Surface Free Energy Determination of APEX Photosensitive Glass
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Glass Processing
2.2. Contact Angle Measurement
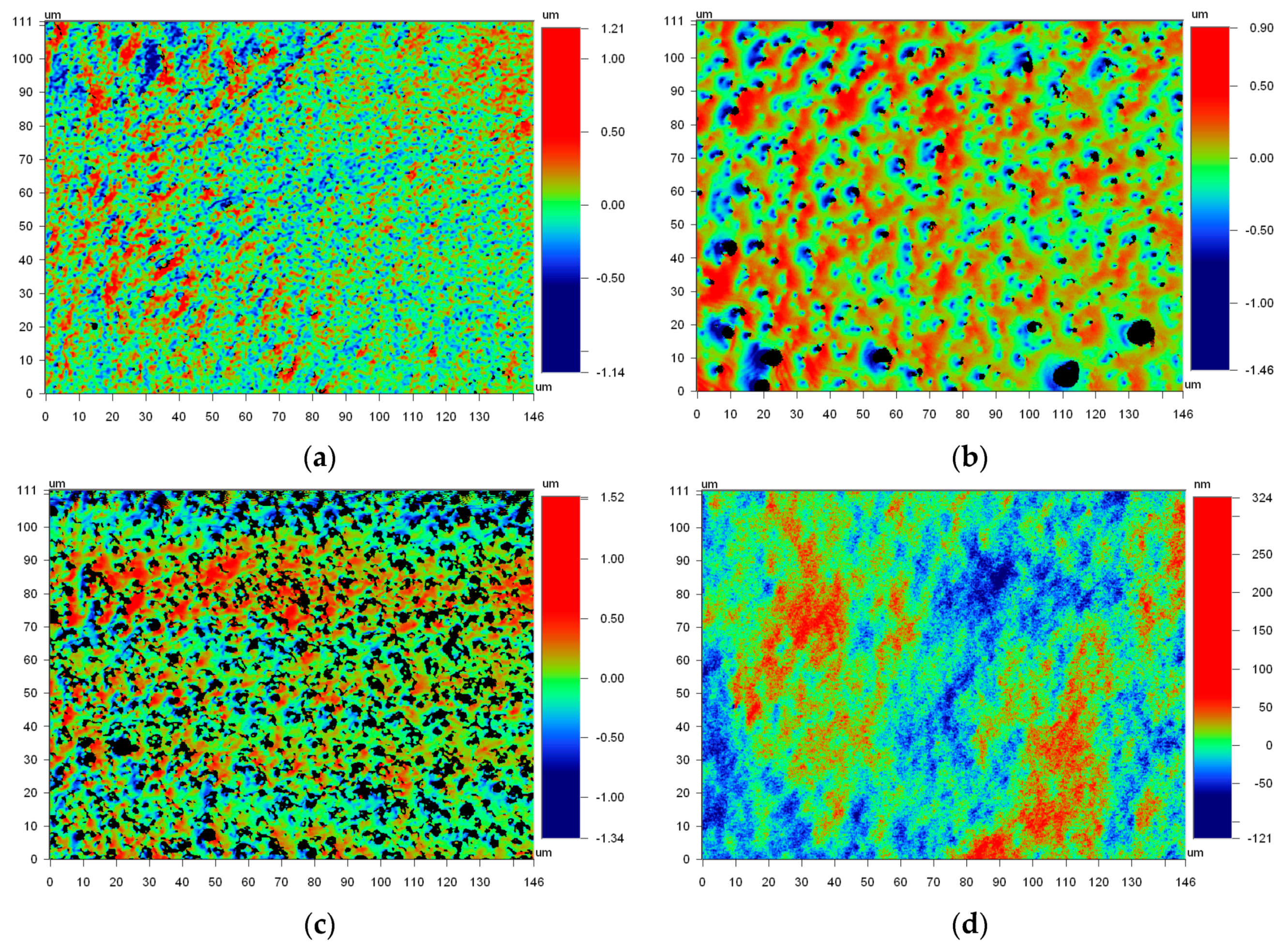
2.3. Surface Roughness Measurement
3. Results and Discussion
3.1. Contact Angle and Surface Roughness
3.2. Surface Free Energy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berezhnoi, A.I. Glass-Ceramics and Photo-Sitalls; Plenum Press: New York, NY, USA, 1970. [Google Scholar]
- Beall, G.H. Design and properties of glass-ceramics. Annu. Rev. Mater. Sci. 1992, 22, 91–119. [Google Scholar] [CrossRef]
- Etoh, S.; Fujimura, T.; Hattori, R.; Kuroki, Y. Fabrication of on-chip microcapillary using photosensitive glass. Microsyst. Technol. 2003, 9, 541–545. [Google Scholar] [CrossRef]
- Gaillard, W.R.; Tantawi, K.H.; Waddell, E.; Fedorov, V.; Williams, J.D. In-plane spectroscopy with optical fibers and liquid-filled APEX™ glass microcuvettes. J. Micromech. Microeng. 2013, 23, 107001. [Google Scholar] [CrossRef]
- Maboudian, R.; Howe, R.T. Critical review: Adhesion in surface micromechanical structures. J. Vac. Sci. Technol. B 1997, 15, 1–20. [Google Scholar] [CrossRef]
- Mastrangelo, C.H.; Hsu, C.H. Mechanical stability and adhesion of microstructures under capillary forces—Part I: Basic theory. J. Microelectromech. Syst. 1993, 2, 33–43. [Google Scholar] [CrossRef]
- Kim, C.J. Microfluidics using the surface tension force in microscale. Proc. SPIE 2000, 4177, 18–24. [Google Scholar]
- Neagu, C.R.; Gardeniers, J.G.E.; Elwenspoek, M.; Kelly, J.J. An electrochemical microactuator: Principle and first results. J. Microelectromech. Syst. 1996, 5, 2–9. [Google Scholar] [CrossRef]
- Hua, S.Z.; Sachs, F.; Yang, D.X.; Chopra, H.D. Microfluidic actuation using electrochemically generated bubbles. Anal. Chem. 2002, 74, 6392–6396. [Google Scholar] [CrossRef] [PubMed]
