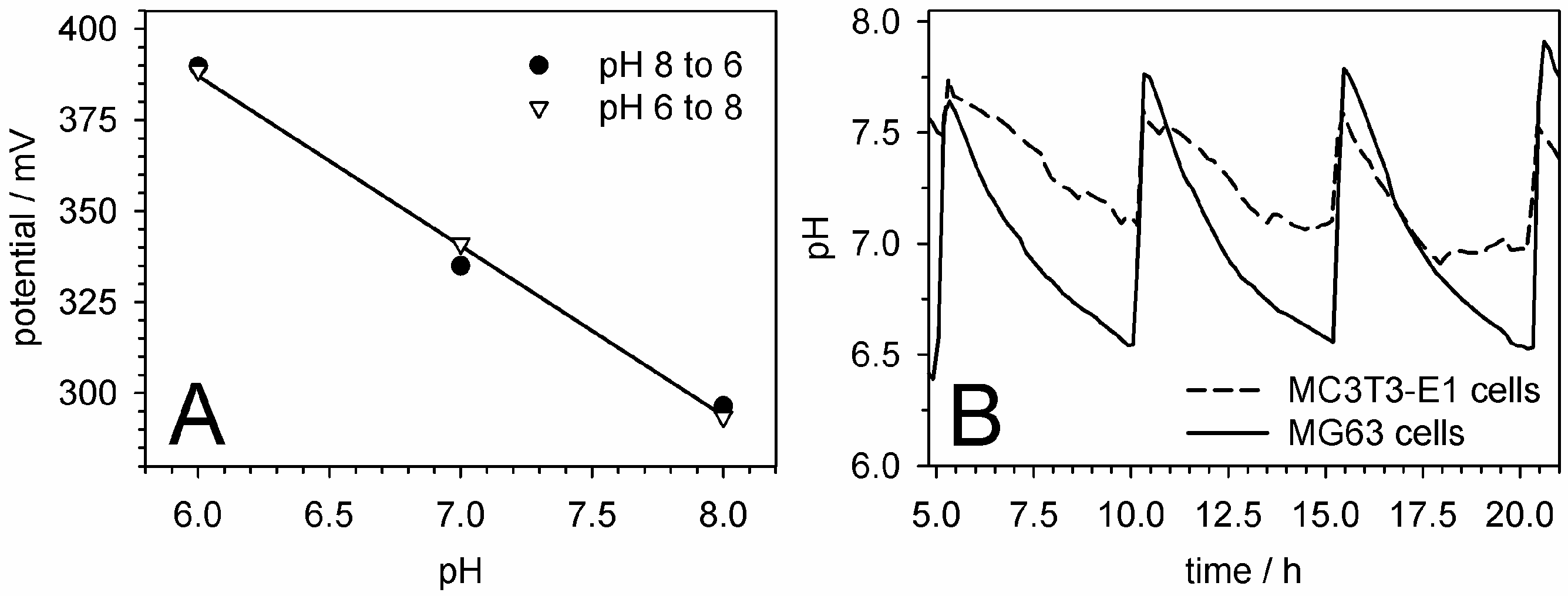
5.5. pH Measurements
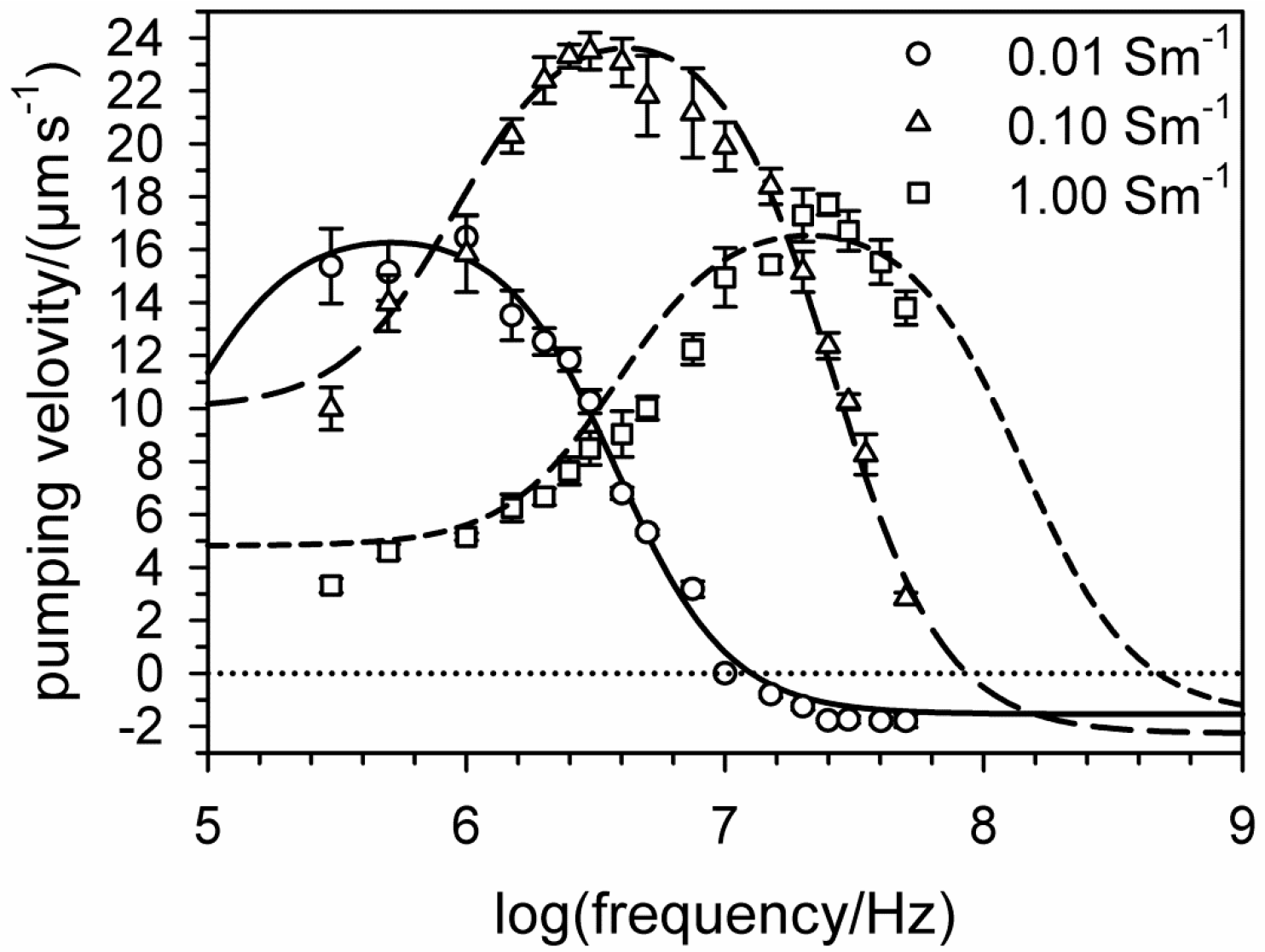
6. Electro-Thermal Micro Pumps (ETµPs)
7. Electro-Poration Electrodes
8. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| AC | Alternating current |
| ANOVA | Analysis of variance |
| CMF | Clausius-Mossotti factor |
| CMMS | Cell monitoring and manipulation systems |
| DC | Direct current |
| DEP | Dielectrophoresis |
| DIV | Days in vitro |
| DMEM | Dulbeccco’s modified Eagle’s medium |
| DSMZ | German Collection of Microorganisms and Cell Cultures |
| E16 | Embryonic day 16 |
| ETµP | Electro-thermal micro-pumps |
| GC3 | Glass cell-culture chip |
| GNC | Glass-neuro chip |
| IDES | Interdigitated electrode structure |
| ISFET | Ion-sensitive field-effect transistor |
| MEA | Multi-electrode array |
| PCR | Polymerase chain reaction |
| PDL | Poly-d-lysine |
| PDMS | Poly-dimethyl-siloxane |
| PNC | Murine embryonic primary neuronal cells |
| Pt | Platinum |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| Si3N4 | Silicon nitride |
References
- Bousse, L.; Mcreynolds, R.J.; Kirk, G.; Dawes, T.; Lam, P.; Bemiss, W.R.; Parce, J.W. Micromachined multichannel systems for the measurement of cellular-metabolism. Sens. Actuators B Chem. 1994, 20, 145–150. [Google Scholar] [CrossRef]
- Bousse, L.; Parce, J.W. Applying silicon micromachining to cellular-metabolism. IEEE Eng. Med. Biol. Mag. 1994, 13, 396–401. [Google Scholar] [CrossRef]
- Fuhr, G.; Mueller, T.; Schnelle, T.; Glasser, H.; Gimsa, J.; Hofmann, U.; Wagner, B. Handling and Investigation of Adherently Growing Cells and Viruses of Medical Relevance in Three-Dimensional Micro-Structures. In Proceedings of IEEE the Tenth Annual International Workshop on Micro Electro Mechanical Systems, Nagoya, Japan, 26–30 January 1997; pp. 344–349.
