A Microfluidic-Based Fabry-Pérot Gas Sensor
Abstract
:1. Introduction
2. Experimental
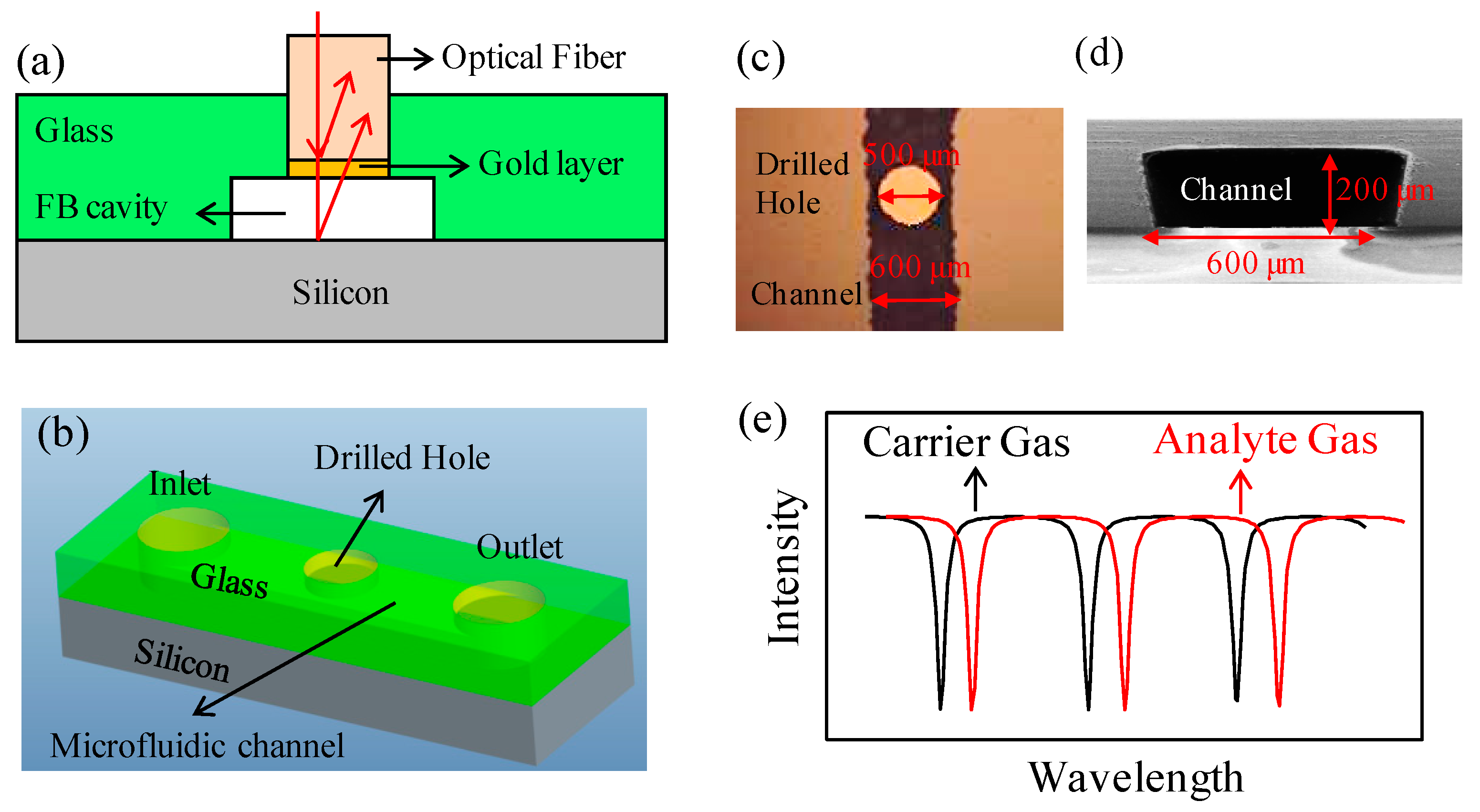
2.1. Detector Fabrication
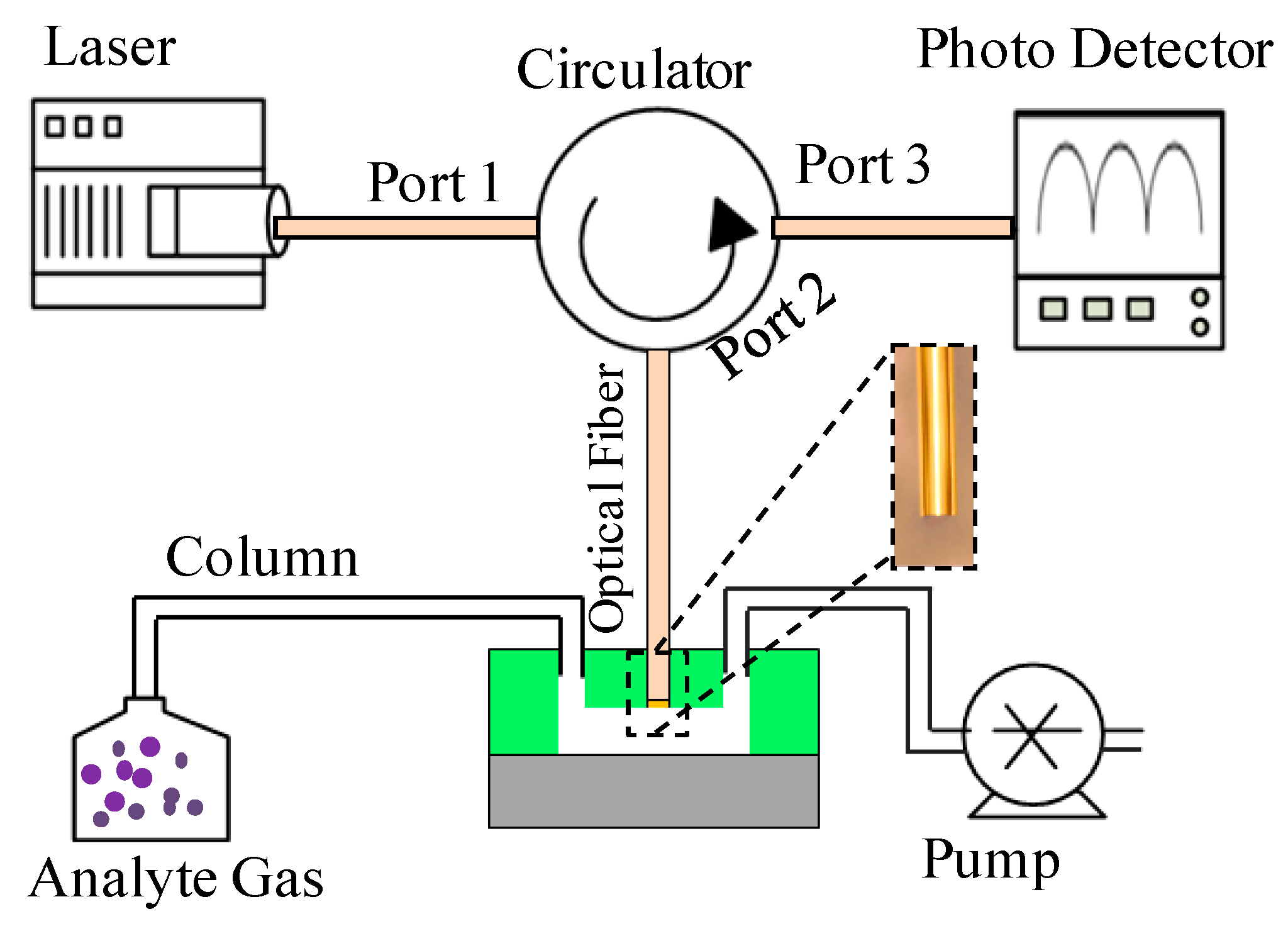
2.2. Experimental Setup
3. Results and Discussion
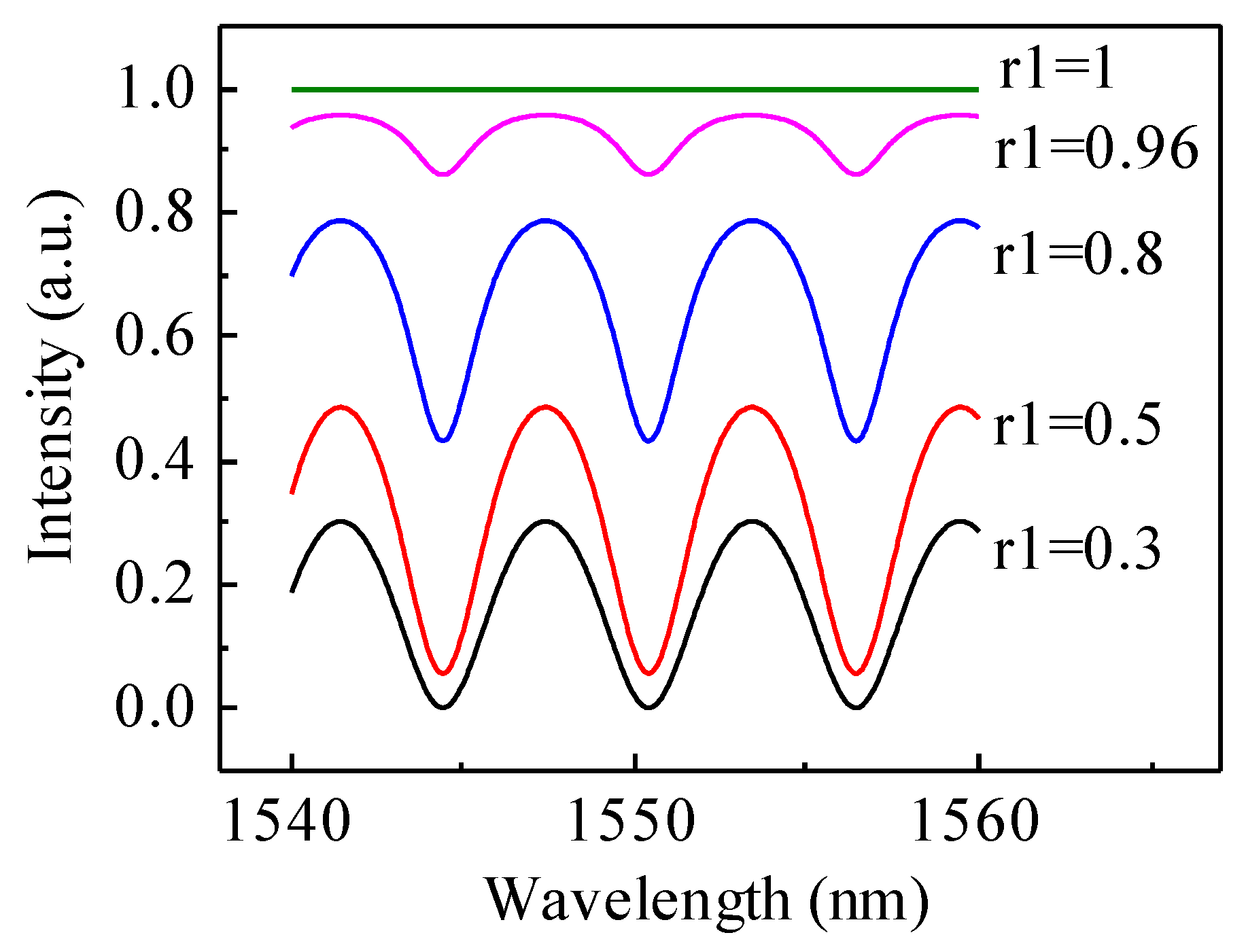
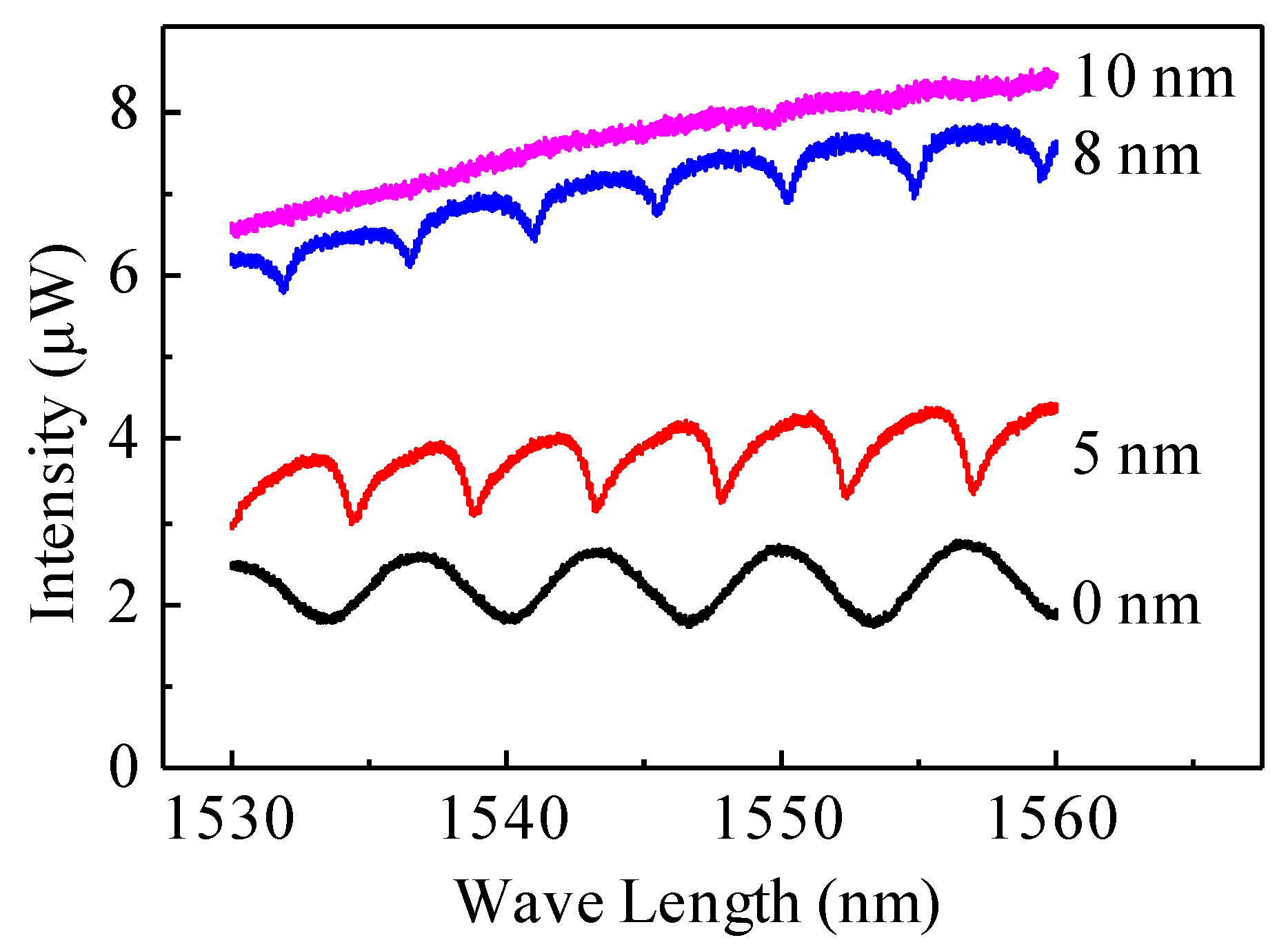
3.1. Gold Coating Calibration
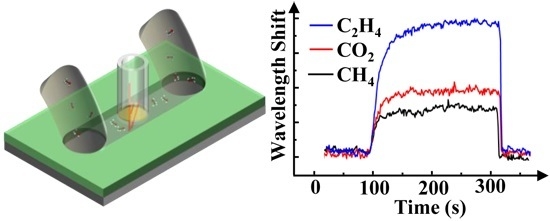
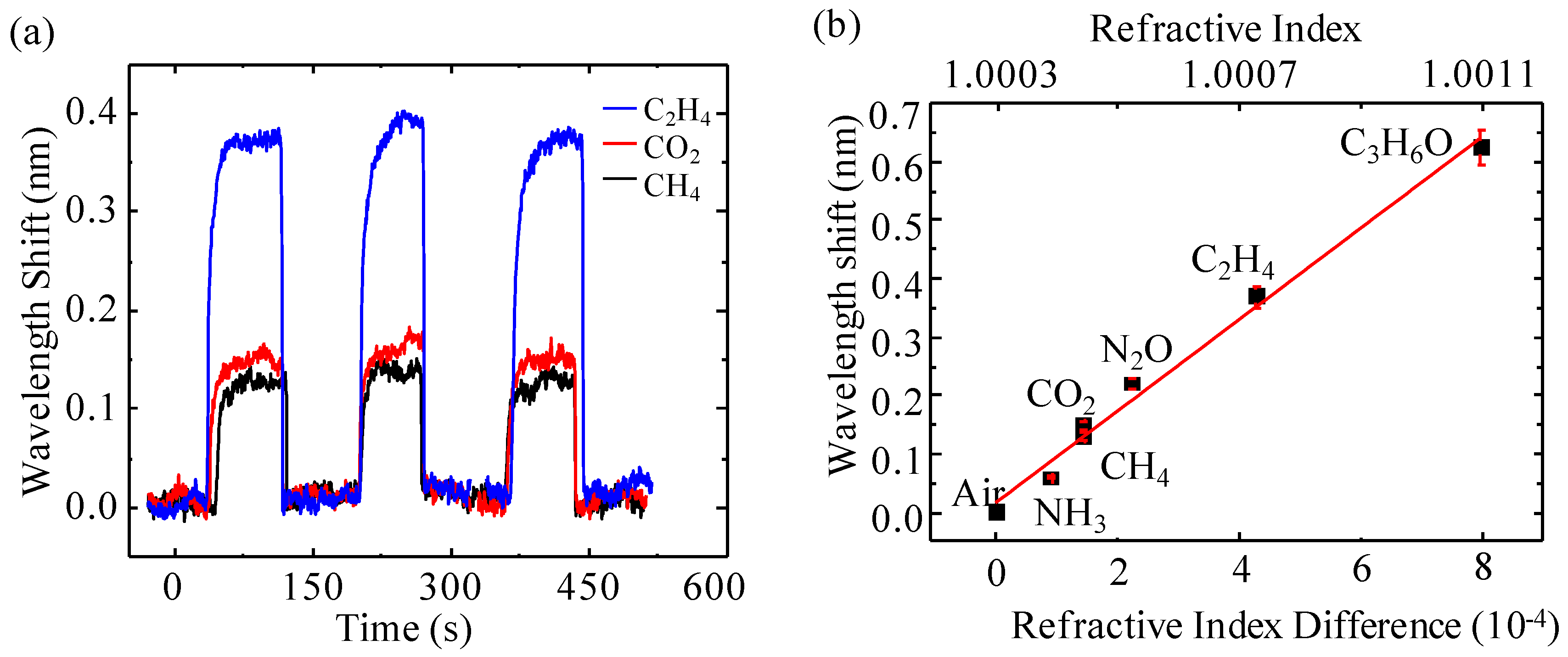
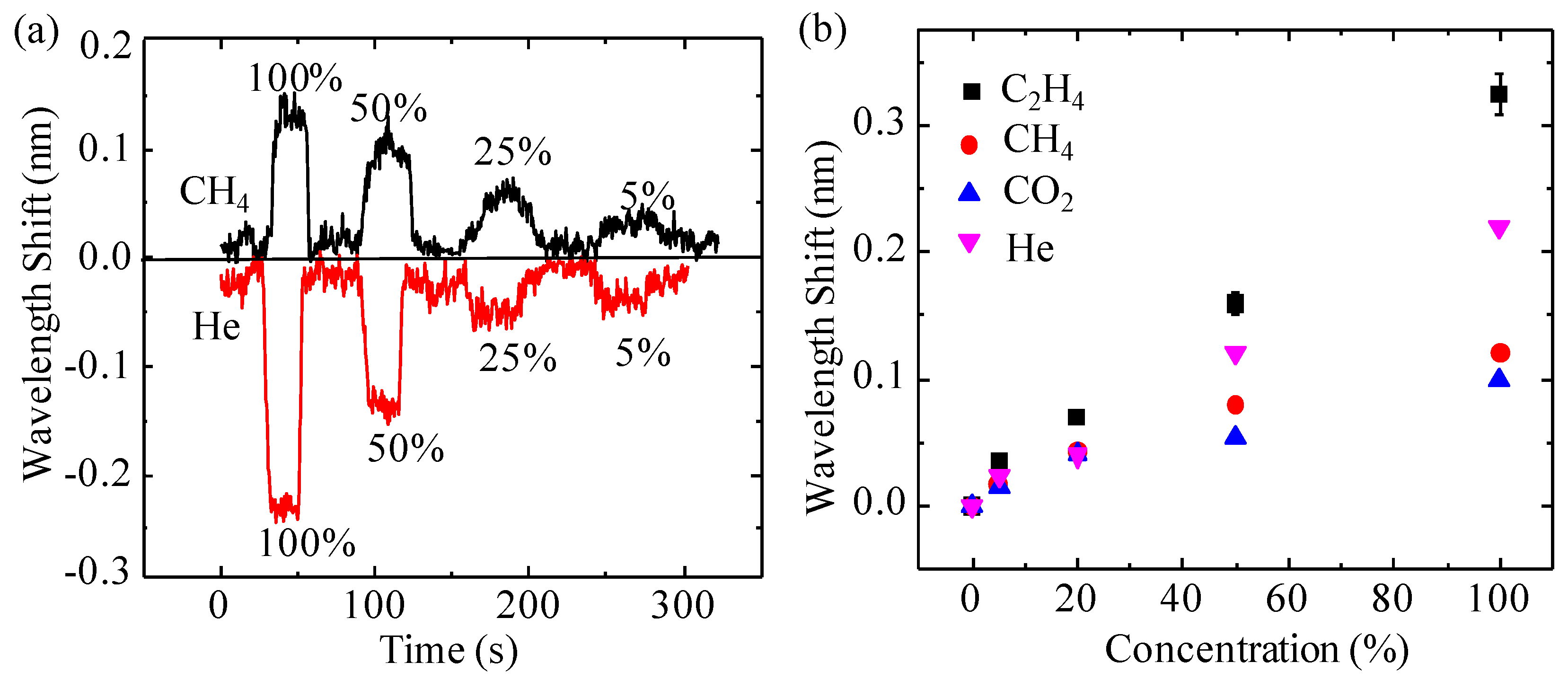
3.2. Real-Time Response
4. Materials and Methods
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Carotta, M.C.; Ferroni, M.; Sadaoka, Y.; Traversa, E. Screen-printed perovskite-type thick films as gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 55, 99–110. [Google Scholar] [CrossRef]
- Moore, D.S. Recent advances in trace explosives detection instrumentation. Sens. Imaging 2007, 8, 9–38. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Sensing of Explosives. Electroanalysis 2007, 19, 415–423. [Google Scholar] [CrossRef]
- Bourrounet, B.; Talou, T.; Gaset, A. Application of a multi-gas-sensor device in the meat industry for boar-taint detection. Sens. Actuators B Chem. 1995, 27, 250–254. [Google Scholar] [CrossRef]