- Tseng, F.G.; Kim, C.J.; Ho, C.M. A novel microinjector with virtual chamber neck. In Proceedings of the Eleventh Annual International Workshop on Micro Electro Mechanical Systems (MEMS 98), Heidelberg, Germany, 25–29 January 1998.
- Basu, A.S.; Gianchandani, Y.B. Virtual microfluidic traps, filters, channels, and pumps using Marangoni flows. J. Micromech. Microeng. 2008, 18, 115031. [Google Scholar] [CrossRef]
- Lee, J.; Kim, C.J. Surface-tension-driven microactuation based on continuous electrowetting. J. Microelectromech. Syst. 2000, 9, 171–180. [Google Scholar]
- Lynn, N.S.; Dandy, D.S. Passive microfluidic pumping using coupled capillary/evaporation effects. Lab Chip 2009, 9, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Bruss, H. Theoretical Microfluidics; Oxford University Press: Oxford, NY, USA, 2011. [Google Scholar]
- Nguyen, N.T.; Wereley, S.T. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2006. [Google Scholar]
- You, I.; Yun, N.; Lee, H. Surface-tension-confined microfluidics and their applications. ChemPhysChem 2013, 14, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Selva, B.; Miralles, V.; Cantat, I.; Jullien, M.C. Thermocapillary actuation by optimized resistor pattern: Bubbles and droplets displacing, switching and trapping. Lab Chip 2010, 10, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Tretheway, D.C.; Meinhart, C.D. Apparent fluid slip at hydrophobic microchannel walls. Phys. Fluids 2002, 14, 9–12. [Google Scholar] [CrossRef]
- Tretheway, D.C.; Meinhart, C.D. A generating mechanism for apparent fluid slip in hydrophobic microchannels. Phys. Fluids 2004, 16, 1509–1515. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Xie, R.; Yu, Y.L.; Chen, G.; Ju, X.J.; Yang, L.; Liang, B.; Chu, L.Y. Effects of surface wettability and roughness of microchannel on flow behaviors of thermo-responsive microspheres therein during the phase transition. J. Colloid Interface Sci. 