Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis
Abstract
:1. Introduction
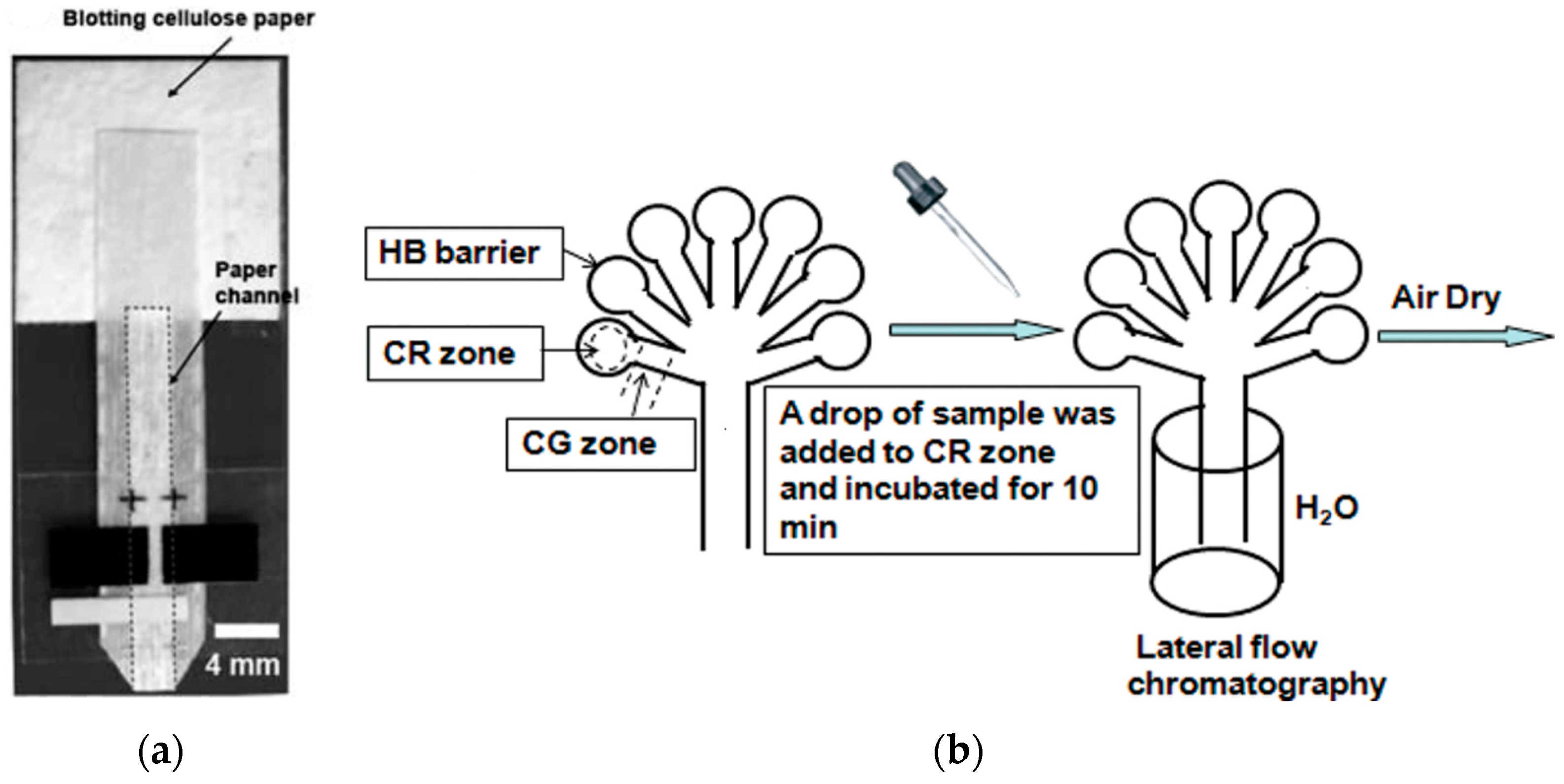
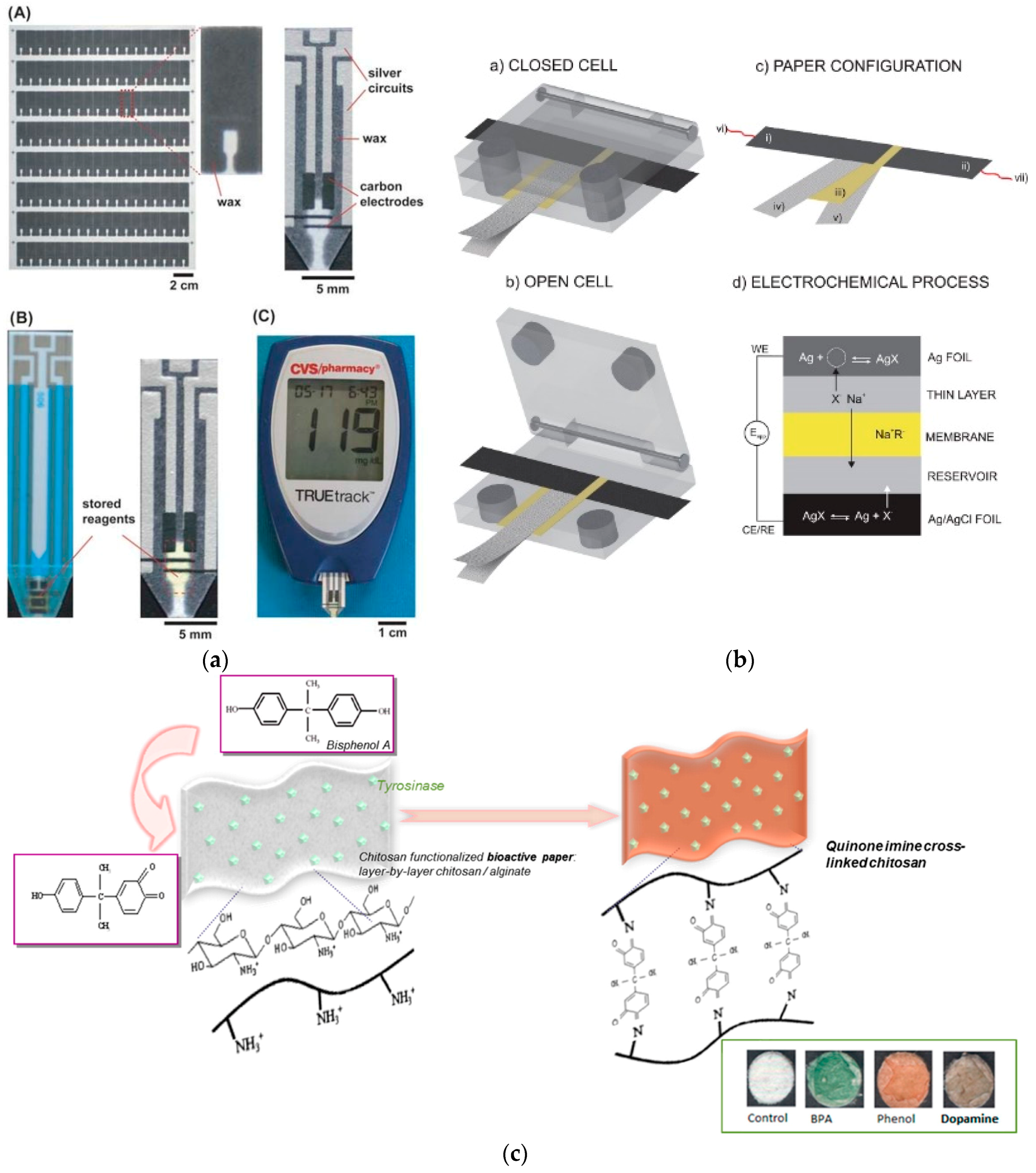
2. Paper in Microfluidics
3. Applications to Food and Water Contamination
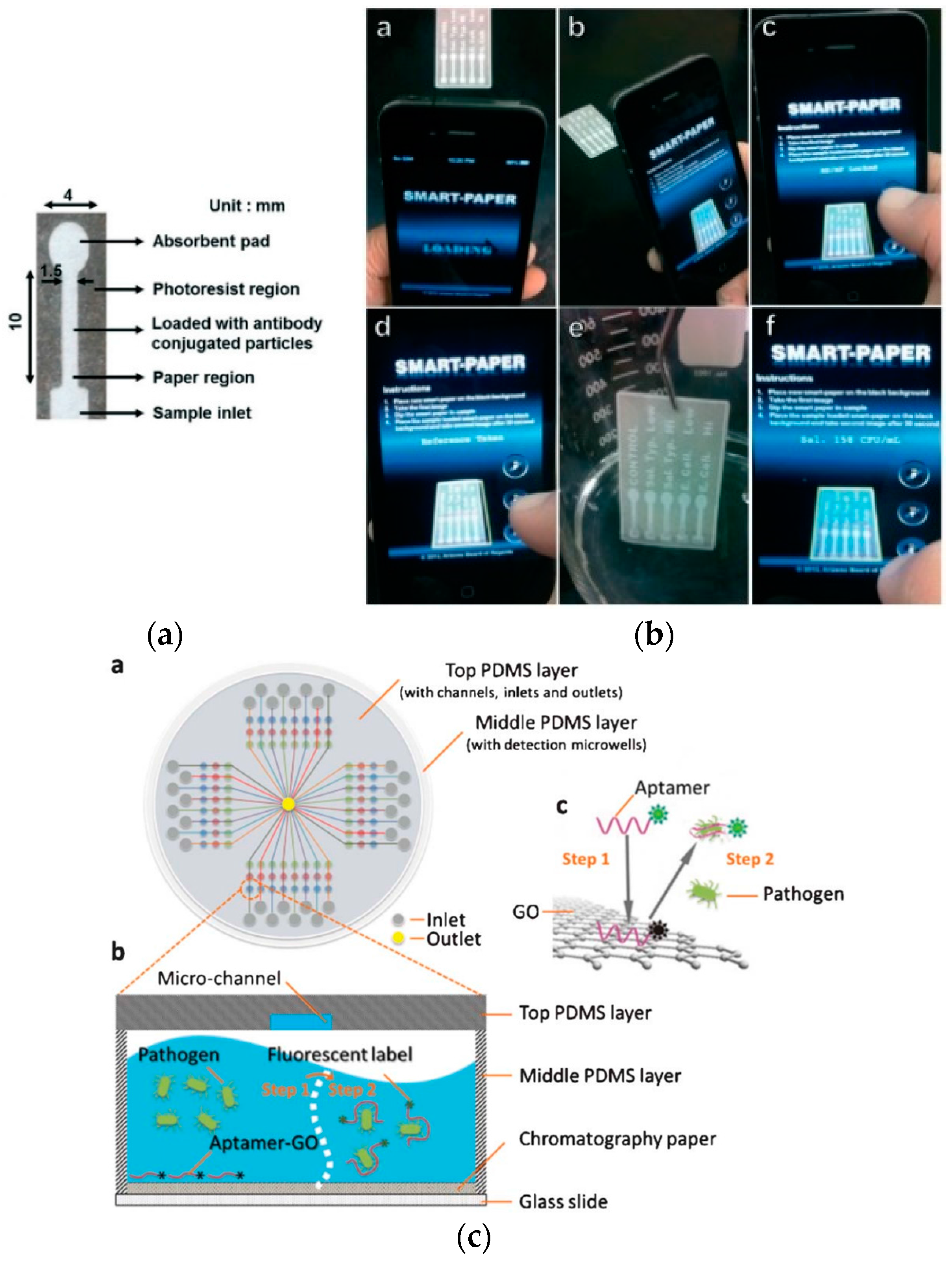
3.1. Detection of Foodborne and Waterborne Pathogens
3.2. Detection of Pesticides and Herbicides
3.3. Detection of Food Additives
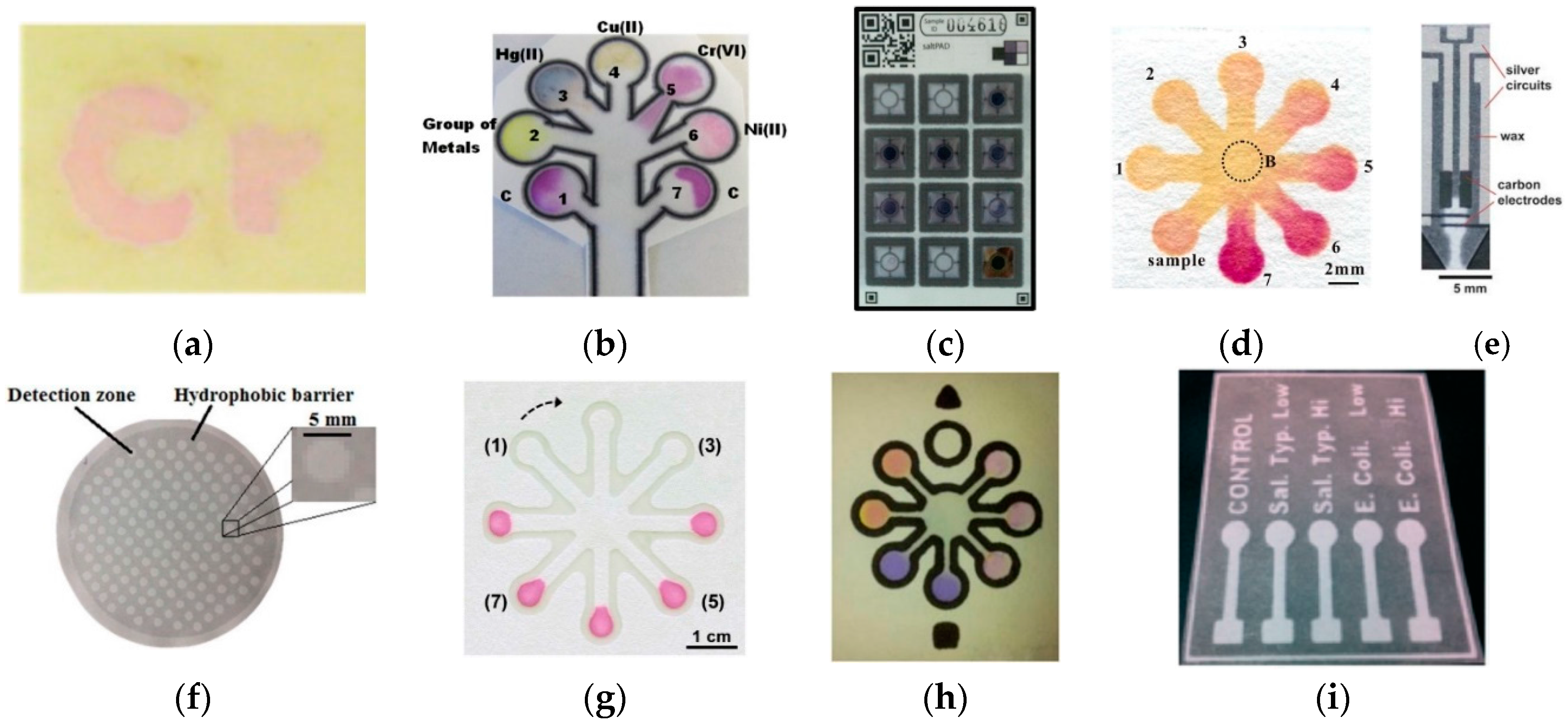
3.4. Detection of Heavy Metals
3.5. Detection of Other Food and Water Contaminants
4. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| Ach | acetylcholinesterase |
| AgNP | silver nanoparticle |
| AgNPl | silver nanoplate |
| ATP | adenosine triphosphate |
| B[a]P | benzo[a]pyrene |
| B-GAL | β-galactosidase |
| BPA | bisphenol A |
| CFU | colony-forming unit |
| CL | chemiluminescence |
| CMYK | cyan-magenta-yellow-key |
| CPRG | chlorophenol red β-galactopyranoside |
| DDV | dichlorvos |
| E. coli | Escherichia coli |
| ECL | electrochemiluminescence |
| FL | fluorescence |
| GO | graphene oxide |
| HRP | horseradish peroxidase |
| L. monocytogenes | Listeria monocytogenes |
| LOD | limit of detection |
| MCE | mixed cellulose esters |
| MC-LR | microcystin-LR |
| MIP | molecularly imprinted polymer |
| NEO | neomycin |
| PCP | pentachlorophenol |
| PDMS | poly(dimethylsiloxane) |
| PEC | photoelectrochemical detection |
| PIM | polymer inclusion membrane |
| Ppy | polypyrrole |
| PS-MS | paper spray mass spectrometry |
| Qdots | quantum dots |
| S. aureus | Staphylococcus aureus |
| S. enterica | Salmonella enterica |
| S. Typhimurium | Salmonella Typhimurium |
| SERS | surface-enhanced Raman spectroscopy |
| SWASV | square wave anodic stripping voltammetry |
| μPAD | microfluidic paper-based analytical device |
Appendix A
| Target | μPAD Wall Fabrication Method | Paper Substrate | Detection Method | Linear Detection Range | LOD | Real Sample Application | Reference |
|---|---|---|---|---|---|---|---|
| Pathogens | |||||||
| E. coli O157:H7, Salmonella Typhimurium, L. monocytogenes | Wax printing | Whatman No. 1 filter paper | Colorimetric | - | 106 CFU mL−1, 104 CFU mL−1, 108 CFU mL−1 | Bologna | [ 32] |
| Salmonella | Wax printing | Whatman No. 1 chromatography paper | CL | - | 2.6 × 107 CFU mL−1 | - | [ 33] |
| S. Typhimurium | Photolithography | Chromatography paper | Optical (Mie scattering) | 102–105 CFU mL−1* | 102 CFU mL−1 | - | [ 46] |
| S. aureus, S. enterica | Cutting by punching (PDMS/paper/glass hybrid) | Whatman chromatography paper | FL | 104–106 CFU mL−1, 42.2–675.0 CFU mL−1 | 800.0 CFU mL−1, 61.0 CFU mL−1 | - | [ 60] |