- Ehret, R.; Baumann, W.; Brischwein, M.; Schwinde, A.; Stegbauer, K.; Wolf, B. Monitoring of cellular behaviour by impedance measurements on interdigitated electrode structures. Biosens. Bioelectron. 1997, 12, 29–41. [Google Scholar] [CrossRef]
- Gimsa, J.; Stubbe, M.; Gimsa, U. A short tutorial contribution to impedance and AC-electrokinetic characterization and manipulation of cells and media: Are electric methods more versatile than acoustic and laser methods? J. Electr. Bioimp. 2014, 5, 74–91. [Google Scholar] [CrossRef]
- Baumann, W.H.; Schwinde, A.; Ehret, R.; Brischwein, M.; Wolf, B. Microelectronic sensor system for microphysiological application on living cells. Sens. Actuators B Chem. 1999, 55, 77–89. [Google Scholar] [CrossRef]
- Daridon, A.; Fascio, V.; Lichtenberg, J.; Wutrich, R.; Langen, H.; Verpoorte, E.; de Rooij, N.F. Multi-layer microfluidic glass chips for microanalytical applications. Fresenius J. Analyt. Chem. 2001, 371, 261–269. [Google Scholar] [CrossRef]
- Vilkner, T.; Janazek, D.; Manz, A. Micro total analysis systems. Recent developments. Anal. Chem. 2004, 76, 3373–3386. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Koester, P.J.; Buehler, S.M.; Stubbe, M.; Tautorat, C.; Niendorf, M.; Baumann, W.; Gimsa, J. Modular glass chip system measuring the electric activity and adhesion of neuronal cells-application and drug testing with sodium valproic acid. Lab Chip 2010, 10, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab Chip 