2009, 336, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Pit, R.; Hervet, H.; Leger, L. Direct experimental evidence of slip in hexadecane: Solid interfaces. Phys. Rev. Lett. 2000, 85, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Granick, S. Limits of the hydrodynamic no-slip boundary condition. Phys. Rev. Lett. 2002, 88, 106102. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.; Jing, J.; Tay, F.E.; Miao, J.; Sun, T. Characterization of masking layers for deep wet etching of glass in an improved HF/HCl solution. Surf. Coat. Technol. 2005, 198, 314–318. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lee, G.-B.; Lin, Y.-H.; Chang, G.-L. A fast prototyping process for fabrication of microfluidic systems on soda-lime glass. J. Micromech. Microeng. 2001, 11, 726–732. [Google Scholar] [CrossRef]
- Zhu, H.; Holl, M.; Ray, T.; Bhushan, S.; Meldrum, D.R. Characterization of deep wet etching of fused silica glass for single cell and optical sensor deposition. J. Micromech. Microeng. 2009, 19, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Nikumb, S. Optical quality micromachining of glass with focused laser-produced metal plasma etching in the atmosphere. Appl. Opt. 2003, 42, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yakar, A.; Byer, R.L.; Harkin, A.; Ashmore, J.; Stone, H.A.; Shen, M.; Mazur, E. Morphology of femtosecond-laser-ablated borosilicate glass surfaces. Appl. Phys. Lett. 2003, 83, 3030–3032. [Google Scholar] [CrossRef]
- Corman, T.; Enoksson, P.; Stemme, G. Deep wet etching of borosilicate glass using an anodically bonded silicon substrate as mask. J. Micromech. Microeng. 1988, 8, 84–87. [Google Scholar] [CrossRef]
- Williams, J.; Schmidt, C.; Serkland, D. Processing Advances in Transparent Foturan Glass MEMS. Appl. Phys. A 2010, 99, 777–782. [Google Scholar] [CrossRef]