| E. coli | - | Millipore MCE membrane filter | Colorimetric and bioluminescence | - | 4 CFU mL−1 | - | [ 57] |
| E. coli | Wax pencil drawing and PDMS screen printing | Whatman No. 1 filter paper | Colorimetric | - | 57 CFU mL−1 | Drinking water | [ 30] |
| Pesticides and Herbicides | |||||||
| 2,4-D | - | Whatman No. 1 chromatography paper | CL | - | 1.0 pM | Tap water, lake water | [ 49] |
| Paraoxon, Malathion | Wax printing | Whatman No. 1 filter paper | Colorimetric | 1 × 10−8–ca. 1 × 10−6 M | 10 nM | - | [ 34] |
| Methyl-paraoxon, Chlorpyrifos-oxon | Polymer screen-printing | Whatman No. 4 filter paper | Colorimetric | 0–0.1 μg·mL−1, 0–60 ng·mL−1 | 18 ng·mL−1, 5.3 ng·mL−1 | For methyl-paraoxon: cabbage, dried green mussel | [ 42] |
| Dichlorvos | Cutting | Whatman 3MM Chr chromatography paper | CL | 10 ng·mL−1–1.0 μg·mL−1 | 3.6 ng·mL−1 | Cucumber, tomato, cabbage | [ 20] |
| Dichlorvos | Cutting | Whatman 3MM Chr chromatography paper | CL | 3.0 ng·mL−1–1.0 μg·mL−1 | 0.8 ng·mL−1 | Cabbage, tomato | [ 21] |
| Methomyl, Profenofos | Cutting | Canson paper | Colorimetric | - | 6.16 × 10−4 mM, 0.27 mM | - | [ 58] |
| PCP | Wax screen-printing | Whatman No. 1 chromatography paper | PEC | 0.01–100 ng·mL−1 | 4 pg·mL−1 | - | [ 50] |
| Methyl viologen (paraquat) | Cutting | Whatman filter paper | FL | 0.39 μmol·L−1–3.89 μmol·L−1 | 0.16 μmol·L−1 | - | [ 67] |
| Food Additives | |||||||
| Glucose | Cutting by punching | Whatman No. 1 filter paper | Electrochemical | 1–5 mM | 0.18 mM | Commercial soda beverages | [ 51] |
| Glucose, Fructose, Sucrose | Wax printing | Whatman No. 1 filter paper | Electrochemical | - | 270 nM, 340 nM, 430 nM | Coca-Cola™, Orange Powerade™, Strawberry Lemonade Powerade™, Red Bull™, Vitamin Water™ | [ 35] |
| Glucose | Paraffin stamping | Whatman grade 1 paper | PS-MS | 1–500 μmol·L−1 | 2.77 μmol·L−1 | Liquors | [ 43] |
| Sunset yellow, Lemon yellow | Cutting | Filter paper | SERS | - | 10−5 M, 10−4 M | Grape juice, orange juice | [ 22] |
| Nitrite | Paraffin stamping | JProLab JP 40 filter paper | Colorimetric | 0–100 μM | 5.6 μM | Ham, sausage, preservative water | [ 44] |
| Nitrite | Alkylsilane assembling and UV-lithography | Whatman No. 1 filter paper | Colorimetric | 0.156–2.50 mM | - | Processed red cubilose | [ 52] |
| Nitrite | Indelible ink contact stamping | Whatman No. 1 filter paper | Colorimetric | - | 0.52 mg·L−1 | - | [ 45] |
| Nitrite, Nitrate | Inkjet printing | Whatman No. 1 and No.4 filter papers | Colorimetric | 10–150 μM, 50–1000 μM | 1.0 μM, 19 μM | Tap water, mineral water, pond water | [ 53] |
