A Rapid Prototyping Technique for Microfluidics with High Robustness and Flexibility
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
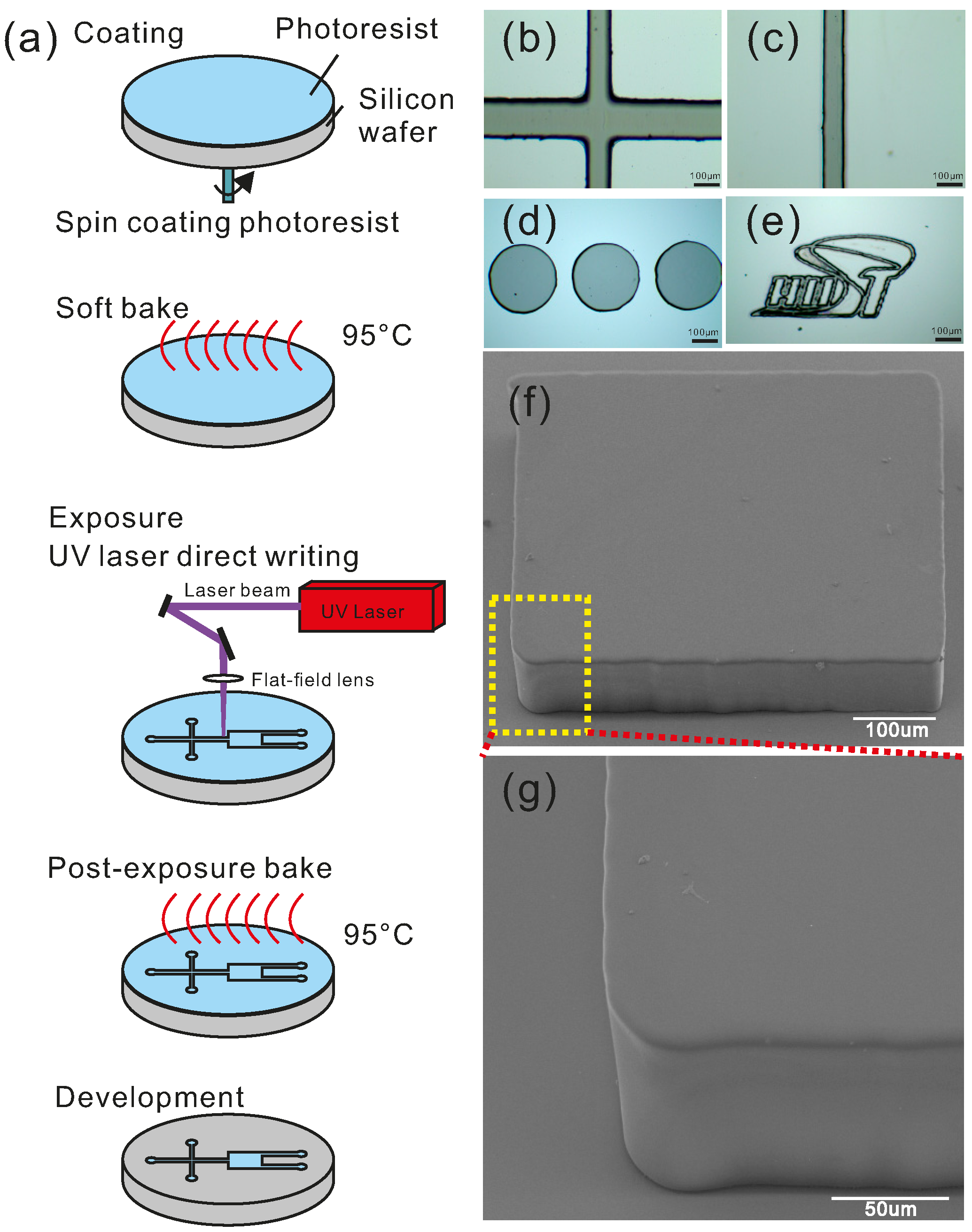
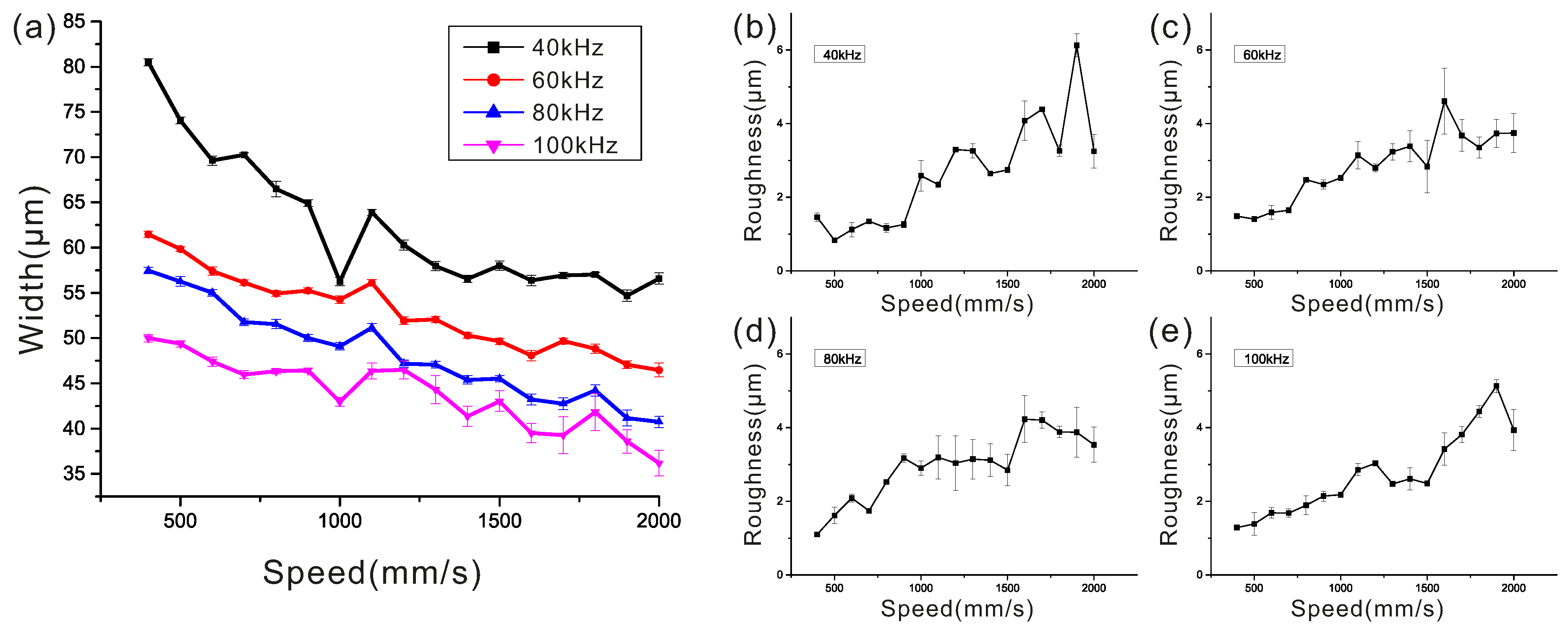
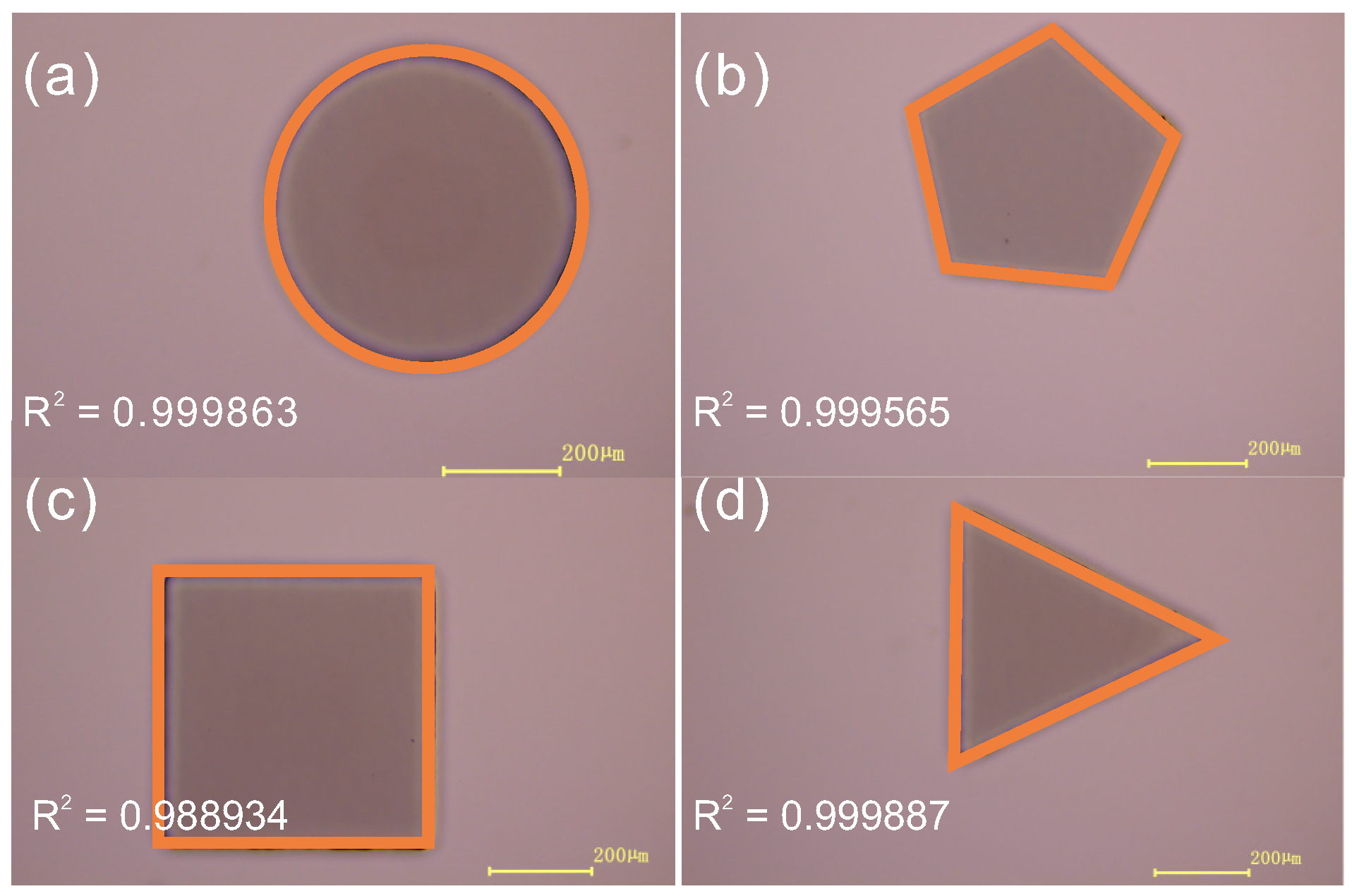
3.1. Fabrication Capability
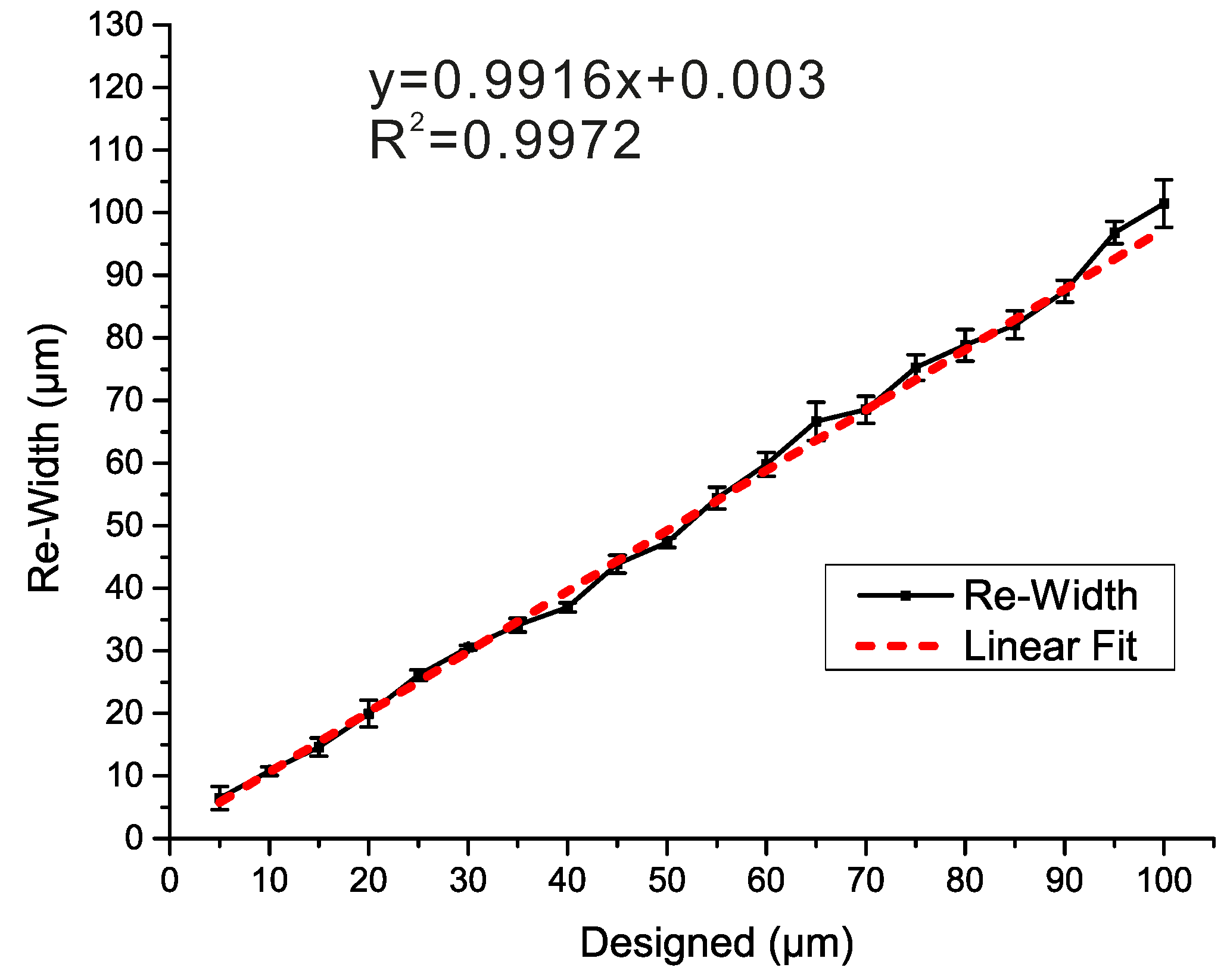
3.2. Bias Calibration
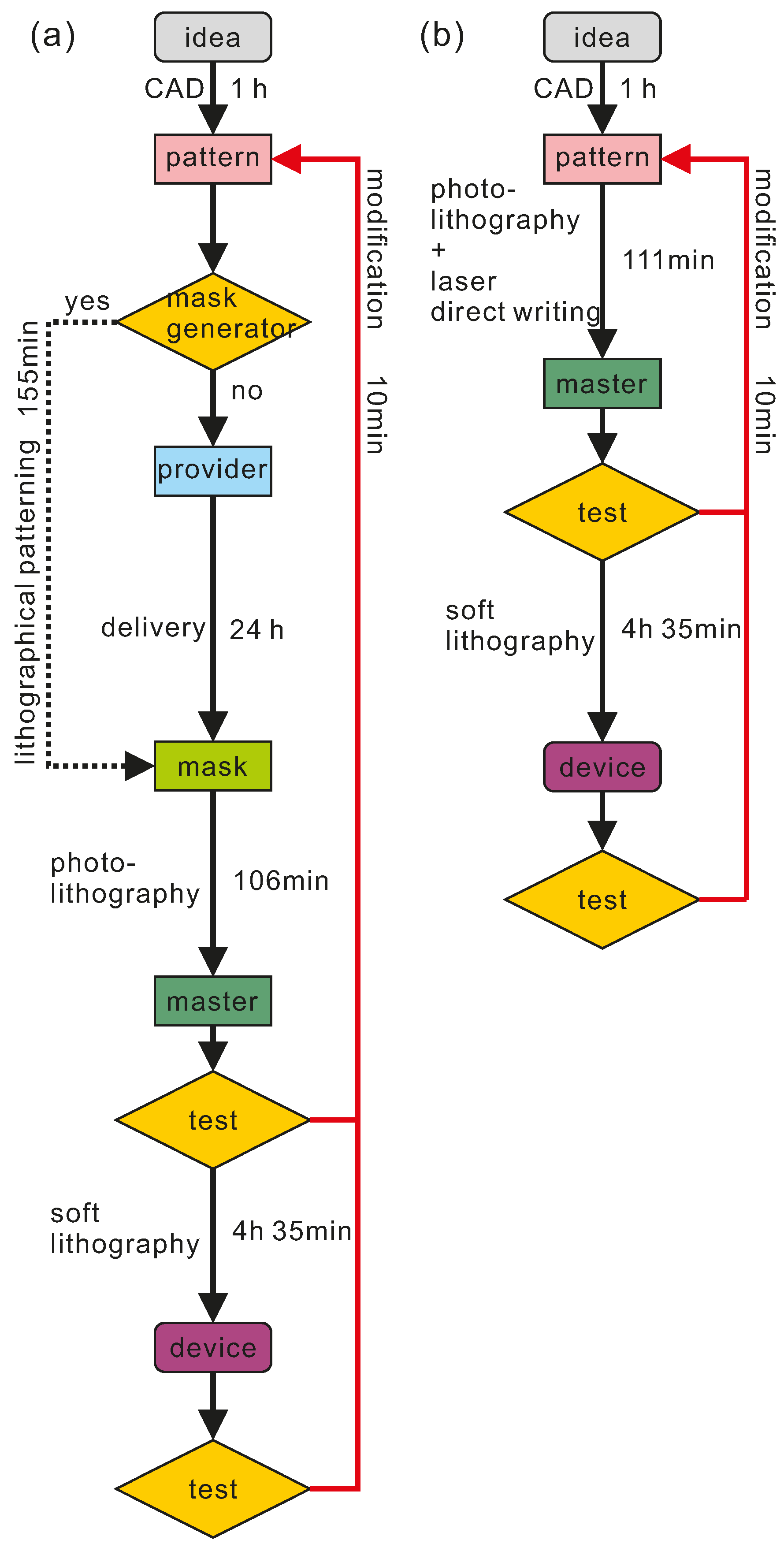
3.3. Prototyping Time Estimation and Instrumental Investment
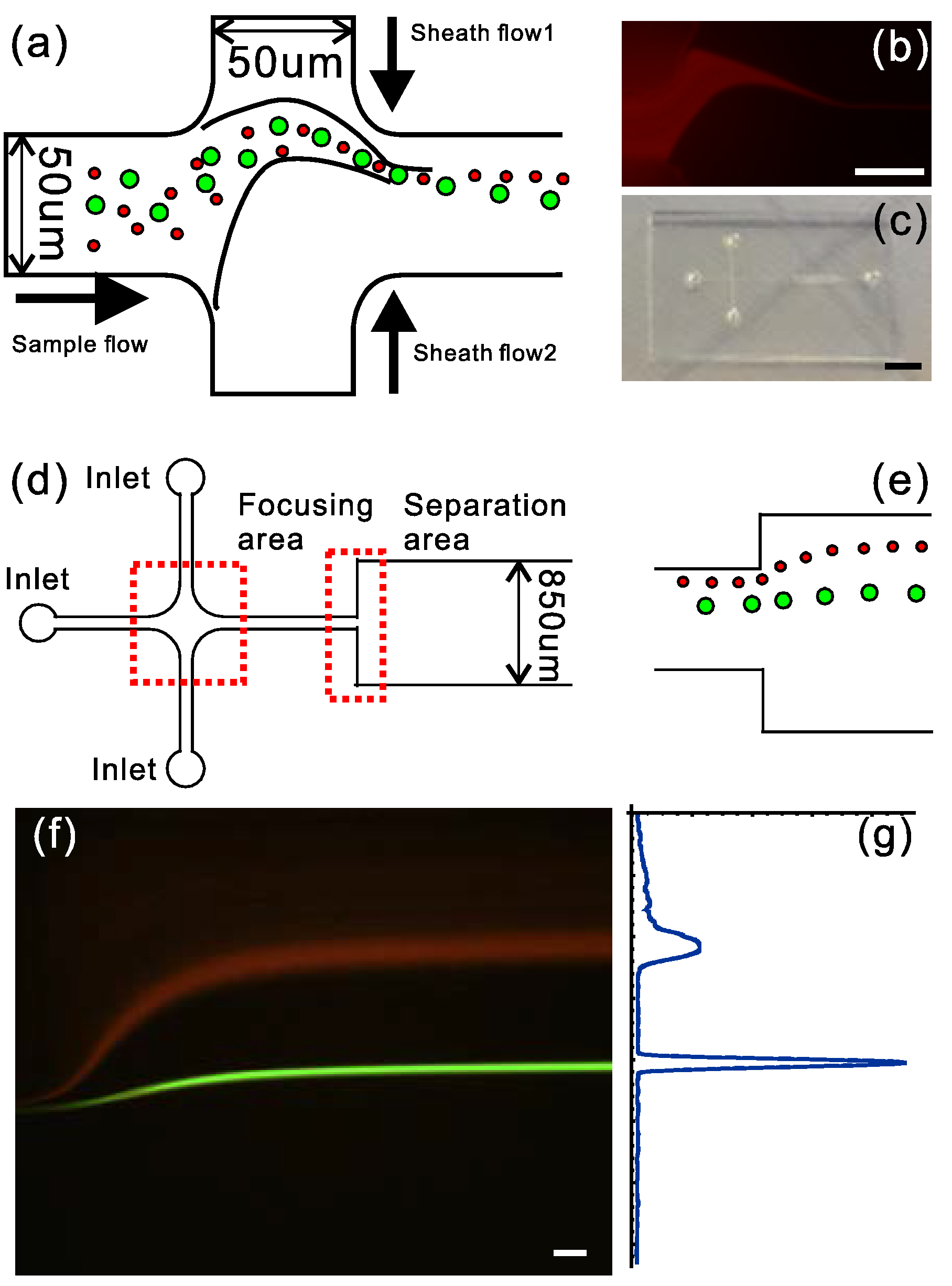
3.4. Microfluidic Application Demonstration
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whitesides, G.M. Cool, or simple and cheap? Why not both? Lab Chip 2013, 13, 11–13. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Price, A.K.; Culbertson, C.T. Chemical analysis of single mammalian cells with microfluidics. Anal. Chem. 2007, 79, 2614–2621. [Google Scholar] [CrossRef]