- Dietrich, T.R.; Ehrfeld, W.; Lacher, M.; Krämer, M.; Speit, B. Fabrication technologies for microsystems utilizing photoetchable glass. Microelectron. Eng. 1996, 30, 497–504. [Google Scholar] [CrossRef]
- Flemming, J.H.; Mack, R.; Buckley, C. Photosensitive glass for ultra high aspect ratio features. In Proceedings of 2009 Nanotech Conference & Expo, Houston, TX, USA, 3–7 May 2009.
- Tantawi, K.H.; Oates, J.; Kamali-Sarvestani, R.; Bergquist, N.; Williams, J.D. Processing of photosensitive APEX™ glass structures with smooth and transparent sidewalls. J. Micromech. Microeng. 2011, 21, 017001. [Google Scholar] [CrossRef]
- Tantawi, K.H.; Waddell, E.; Williams, J.D. Structural and composition analysis of Apex™ and Foturan™ photodefinable glasses. J. Mater. Sci. 2013, 48, 5316–5323. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Shafrin, E.G.; Zisman, W.A. Effect of adsorbed water on the spreading of organic liquids on soda-lime glass. J. Am. Ceram. Soc. 1967, 50, 478–484. [Google Scholar] [CrossRef]
- Sumner, A.L.; Menke, E.J.; Dubowski, Y.; Newberg, J.T.; Penner, R.M.; Hemminger, J.C.; Wingen, L.M.; Brauers, T.; Finlayson-Pitts, B.J. The nature of water on surfaces of laboratory systems and implications for heterogeneous chemistry in the troposphere. Phys. Chem. Chem. Phys. 2004, 6, 604–613. [Google Scholar] [CrossRef]
- Menawat, A.; Henry, J.; Siriwardane, R. Control of surface energy of glass by surface reactions: Contact angle and stability. J. Colloid Interface Sci. 1984, 101, 110–119. [Google Scholar] [CrossRef]
- Olsen, D.A.; Osteraas, A.J. The critical surface tension of glass. J. Phys. Chem. 1964, 68, 2730–2731. [Google Scholar] [CrossRef]
- Gulec, H.A.; Sarioglu, K.; Mutlu, M. Modification of food contacting surfaces by plasma polymerization technique. Part I: Determination of hydrophilicity, hydrophobicity and surface free energy by contact angle method. J. Food Eng. 2006, 75, 187–195. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; CRC Press: New York, NY, USA, 1982. [Google Scholar]