2014, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Thedinga, E.; Kob, A.; Holst, H.; Keuer, A.; Drechsler, S.; Niendorf, R.; Baumann, W.; Freund, I.; Lehmann, M.; Ehret, R. Online monitoring of cell metabolism for studying pharmacodynamic effects. Toxicol. Appl. Pharmacol. 2007, 220, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Buehler, S.M.; Stubbe, M.; Gimsa, U.; Baumann, W.; Gimsa, J. A decrease of intracellular ATP is compensated by increased respiration and acidification at sub-lethal parathion concentrations in murine embryonic neuronal cells: Measurements in metabolic cell-culture chips. Toxicol. Lett. 2011, 207, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Baumann, W.; Gimsa, J. Population bursts of parvalbumin-positive interneurons inhibit spiking pyramidal cells in spontaneously active cortical in vitro networks. J. Chem. Chem. Eng. 2012, 6, 1033–1042. [Google Scholar]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. The Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Kaufmann, W. Current status of developmental neurotoxicity: an industry perspective. Toxicol. Lett. 2003, 140, 161–169. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Final Report of the OECD Workshop on Harmonization of Validation and Acceptance Criteria for Alternative Toxicological Test Methods; OECD: Paris, France, 1996. [Google Scholar]
- Giordano, G.; Costa, L.G. Developmental neurotoxicity: some old and new issues. ISRN Toxicol. 2012, 2012, 814795. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Health Effects Test Guidelines OPPTS 870.6300 Developmental Neurotoxicity Study; U.S. Environmental Protection Agency: Washington, DC, USA, 1998.