- Schweizer-Berberich, P.M.; Vaihinger, S.; Göpel, W. Characterisation of food freshness with sensor arrays. Sens. Actuators B Chem. 1994, 18, 282–290. [Google Scholar] [CrossRef]
- Ólafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Ricco, A.J.; Martin, S.J.; Zipperian, T.E. Surface acoustic wave gas sensor based on film conductivity changes. Sens. Actuators B Chem. 1985, 8, 319–333. [Google Scholar] [CrossRef]
- Abraham, J.K.; Philip, B.; Witchurch, A.; Varadan, V.K.; Reddy, C.C. A compact wireless gas sensor using a carbon nanotube/PMMA thin film chemiresistor. Smart Mater. Struct. 2004, 13, 1045–1049. [Google Scholar] [CrossRef]
- Paul, R.K.; Badhulika, S.; Saucedo, N.M.; Mulchandani, A. Graphene nanomesh as highly sensitive chemiresistor gas sensor. Anal. Chem. 2012, 84, 8171–8178. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Tsou, Y.H. Chemiresistor-type NO gas sensor based on nickel phthalocyanine thin films. Sens. Actuators B Chem. 2001, 77, 253–259. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Simon, I.; Bârsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B Chem. 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Z.; Zhang, Y.; Gao, S.; He, S. Active Fiber Gas Sensor for Methane Detecting Based on a Laser Heated Fiber Bragg Grating. IEEE Photonics Technol. Lett. 2014, 26, 1069–1072. [Google Scholar] [CrossRef]
- Bingham, J.M.; Anker, J.N.; Kreno, L.E.; Van Duyne, R.P. Gas Sensing with High-Resolution Localized Surface Plasmon Resonance Spectroscopy. JACS 2010, 132, 17358–17359. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-optic sensors based on surface plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Rosengren, L.G. An opto-thermal gas concentration detector. Infrared Phys. 1973, 13, 173–182. [Google Scholar] [CrossRef]
- Morrison, S.R. Selectivity in semiconductor gas sensors. Sens. Actuators 1987, 12, 425–440. [Google Scholar] [CrossRef]
- Coles, G.S.V.; Williams, G.; Smith, B. Selectivity studies on tin oxide-based semiconductor gas sensors. Sens. Actuators B Chem. 1991, 3, 7–14. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Howard, D.J.; Frye-Mason, G.; Thompson, A.K.; Ja, S.J.; Wang, S.K.; Bai, M.; Taub, H.; Almasri, M.; Fan, X. Fabry-Pérot cavity sensors for multipoint on-column micro gas chromatography detection. Anal. Chem. 2010, 82, 4370–4375. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.J.; Misselbrook, T.H.; Pain, B.F. Assessment of Odours from Livestock Wastes by a Photoionization Detector, an Electronic Nose, Olfactometry and Gas Chromatography-Mass Spectrometry. J. Agric. Eng. Res. 1995, 60, 137–144. [Google Scholar] [CrossRef]
- McWilliam, I.G.; Dewar, R.A. Flame ionization detector for gas chromatography. Nature 1958, 181, 760. [Google Scholar] [CrossRef]
- Marriott, P.J.; Chin, S.T.; Maikhunthod, B.; Schmarr, H.G.; Bieri, S. Multidimensional gas chromatography. Trends Anal. Chem. 2012, 34, 1–20. [Google Scholar] [CrossRef]
- Seeley, J.V. Recent advances in flow-controlled multidimensional gas chromatography. J. Chromatogr. A 2012, 1255, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Seo, J.H.; Li, Y.; Chen, D.; Kurabayashi, K.; Fan, X. Smart multi-channel two-dimensional micro-gas chromatography for rapid workplace