- Toussaint, A.F.; Luner, P. The wetting properties of grafted cellulose films. In Contact Angle, Wettability and Adhesion; Mittal, K.L., Ed.; VSP: Utrecht, The Netherlands, 1993; pp. 383–396. [Google Scholar]
- Jancuk, B.; Wojcik, W.; Zdziennicka, A. Determination of the components of the surface tension of some liquids from interfacial liquid-liquid tension measurements. J. Colloid Interface Sci. 1993, 157, 384–393. [Google Scholar] [CrossRef]
- Belaud, V.; Valette, S.; Stremsdoerfer, G.; Bigerelle, M.; Benayoun, S. Wettability versus roughness: Multi-scales approach. Tribol. Int. 2015, 82, 343–349. [Google Scholar] [CrossRef]
- Eliaz, N.; Shmueli, S.; Shur, I.; Benayahu, D.; Aronov, D.; Rosenman, G. The effect of surface treatment on the surface texture and contact angle of electrochemically deposited hydroxyapatite coating and on its interaction with bone-forming cells. Acta Biomater. 2009, 5, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.; de Palma, R.; Verlaak, S.; Heremans, P.; Dehaen, W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Sold Films 2006, 515, 1433–1438. [Google Scholar] [CrossRef]
- De Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Cras, J.J.; Rowe-Taitt, C.A.; Nivens, D.A.; Ligler, F.S. Comparison of chemical cleaning methods of glass in preparation for silanization. Biosens. Bioelectron. 1999, 14, 683–688. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Vicente, C.M.S.; Andre, P.S.; Ferreira, F.A.S. Simple measurement of surface free energy using a web cam. Rev. Bras. Ensino Fis. 2012, 34, 3312. [Google Scholar] [CrossRef]
- Fluegel, A.; Earl, D.A.; Varshneya, A.K.; Oksoy, D. Statistical Analysis of Viscosity, Electrical Resistivity, and Further Glass Melt Properties. In High Temperature Glass Melt Property Database for Process Modeling; Seward, T.P., Vascott, T., Eds.; The American Ceramic Society: Westerville, OH, USA, 2005; Chapter 9. [Google Scholar]