- Gross, G.W.; Rieske, E.; Kreutzberg, G.W.; Meyer, A. A New fixed-array multi-electrode system designed for long-term monitoring of extracellular single unit neuronal-activity in vitro. Neurosci. Lett. 1977, 6, 101–105. [Google Scholar] [CrossRef]
- Duwensee, H.; Mix, M.; Stubbe, M.; Gimsa, J.; Adler, M.; Flechsig, G.U. Electrochemical product detection of an asymmetric convective polymerase chain reaction. Biosens. Bioelectron. 2009, 25, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.C.; Stephen, C.; Jacobson, L.C.; Waters, N.K.; Khandurina, J.; Foote, R.S.; Justice, M.J.; Stubbs, L.J.; Ramsey, J.M. PCR-amplification and analysis of simple sequence-length polymorphisms in mouse DNA, using a single-microchip device. Anal. Biochem. 2000, 277, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Gradl, G.; Howitz, S.; Shirley, S.; Schnelle, T.; Fuhr, G. A 3-D microelectrode system for handling and caging single cells and particles. Biosens. Bioelectron. 1999, 14, 247–256. [Google Scholar] [CrossRef]
- Müller, T.; Pfennig, A.; Klein, P.; Gradl, G.; Jäger, M.; Schnelle, T. The potential of dielectrophoresis for single-cell experiments. IEEE Eng. Med. Biol. Mag. 2003, 22, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gimsa, J.; Müller, T.; Schnelle, T.; Fuhr, G. Dielectric spectroscopy of single human erythrocytes at physiological ionic strength: Dispersion of the cytoplasm. Biophys. J. 1996, 71, 495–506. [Google Scholar] [CrossRef]
- Gimsa, J. A comprehensive approach to electroorientation, electrodeformation, dielectrophoresis and electrorotation of ellipsoidal particles and biological cells. Bioelectrochemistry 2001, 54, 23–31. [Google Scholar] [CrossRef]
- Maswiwat, K.; Wachner, D.; Gimsa, J. Effects of cell orientation and electric field frequency on the transmembrane potential induced in ellipsoidal cells. Bioelectrochemistry 2008, 74, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Heida, T.; Rutten, W.L.C.; Marani, E. Dielectrophoretic trapping of dissociated fetal cortical rat neuron. IEEE Trans. Biomed. Eng. 2001, 48, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.J.; Li, J.; Sukumar, P.; Majeed, Y.; Dallas, M.L.; English, A.; Emery, P.; Porter, K.E.; Smith, A.M.; McFadzean, I.; et al. Robotic multiwall planar patch-clamp for native and primary mammalian cells. Nat. Protoc. 2009, 4, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Stoelzle, S.; Haythornthwaite, A.; Kettenhofen, R.; Kolossov, E.; Bohlen, H.; George, M.; Brüggemann, A.; Fertig, N. Automated patch clamp on mESC-derived cardiomyocytes for cardiotoxicity prediction. J. Biomol. Screen. 2011, 16, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Stoelzle, S.; Obergrussberger, A.; Brüggemann, A.; Haarmann, C.; George, M.; Kettenhofen, R.; Fertig, N. State-of-the-art automated patch clamp devices: heat activation, action potentials, and high throughput in ion channel screening. Front. Pharmacol. 2011, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Pine, J. Recording action-potential from cultured neurons with extracellular micro-circuit electrodes. J. Neurosci. 1980, 2, 19–31. [Google Scholar]