hazardous volatile organic compounds measurement. Lab Chip 2013, 13, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Seo, J.H.; Liu, J.; Kurabayashi, K.; Fan, X. Smart Three-Dimensional Gas Chromatography. Anal. Chem. 2013, 85, 6871–6875. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.; Chang, J.P.; Showalter, S.K.; Gelbard, F.; Manginell, R.P.; Blain, M.G. Microfabricated thermal conductivity detector for the micro-ChemLab™. Sens. Actuators B Chem. 2007, 121, 414–422. [Google Scholar] [CrossRef]
- Simon, I.; Arndt, M. Thermal and gas-sensing properties of a micromachined thermal conductivity sensor for the detection of hydrogen in automotive applications. Sens. Actuators A Phys. 2002, 97, 104–108. [Google Scholar] [CrossRef]
- Kuo, J.T.; Yu, L.; Meng, E. Micromachined thermal flow sensors—A review. Micromachines 2012, 3, 550–573. [Google Scholar] [CrossRef]
- Zhong, Q.; Steinecker, W.H.; Zellers, E.T. Characterization of a high-performance portable GC with a chemiresistor array detector. Analyst 2009, 134, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Fan, X. Highly versatile fiber-based optical Fabry-Pérot gas sensor. Opt. Express 2009, 17, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Guo, Y.; Liu, J.; Lee, W.; Oo, M.K.K.; Fan, X. On-chip Fabry-Pérot interferometric sensors for micro-gas chromatography detection. Sens. Actuators B Chem. 2011, 159, 60–65. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Paknahad, M.; Ghafarinia, V. A miniature gas analyzer made by integrating a chemoresistor with a microchannel. Lab Chip 2012, 12, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Jiang, Y.; Ding, W.; Wang, Z.; Liu, D. Filmed extrinsic Fabry-Perot interferometric sensors for the measurement of arbitrary refractive index of liquid. Sens. Actuators B Chem. 2013, 177, 924–928. [Google Scholar] [CrossRef]
- Reddy, K.; Guo, Y.; Liu, J.; Lee, W.; Oo, M.K.K.; Fan, X. Rapid, sensitive, and multiplexed on-chip optical sensors for micro-gas chromatography. Lab Chip 2012, 12, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Han, Y.; Li, Y.; Tsai, H.L.; Xiao, H. Temperature-insensitive miniaturized fiber inline Fabry-Perot interferometer for highly sensitive refractive index measurement. Opt. Express 2008, 16, 5764–5769. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Jiang, L.; Xiao, H.; Chai, Y.H.; Chen, S.J.; Tsai, H.L. Fabry-Perot interferometer embedded in a glass chip fabricated by femtosecond laser. Opt. Lett. 2009, 34, 2408–2410. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, W.; Wu, N.; Zou, X.; Guthy, C.; Wang, X. A miniature fiber optic refractive index sensor built in a MEMS-based microchannel. Sensors 2011, 11, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Z.; Adnet, A.; Zhang, Z.; Sun, F.G.; Grover, C.P. Monitoring changes in the refractive index of gases by means of a fiber optic Fabry-Perot interferometer sensor. Sens. Actuators A Phys. 2005, 118, 177–182. [Google Scholar] [CrossRef]
- Duan, D.W.; Rao, Y.J.; Zhu, T. High sensitivity gas refractometer based on all-fiber open-cavity Fabry-Perot interferometer formed by large lateral offset splicing. JOSA B 2012, 29, 912–915. [Google Scholar] [CrossRef]
- Kou, J.L.; Feng, J.; Ye, L.; Xu, F.; Lu, Y.Q. Miniaturized fiber taper reflective interferometer for high temperature measurement. Opt. Express 2010, 18, 14245–14250. [Google Scholar] [CrossRef] [PubMed]