| Metals | |||||||
| Pb(II) | Photolithography | Whatman No. 1 chromatography paper | Electrochemical | 0–100 ppb | 1.0 ppb | - | [ 47] |
| Hg(II), Cu(II), Cr(VI), Ni(II) | Wax printing | Whatman No. 1 paper | Colorimetric | - | ~0.5–1 ppm | - | [ 31] |
| Pb(II), Cd(II) | Cutting | Whatman No. 1 filter paper | Electrochemical | 10–100 ppb | 2.0 ppb, 2.3 ppb | Carbonated electrolyte drinks | [ 54] |
| As(III) | Cutting | Whatman filter paper | Colorimetric | - | 1.0 ppb | - | [ 23] |
| Pb(II), Cu(II) | Cutting | Whatman filter paper | Colorimetric | - | ≤10.0 ppb for both | - | [ 24] |
| Cu(II) | Cutting | Whatman No. 1 filter paper | Colorimetric | 7.8–62.8 μM | 7.8 nM or 0.5 μg·L−1 | Drinking water | [ 25] |
| Cu(II) | Wax printing | Whatman No. 1 filter paper | Colorimetric | 0.5–200 ng·mL−1 | 0.3 ng·mL−1 | Drinking water, ground water, tomato, rice | [ 36] |
| Cu(II) | Inkjet printing | Whatman No. 4 filter paper | Colorimetric | 0.1–30.0 mg·L−1 | 0.6 mg·L−1 | Hot tap water | [ 55] |
| Hg(II) | Wax screen printing | Whatman No. 1 filter paper | Colorimetric | 5–75 ppm | 0.12 ppm | Commercial bottled drinking water, tap water | [ 41] |
| Hg(II), Ag(I), NEO | Wax printing | Whatman No. 1 chromatography paper | FL | 0–3 μM, 0–1.75 μM, 0–2 μM | 121 nM, 47 nM, 153 nM | - | [ 37] |
| Cu(II), Cr(VI), Ni(II) | Wax patterning | Whatman No. 1 chromatography paper | Colorimetric | - | ≥0.8 mg·L−1, >0.5 mg·L−1, ≥0.5 mg·L−1 | Tap water | [ 28] |
| Fe | Photolithography | Advantec No. 51B chromatography paper | Colorimetric | 8.9–89 μM | 3.96 μM | Tap water, river water | [ 48] |
| Others | |||||||
| Ethanol | Wax printing | Whatman No. 1 chromatography paper | Electrochemical | 0.1–3 mM | 0.1 mM | Water | [ 38] |
| Phenol, Bisphenol A, Dopamine, Catechol, m-Cresol p-Cresol | Cutting by hole punching | Fisherbrand P5 filter paper | Colorimetric | 1–400 μg·L−1, 1–200 μg·L−1, 1–300 μg·L−1, 1–300 μg·L−1, 1–500 μg·L−1, 1–200 μg·L−1 | 0.86 (±0.102) μg·L−1 for each of the phenolic compounds | Tap water, river water | [ 59] |
| Bromide, Iodide, Chloride | - | Whatman RC60 regenerated cellulose membrane filter | Electrochemical | 10−4.8–0.1 M for bromide and iodide, 10−4.8–0.6 M for chloride | 10−5 M | Food supplement, seawater, mineral water, tap water, river water | [ 56] |
| Iodate | Wax printing | Ahlstrom 319 paper | Colorimetric | 0.8–15 ppm iodine atoms from iodate | 0.8 ppm iodine atoms | Iodized salt | [ 39] |