- Gross, G.W.; Williams, A.N.; Lucas, J.H. Recording of spontaneous activity with photoetched microelectrode from mouse spinal neurons in culture. J. Neurosci. 1982, 5, 13–22. [Google Scholar] [CrossRef]
- Keefer, E.W.; Gramowski, A.; Stenger, D.A.; Pancrazio, J.J.; Gross, G.W. Characterization of acute neurotoxic effects of trimethylolpropane phosphate via neuronal network biosensors. Biosens. Bioelectron. 2001, 16, 513–525. [Google Scholar] [CrossRef]
- Prasad, S.; Yang, M.; Zhang, X.; Ozkan, C.S.; Ozkan, M. Electrical field assisted patterning of neuronal networks for the study of brain functions. J. Biomed. Micro Dev. 2003, 5, 125–137. [Google Scholar] [CrossRef]
- Gramowski, A.; Juegelt, K.; Weiss, D.G.; Gross, G.W. Substance identification by quantitative characterization of oscillatory activity in murine spinal cord networks on microelectrode arrays. Eur. J. Neurosci. 2004, 19, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [PubMed]
- Matsuo, T.; Wise, K.P. An integrated field effect electrode for biopotential recording. IEEE Trans. Biomed. Eng. 1974, 6, 485–487. [Google Scholar] [CrossRef]
- Bousse, L.; Hafeman, D.; Tran, N. Time-dependence of the chemical response of silicon nitride surfaces. Sens. Actuators B Chem. 1990, 1, 361–367. [Google Scholar] [CrossRef]
- Stubbe, M.; Holtappels, M.; Gimsa, J. A new working principle for AC electro-hydrodynamic on-chip micro-pumps. J. Phys. D Appl. Phys. 2007, 40, 6850. [Google Scholar] [CrossRef]
- Stubbe, M.; Gimsa, J. A short review on AC electro-thermal micropumps based on smeared structural polarizations in the presence of a temperature gradient. Coll. Surf. A Physicochem. Eng. Asp. 2011, 376, 97–101. [Google Scholar] [CrossRef]
- Holtappels, M.; Stubbe, M.; Gimsa, J. AC-field-induced fluid pumping in microsystems with asymmetric temperature gradients. Phys. Rev. E 2009, 79, 026309. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, M.; Gyurova, A.; Gimsa, J. Experimental verification of an equivalent circuit for the characterization of electrothermal micropumps: High pumping velocities induced by the external inductance at driving voltages below 5 V. Electrophoresis 2013, 34, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.W.; Gimsa, J. Gradual changes of membrane properties at high transmembrane electric potential simulate a breakdown threshold. In Electricity and Magnetism in Biology and Medicine; Ferdinando, B., Ed.; Springer US: New York, NY, USA, 1993; pp. 135–137. [Google Scholar]
- Mir, L.M.; Orlowski, S. Mechanisms of electrochemotherapy. Adv. Drug Deliver. Rev. 1999, 35, 107–118. [Google Scholar] [CrossRef]
- Neumann, E. The relaxation hysteresis of membrane electroporation. In Electroporation and Electrofusion in Cell Biology; Neumann, E., Sowers, A.E., Jordan, C.A., Eds.; Plenum Press: New York, NY, USA, 1989; pp. 61–82. [Google Scholar]
- Pliquett, U. Joule heating during solid tissue electroporation. Med. Biol. Eng. Comput. 2003, 41, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Neon® Transfection System. Available online: https://www.thermofisher.com/cn/zh/home/life-science/cell-culture/transfection/transfection---selection-misc/neon-transfection-system.html (accessed on 26 April 2016).