- Maharana, P.K.; Jha, R.; Padhy, P. On the electric field enhancement and performance of SPR gas sensor based on graphene for visible and near infrared. Sens. Actuators B Chem. 2015, 207, 117–122. [Google Scholar] [CrossRef]
- Goyal, A.K.; Pal, S. Design and simulation of high-sensitive gas sensor using a ring-shaped photonic crystal waveguide. Phys. Scr. 2015, 90, 025503. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Zhang, Q.; Xiao, Y.; Li, X.; Yao, P.; Pang, W.; Zhang, H.; Duan, X.; Zhang, D.; Liu, J. A Microfluidic-Based Fabry-Pérot Gas Sensor. Micromachines 2016, 7, 36. https://doi.org/10.3390/mi7030036
Tao J, Zhang Q, Xiao Y, Li X, Yao P, Pang W, Zhang H, Duan X, Zhang D, Liu J. A Microfluidic-Based Fabry-Pérot Gas Sensor. Micromachines. 2016; 7(3):36. https://doi.org/10.3390/mi7030036
Chicago/Turabian StyleTao, Jin, Qiankun Zhang, Yunfeng Xiao, Xiaoying Li, Pei Yao, Wei Pang, Hao Zhang, Xuexin Duan, Daihua Zhang, and Jing Liu. 2016. "A Microfluidic-Based Fabry-Pérot Gas Sensor" Micromachines 7, no. 3: 36. https://doi.org/10.3390/mi7030036