| MC-LR | Wax printing | Whatman No. 1 chromatography paper | Electrochemical | 0.01–200 μg·mL−1 | 0.004 μg·mL−1 | - | [ 40] |
| B[a]P | Wax patterning and screen printing | Whatman No. 1 filter paper | ECL | 0.15–12.5 μM | ~150 nM | Chicken skin | [ 29] |
References
- Hamburg, M.A. Advancing regulatory science. Science 2011, 331, 987. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A. Lights and shadows on food microfluidics. Lab Chip 2014, 14, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.F.; Kawaguchi, S.; Kamaya, A.; Ohshita, M.; Kabasawa, K.; Iwama, K.; Taniguchi, K.; Tsuda, S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 519, 103–119. [Google Scholar] [CrossRef]
- International Agency for Research in Cancer (IARC). 2-(2-Furyl)-3-(5-nitro-2-fyryl)acrylamide (AF-2). In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Some Food Additives, Feed Additives and Naturally Occurring Substances; World Health Organization: Lyon, France, 1983; Volume 31, p. 41. [Google Scholar]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2014, 19, 689–705. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Lüdi, H.; Widmer, H.M. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems. J. Chromatogr. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Ruano, J.M.; Benoit, V.; Aitchison, J.S.; Cooper, J.M. Flame hydrolysis deposition of glass on silicon for the integration of optical and microfluidic devices. Anal. Chem. 2000, 72, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Queste, S.; Salut, R.; Clatot, S.; Rauch, J.-Y.; Khan Malek, C.G. Manufacture of microfluidic glass chips by deep plasma etching, femtosecond laser ablation, and anodic bonding. Microsyst. Technol. 2010, 16, 1485–1493. [Google Scholar] [CrossRef]
- Harris, N.R.; Hill, M.; Beeby, S.; Shen, Y.; White, N.M.; Hawkes, J.J.; Coakley, W.T. A silicon microfluidic ultrasonic separator. Sens. Actuators B Chem. 2003, 95, 425–434. [Google Scholar] [CrossRef]
- Sanjoh, A.; Tsukihara, T. Spatiotemporal protein crystal growth studies using microfluidic silicon devices. J. Cryst. Growth 1999, 196, 691–702. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Sakai, Y.; Fujii, T. Microfluidic PDMS (polydimethylsiloxane) bioreactor for large-scale culture of hepatocytes. Biotechnol. Prog. 2004, 20, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R. Bioactive paper provides a low-cost platform for diagnostics. TrAC Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Maeki, M.; Mohamadi, R.M.; Ishida, A.; Tani, H.; Tokeshi, M. An instrument-free, screen-printed paper microfluidic device that enables bio and chemical sensing. Analyst 2015, 140, 6493–6499. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kou, J.; Xing, H.; Li, B. Paper-based chromatographic chemiluminescence chip for the detection of dichlorvos in vegetables. Biosens. Bioelectron. 2014, 52, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, Y.; Luo, J.; Kou, J.; Zheng, H.; Li, B.; Zhang, Z. A molecularly imprinted polymer based a lab-on-paper chemiluminescence device for the detection of dichlorvos. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 141, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, L.; Yang, L. Designing of the functional paper-based surface-enhanced Raman spectroscopy substrates for colorants detection. Mater. Res. Bull. 2015, 63, 199–204. [Google Scholar] [CrossRef]
- Nath, P.; Arun, R.K.; Chanda, N. A paper based microfluidic device for the detection of arsenic using a gold nanosensor. RSC Adv. 2014, 4, 59558–59561. [Google Scholar] [CrossRef]
- Nath, P.; Arun, R.K.; Chanda, N. Smart gold nanosensor for easy sensing of lead and copper ions in solution and using paper strips. RSC Adv. 2015, 5, 69024–69031. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Simple silver nanoparticle colorimetric sensing for copper by paper-based devices. Talanta 2012, 99, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.Z.; Luckham, R.E.; Smith, A.M.; Lebert, J.M.; Davies, L.M.; Pelton, R.H.; Filipe, C.D.M.; Brennan, J.D. Development of a bioactive paper sensor for detection of neurotoxins using piezoelectric inkjet printing of sol-gel-derived bioinks. Anal. Chem. 2009, 81, 5474–5483. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.Z.; Luckham, R.E.; McFadden, M.J.; Brennan, J.D. Reagentless bidirectional lateral flow bioactive paper sensors for detection of pesticides in beverage and food samples. Anal. Chem. 2009, 81, 9055–9064. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, R.; Nilghaz, A.; Guan, L.; Zhang, X.; Shen, W. “Periodic-table-style” paper device for monitoring heavy metals in water. Anal. Chem. 2015, 87, 2555–2559. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Kadimisetty, K.; Malla, S.; Joshi, A.A.; Rusling, J.F. Paper-based electrochemiluminescent screening for genotoxic activity in the environment. Environ. Sci. Technol. 2013, 47, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, Y.; Liu, J.; Wu, J. Visible paper chip immunoassay for rapid determination of bacteria in water distribution system. Talanta 2014, 120, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.Z.; Brennan, J.D. β-Galactosidase-based colorimetric paper sensor for determination of heavy metals. Anal. Chem. 2011, 83, 8772–8778. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-Q.; Guo, S.-M.; Zuo, P.; Ye, B.-C. A cost-effective Z-folding controlled liquid handling microfluidic paper analysis device for pathogen detection via ATP quantification. Biosens. Bioelectron. 2014, 63, 379–383. [Google Scholar] [CrossRef] [PubMed]
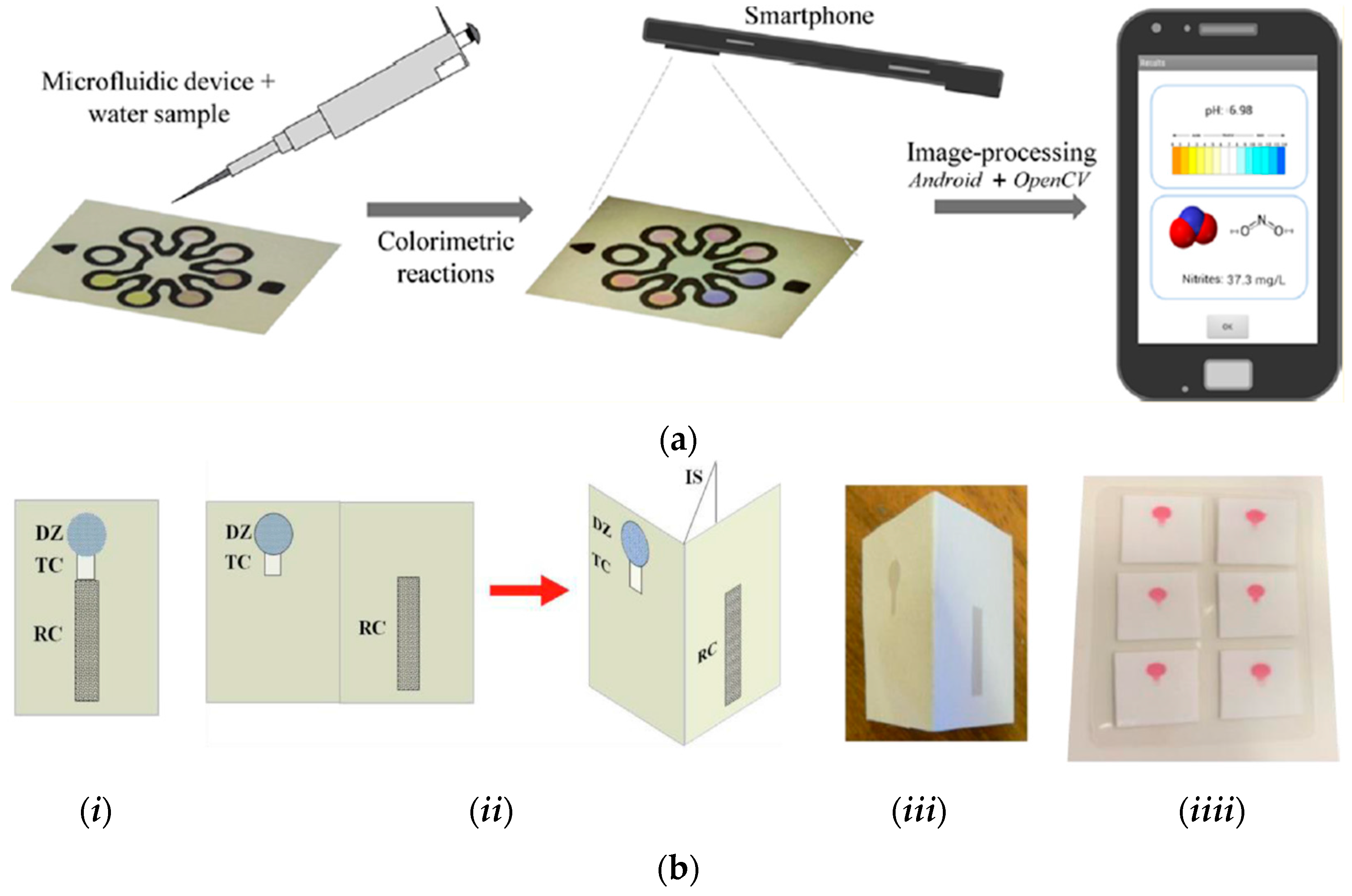
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef]
- Adkins, J.A.; Henry, C.S. Electrochemical detection in paper-based analytical devices using microwire electrodes. Anal. Chim. Acta 2015, 891, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Chaiyo, S.; Siangproh, W.; Apilux, A.; Chailapakul, O. Highly selective and sensitive paper-based colorimetric sensor using thiosulfate catalytic etching of silver nanoplates for trace determination of copper ions. Anal. Chim. Acta 2015, 866, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, P.; Ye, B.-C. A low-cost and simple paper-based microfluidic device for simultaneous multiplex determination of different types of chemical contaminants in food. Biosens. Bioelectron. 2015, 68, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Deiss, F.; Liu, X.; Akbulut, O.; Whitesides, G.M. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 2010, 10, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.M.; Kernisan, E.N.; Lieberman, M. Lab on paper: Iodometric titration on a printed card. Anal. Chem. 2015, 87, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Liu, W.; Ge, L.; Yan, M.; Yan, J.; Huang, J.; Yu, J. In situ assembly of porous Au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for Microcystin-LR immunoassay. Biosens. Bioelectron. 2013, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Apilux, A.; Siangproh, W.; Praphairaksit, N.; Chailapakul, O. Simple and rapid colorimetric detection of Hg(II) by a paper-based device using silver nanoplates. Talanta 2012, 97, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Nouanthavong, S.; Nacapricha, D.; Henry, C.; Sameenoi, Y. Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Analyst 2016, 141, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Colletes, T.C.; Garcia, P.T.; Campanha, R.B.; Abdelnur, P.V.; Romão, W.; Coltro, W.K.T.; Vaz, B.G. A new insert sample approach to paper spray mass spectrometry: Paper substrate with paraffin barriers. Analyst 2016, 141, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.M.G.; Garcia, P.T.; Coltro, W.K.T. Colorimetric determination of nitrite in clinical, food and environmental samples using microfluidic devices stamped in paper platforms. Anal. Methods 2015, 7, 7311–7317. [Google Scholar] [CrossRef]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-based simultaneous pH and nitrite colorimetric determination for paper microfluidic devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Li, W.; McCracken, K.E.; Yoon, J.