- Sukhorukov, V.L.; Mussauer, H.; Zimmermann, U. The effect of electrical deformation forces on the electropermeabilization of erythrocyte membranes in low- and high-conductivity media. J. Membr. Biol. 1998, 163, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bonk, S.M.; Stubbe, M.; Bühler, S.M.; Tautorat, C.; Baumann, W.; Klinkenberg, E.D.; Gimsa, J. Design and characterization of a sensorized microfluidic cell-culture system with electro-thermal micro-pumps and sensors for cell adhesion, oxygen, and pH on a glass chip. Biosensors 2015, 5, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, G.; Gimsa, J.; Mueller, T.; Schnelle, T. Process and Device for Generating Resonance Phenomena in Particle Suspensions. U.S. Patent 6,056,861, 2 May 2000. [Google Scholar]
- Cruikshank, S.J.; Hopperstad, M.; Younger, M.; Connors, B.W.; Spray, D.C.; Srinivas, M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc. Natl. Acad. Sci. USA 2004, 33, 12364–12369. [Google Scholar] [CrossRef] [PubMed]
- Kinosita, K.; Tsong, T.Y. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim. Biophys. Acta. 1977, 471, 227–242. [Google Scholar] [CrossRef]
- Neumann, E.; Toensing, K.; Kakorin, S.; Budde, P.; Frey, J. Mechanism of electroporative dye uptake by mouse B cells. Biophys. J. 1998, 74, 98–108. [Google Scholar] [CrossRef]
- Chang, D.C.; Hunt, J.R.; Zheng, Q.; Gao, P.Q. Electroporation and electrofusion using a pulsed radio-frequency electric field. In Guide to Electroporation and Electrofusion; Chang, D.C., Chassy, B.M., Saunders, J.A., Sowers, A.E., Eds.; Academic Press: San Diego, CA, USA, 1992; pp. 303–326. [Google Scholar]
- Zimmermann, U.; Neil, G.A. Electromanipulation. of Cells; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Wolf, H.; Rols, M.P.; Boldt, E.; Neumann, E.; Teissie, J. Control by pulse parameters of electric field-mediated gene transfer in mammalian cells. Biophys. J. 1994, 66, 524–531. [Google Scholar] [CrossRef]
- Kotnik, T.; Pucihar, G.; Rebersek, M.; Miklavcic, D.; Mir, L.M. Role of pulse shape in cell membrane electropermeabilization. Biochim. Biophys. Acta 2003, 1614, 193–200. [Google Scholar] [CrossRef]
- Gimsa, J.; Donath, E.; Glaser, R. Evaluation of the data of simple cells by electrorotation using square-topped fields. Bioelectrochem. Bioenerg. 1988, 19, 389–396. [Google Scholar] [CrossRef]
- Marszalek, P.; Liu, D.S.; Tsong, T.Y. Schwan equation and transmembrane potential induced by alternating electric field. Biophys. J. 1990, 58, 1053–1058. [Google Scholar] [CrossRef]
- Gabriel, B.; Teissié, J. Time courses of mammalian cell electropermeabilization observed by millisecond imaging of membrane property changes during the pulse. Biophys. J. 1999, 76, 2158–2165. [Google Scholar] [CrossRef]
- Maswiwat, K.; Wachner, D.; Warnke, R.; Gimsa, J. Simplified equations for the transmembrane potential induced in ellipsoidal cells of rotational symmetry. J. Phys. D Appl. Phys. 2007, 40, 914–923. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Sukharev, S.I.; Popov, S.V.; Pastushenko, V.F.; Sokirko, A.V.; Abidor, I.G.; Chizmadzhev, Y.A. The electrical breakdown of cell and lipide membranes: the similarity of phenomenologies. Biochim. Biophys. Acta 1987, 902, 360–373. [Google Scholar] [CrossRef]
- Glaser, R.W.; Leikin, S.L.; Chernomordik, L.V.; Pastushenko, V.F.; Sokirko, A.I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim. Biophys. Acta 1988, 940, 275–287. [Google Scholar] [CrossRef]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Xie, T.D.; Tsong, T.Y. Study of mechanisms of electric field-induced DNA transfection. III. Electric parameters and other conditions for effective transfection. Biophys. J. 1992, 63, 28–34. [Google Scholar] [CrossRef]