- Crowe, L.L.; Tolbert, L.M. Silica passivation efficiency monitored by a surface-bound fluorescent dye. Langmuir 2008, 24, 8541–8546. [Google Scholar] [CrossRef] [PubMed]
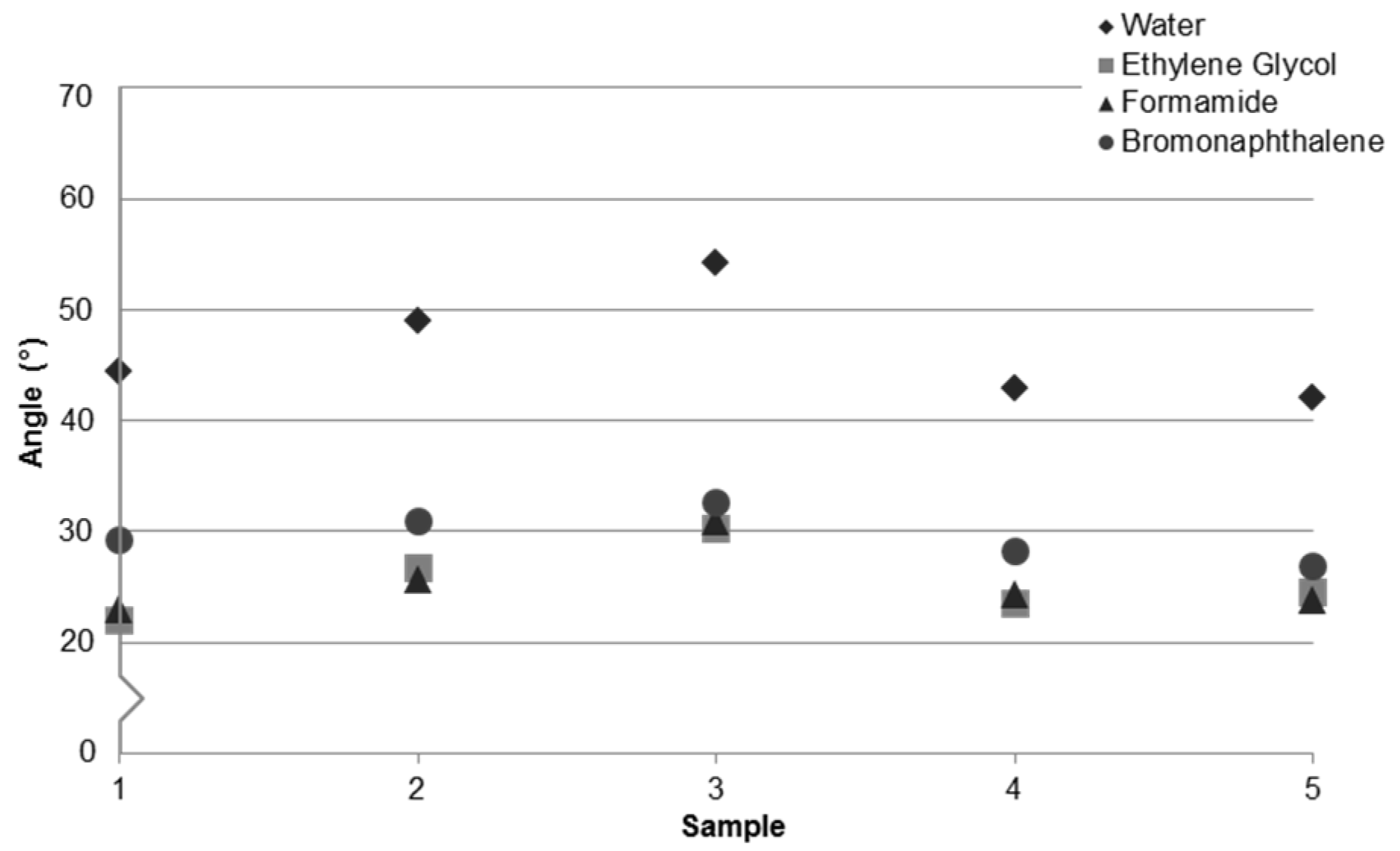



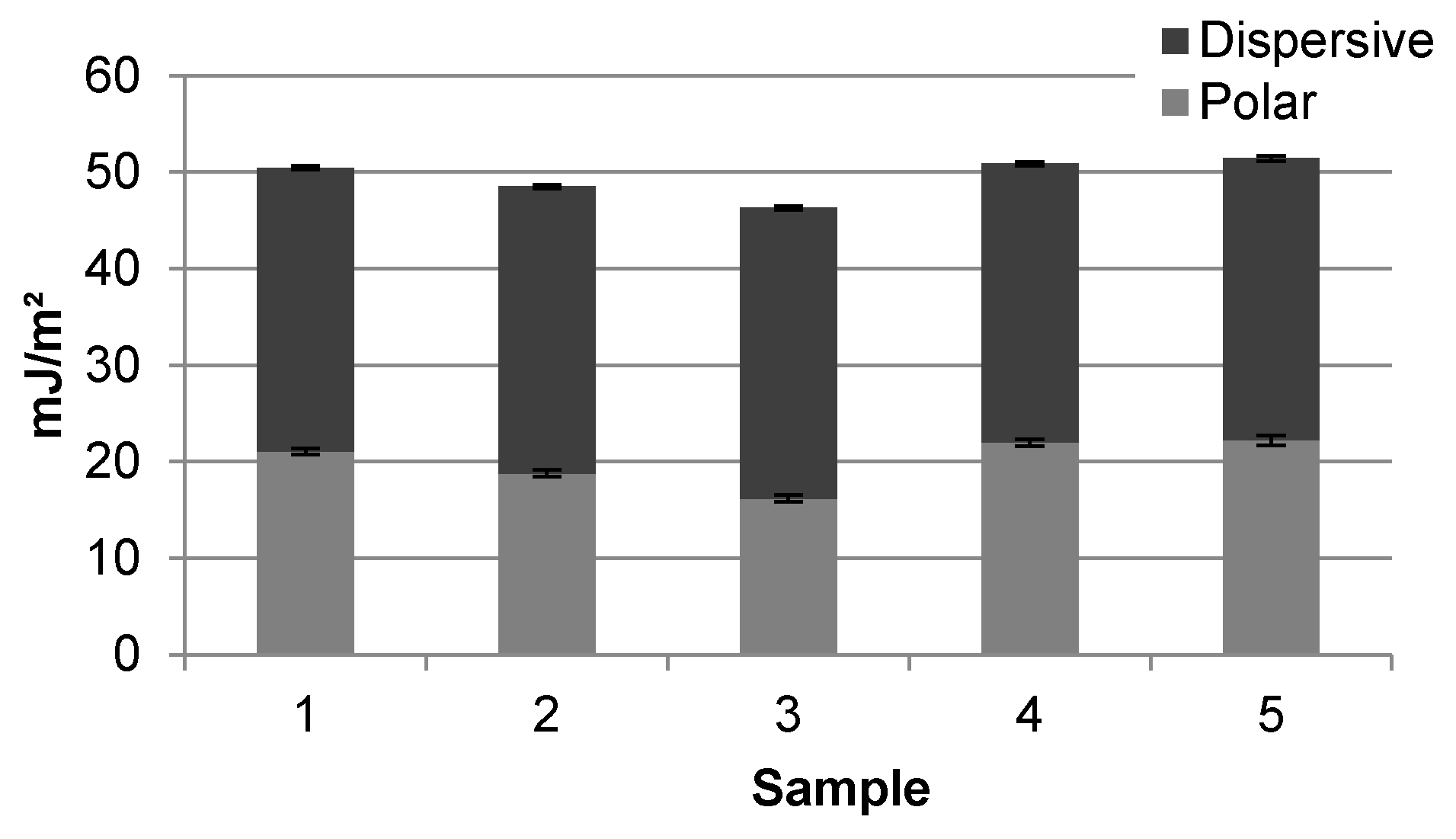
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| UV | - | - | - | - | - |
| Bake | - | - | x | x | x |
| HF | - | x | x | x | - |
| Anneal | - | - | - | x | x |
| Test Liquid | Polar | Dispersive |
|---|---|---|
| Distilled Water [40] | 50.3 | 22.5 |
| Ethylene Glycol [41] | 16.0 | 32.8 |
| Formamide [41] | 23.5 | 34.4 |
| Bromonaphthalene [42] | 0.0 | 44.4 |
| Sample | Sdr | Range |
|---|---|---|
| 1 | 0.29 | ±0.06 |
| 2 | 0.26 | ±0.04 |
| 3 | 2.22 | ±0.71 |
| 4 | 0.39 | ±0.10 |
| 5 | 0.36 | ±0.08 |
| Component | Soda-Lime (Limits) | Soda-Lime (Typical) | APEX |
|---|---|---|---|
| SiO2 | 58.22–84.15 | 73.26 | 58.50 |
| Na2O | 9.3–15.19 | 13.81 | 1.78 |
| CaO | 6.55–12.83 | 8.78 | 0 |
| MgO | 0–3.95 | 3.86 | 0 |
| Al2O3 | 0–3.33 | 0.14 | 16.64 |
| K2O | 0–2.31 | 0.03 | 4.96 |
| Fe2O3 | 0–1.57 | 0.11 | 0 |
| TiO2 | 0–1.04 | 0.01 | 0 |
| Li2O and B2O3 | 0 | 0 | 16.16 |
| ZnO | 0 | 0 | 1.28 |
| Ag2O | 0 | 0 | 0.20 |
| Sb2O3 | 0 | 0 | 0.25 |
| Ce2O3 | 0 | 0 | 0.22 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaillard, W.R.; Waddell, E.; Williams, J.D. Surface Free Energy Determination of APEX Photosensitive Glass. Micromachines 2016, 7, 34. https://doi.org/10.3390/mi7030034
Gaillard WR, Waddell E, Williams JD. Surface Free Energy Determination of APEX Photosensitive Glass. Micromachines. 2016; 7(3):34. https://doi.org/10.3390/mi7030034
Chicago/Turabian StyleGaillard, William R., Emanuel Waddell, and John D. Williams. 2016. "Surface Free Energy Determination of APEX Photosensitive Glass" Micromachines 7, no. 3: 34. https://doi.org/10.3390/mi7030034