-Y. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip 2013, 13, 4832–4840. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, L.; Li, L.; Yan, M.; Ge, S.; Yu, J. Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosens. Bioelectron. 2013, 50, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kuek Lawrence, C.S.; Tan, S.N.; Floresca, C.Z. A “green” cellulose paper based glucose amperometric biosensor. Sens. Actuators B Chem. 2014, 193, 536–541. [Google Scholar] [CrossRef]
- He, Q.; Ma, C.; Hu, X.; Chen, H. Method for fabrication of paper-based microfluidic devices by alkylsilane self-assembling and UV/O3-patterning. Anal. Chem. 2013, 85, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tang, F.; Xing, H.; Zheng, H.; Lianhua, B.; Wei, W. Electrochemical detection of Pb and Cd in paper-based microfluidic devices. J. Braz. Chem. Soc. 2012, 23, 1124–1130. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Coo, L.dlC.; Cattrall, R.W.; Kolev, S.D. The use of a polymer inclusion membrane in a paper-based sensor for the selective determination of Cu(II). Anal. Chim. Acta 2013, 803, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Crespo, G.A.; Bakker, E. Paper-based thin-layer coulometric sensor for halide determination. Anal. Chem. 2015, 87, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Burnham, S.; Hu, J.; Anany, H.; Brovko, L.; Deiss, F.; Derda, R.; Griffiths, M.W. Towards rapid on-site phage-mediated detection of generic Escherichia coli in water using luminescent and visual readout. Anal. Bioanal. Chem. 2014, 406, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; El-Aswad, A.F. Bioactive paper sensor based on the acetylcholinesterase for the rapid detection of organophosphate and carbamate pesticides. Int. J. Anal. Chem. 2014, 2014, 536823. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.S.J.; Ornatska, M.; Andreescu, S. Colorimetric paper bioassay for the detection of phenolic compounds. Anal. Chem. 2012, 84, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Li, X.; Dominguez, D.C.; Ye, B.-C. A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 2013, 13, 3921–3928. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Sample treatment and determination of pesticide residues in fatty vegetable matrices: A review. Talanta 2009, 79, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Tisdell, C. Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Copley, S.D. Genome shuffling improves degradation of the anthropogenic pesticide pentachlorophenol by sphingobium chlorophenolicum ATCC 39723. Appl. Environ. Microbiol. 2004, 70, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.S.; Briceño, G.E.; Saez, J.M.; Benimeli, C.S.; Diez, M.C.; Amoroso, M.J. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized streptomyces strains. Biomed Res. Int. 2013, 2013, 392573. [Google Scholar] [CrossRef] [PubMed]
- Nesakumar, N.; Ramachandra, B.L.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Evaluation of inhibition efficiency for the detection of captan, 2,3,7,8-Tetrachlorodibenzodioxin, pentachlorophenol and carbosulfan in water: An electrochemical approach. Bull. Environ. Contam. Toxicol. 2016, 96, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ma, S.; Jiang, K.; Han, X. CdTe-paper-based Visual Sensor for Detecting Methyl Viologen. Chin. J. Chem. 2015, 33, 446–450. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.-M.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, S.; Dong, Y.; Chen, X.; Xu, Y.; Ma, Y.; Chen, X. A sensitive colorimetric method for the determination of nitrite in water supplies, meat and dairy products using ionic liquid-modified methyl red as a colour reagent. Food Chem. 2014, 151, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kull, T.P.J.; Backlund, P.H.; Karlsson, K.M.; Meriluoto, J.A.O. Oxidation of the cyanobacterial hepatotoxin microcystin-LR by chlorine dioxide: Reaction kinetics, characterization, and toxicity of reaction products. Environ. Sci. Technol. 2004, 38, 6025–6031. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Mutharasan, R. Highly sensitive and rapid detection of microcystin-LR in source and finished water samples using cantilever sensors. Environ. Sci. Technol. 2011, 45, 1490–1496. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines 2016, 7, 86. https://doi.org/10.3390/mi7050086
Busa LSA, Mohammadi S, Maeki M, Ishida A, Tani H, Tokeshi M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines. 2016; 7(5):86. https://doi.org/10.3390/mi7050086
Chicago/Turabian StyleBusa, Lori Shayne Alamo, Saeed Mohammadi, Masatoshi Maeki, Akihiko Ishida, Hirofumi Tani, and Manabu Tokeshi. 2016. "Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis" Micromachines 7, no. 5: 86. https://doi.org/10.3390/mi7050086