- Shirakashi, R.; Yasui, T.; Memmel, S.; Sukhorukov, V.L. Electro-microinjection of fish eggs with an immobile capillary electrode. Biomicrofluidics 2015, 9, 064109. [Google Scholar] [CrossRef] [PubMed]
- Cukjati, D.; Batiuskaite, D.; Andre, F.; Miklavcic, D.; Mir, L.M. Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry 2007, 70, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, A.; Mir, L.M.; Gehl, J. Electrochemotherapy: Results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat. Rev. 2003, 29, 371–387. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Katsuki, S.; Stark, R.H.; Buescher, E.S.; Beebe, S.J. Bioelectrics-new applications for pulsed power technology. IEEE Trans. Plasma Sci. 2002, 30, 293–300. [Google Scholar] [CrossRef]
- Smith, K.C.; Neu, J.C.; Krassowska, W. Model of creation and evolution of stable electropores for DNA delivery. Biophys. J. 2004, 86, 2813–2826. [Google Scholar] [CrossRef]
- Sun, D.; Guo, T.; Ran, Y.; Huang, Y.; Guan, B.O. In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 2014, 61, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Schmidt, M.A.; Jensen, K.F. A microfluidic electroporation device for cell lysis. Lab Chip 2005, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Available online: http://ec.europa.eu/environment/chemicals/reach/reach_en.htm (accessed on 12 May 2016).
- Van de Waterbeemd, H.; Gifford, E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Bonk, S.M.; Oldorf, P.; Peters, R.; Baumann, W.; Gimsa, J. Fast prototyping of sensorized cell culture chips and microfluidic systems with ultrashort laser pulses. Micromachines 2015, 6, 364–374. [Google Scholar] [CrossRef]
- Visan, A.; Hayess, K.; Sittner, D.; Pohl, E.E.; Riebeling, C.; Slawik, B.; Gulich, K.; Oelgeschläger, M.; Luch, A.; Seiler, A.E.M. Differentiation of mouse embryonic stem cells as a tool to assess developmental neurotoxicity in vitro. Neurotoxicology 2012, 33, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.; Buehler, S.M.; Stubbe, M.; Gimsa, J. Neuronal in vitro activity is more sensitive to valproate than intracellular ATP: Considerations on conversion problems of IC50 in vitro data for animal replacement. Biosystems 2016, 144, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Philippi, N.; Walter, D.; Schlatter, R.; Ferreira, K.; Ederer, M.; Sawodny, O.; Timmer, J.; Borner, C.; Dandekar, T. Modeling system states in liver cells: Survival, apoptosis and their modifications in response to viral infection. BMC Syst. Biol. 2009, 3, 97. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buehler, S.M.; Stubbe, M.; Bonk, S.M.; Nissen, M.; Titipornpun, K.; Klinkenberg, E.-D.; Baumann, W.; Gimsa, J. Cell Monitoring and Manipulation Systems (CMMSs) based on Glass Cell-Culture Chips (GC3s). Micromachines 2016, 7, 106. https://doi.org/10.3390/mi7070106
Buehler SM, Stubbe M, Bonk SM, Nissen M, Titipornpun K, Klinkenberg E-D, Baumann W, Gimsa J. Cell Monitoring and Manipulation Systems (CMMSs) based on Glass Cell-Culture Chips (GC3s). Micromachines. 2016; 7(7):106. https://doi.org/10.3390/mi7070106
Chicago/Turabian StyleBuehler, Sebastian M., Marco Stubbe, Sebastian M. Bonk, Matthias Nissen, Kanokkan Titipornpun, Ernst-Dieter Klinkenberg, Werner Baumann, and Jan Gimsa. 2016. "Cell Monitoring and Manipulation Systems (CMMSs) based on Glass Cell-Culture Chips (GC3s)" Micromachines 7, no. 7: 106. https://doi.org/10.3390/mi7070106