- Barry, R.; Ivanov, D. Microfluidics in biotechnology. J. Nanobiotechnol. 2004, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lafleur, J.P.; Jönsson, A.; Senkbeil, S.; Kutter, J.P. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef]
- Connelly, J.T.; Rolland, J.P.; Whitesides, G.M. A “paper machine” for molecular diagnostics. Anal. Chem. 2015, 87, 7595–7601. [Google Scholar] [CrossRef]
- Figeys, D.; Pinto, D. Lab-on-a-chip: A revolution in biological and medical sciences. Anal. Chem. 2000, 72, 330A–335A. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, Z.G. Microfluidic electronics. Lab Chip 2012, 12, 2782–2791. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, Z.G. Microfluidic stretchable RF electronics. Lab Chip 2010, 10, 3227–3234. [Google Scholar] [CrossRef]
- Tolley, M.T.; Shepherd, R.F.; Mosadegh, B.; Galloway, K.C.; Wehner, M.; Karpelson, M.; Wood, R.J.; Whitesides, G.M. A resilient, untethered soft robot. Soft Robot. 2014, 1, 213–223. [Google Scholar] [CrossRef]
- Morin, S.A.; Shepherd, R.F.; Kwok, S.W.; Stokes, A.A.; Nemiroski, A.; Whitesides, G.M. Camouflage and display for soft machines. Science 2012, 337, 828–832. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Nandagopal, S.; Lin, F. Microfluidics for food, agriculture and biosystems industries. Lab Chip 2011, 11, 1574–1586. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef]
- Abdelgawad, M.; Watson, M.W.L.; Young, E.W.K.; Mudrik, J.M.; Ungrin, M.D.; Wheeler, A.R. Soft lithography: Masters on demand. Lab Chip 2008, 8, 1379–1385. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yang, H.; Kim, K.; Lim, Y.T.; Pyo, H.-B. Study of SU-8 to make a Ni master-mold: Adhesion, sidewall profile, and removal. Electrophoresis 2006, 27, 3284–3296. [Google Scholar] [CrossRef]
- Jeong, S.H.; Hjort, K.; Wu, Z.G. Adhesive transfer soft lithography: Low-cost and flexible rapid prototyping of microfluidic devices. Micro Nanosyst. 2014, 6, 42–49. [Google Scholar] [CrossRef]
- Sun, Y.; Kwok, Y.C.; Nguyen, N.-T. Low-pressure, high-temperature thermal bonding of polymeric microfluidic devices and their applications for electrophoretic separation. J. Micromech. Microeng. 2006, 16, 1681–1688. [Google Scholar] [CrossRef]
- Mosadegh, B.; Lockett, M.R.; Minn, K.T.; Simon, K.A.; Gilbert, K.; Hillier, S.; Newsome, D.; Li, H.; Hall, A.B.; Boucher, D.M.; et al. A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaterials 2015, 52, 262–271. [Google Scholar] [CrossRef]
- Zimmermann, M.; Schmid, H.; Hunziker, P.; Delamarche, E. Capillary pumps for autonomous capillary systems. Lab Chip 2007, 7, 119–125. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Liu, M.; Yang, J. A lab-on-CD prototype for high-speed blood separation. J. Micromech. Microeng. 2008, 18, 125025–125031. [Google Scholar] [CrossRef]
- Kaigala, G.V.; Ho, S.; Penterman, R.; Backhouse, C.J. Rapid prototyping of microfluidic devices with a wax printer. Lab Chip 2007, 7, 384–387. [Google Scholar] [CrossRef]
- Becker, H.; Heim, U. Hot embossing as a method for the fabrication of polymer high aspect ratio structures. Sens. Actuators A Phys. 2000, 83, 130–135. [Google Scholar] [CrossRef]
- Grimes, A.; Breslauer, D.N.; Long, M.; Pegan, J.; Lee, L.P.; Khine, M. Shrinky-Dink microfluidics: Rapid generation of deep and rounded patterns. Lab Chip 2008, 8, 170–172. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 33, 491–499. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 processing for low-cost microstructures fabrication without cleanroom facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; van der Wijngaart, W.; Griss, P.; Niklaus, F.; Stemme, G. Hydrophobic valves of plasma deposited octafluorocyclobutane in DRIE channels. Sens. Actuators B Chem. 2001, 75, 136–141. [Google Scholar] [CrossRef]
- Esch, M.B.; Kapur, S.; Irizarry, G.; Genova, V. Influence of master fabrication techniques on the characteristics of embossed microfluidic channels. Lab Chip 2003, 3, 121–127. [Google Scholar] [CrossRef]
- Singleton, L. Manufacturing aspects of LIGA technologies. J. Photopolym. Sci. Technol. 2003, 16, 413–421. [Google Scholar] [CrossRef]
- Husny, J.; Jin, H.; Harvey, E.C.; Copper-White, J. The creation of drops in T-shaped microfluidic devices with the ‘modified’ laser LIGA technique: I. Fabrication. Smart Mater. Struct. 2005, 15, S117–S123. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.D.; Liu, C.; Lou, Z.F.; Chu, D.N.; Yu, D.H. Swelling of SU-8 structure in Ni mold fabrication by UV-LIGA technique. Microsyst. Technol. 2005, 11, 1272–1275. [Google Scholar]
- Tsai, Y.-C.; Jen, H.-P.; Lin, K.-W.; Hsieh, Y.-Z. Fabrication of microfluidic devices using dry film photoresist for microchip capillary electrophoresis. J. Chromatogr. A 2006, 1111, 267–271. [Google Scholar] [CrossRef]
- Stephan, K.; Pittet, P.; Renaud, L.; Kleimann, P.; Morin, P.; Ouaini, N.; Ferrigno, R. Fast prototyping using a dry film photoresist: Microfabrication of soft-lithography masters for microfluidic structures. J. Micromech. Microeng. 2007, 17, N69–N74. [Google Scholar] [CrossRef]
- Pinto, E.; Faustino, V.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Miranda, J.M.; Lima, R. A Rapid and low-cost nonlithographic method to fabricate biomedical microdevices for blood flow analysis. Micromachines 2015, 6, 121–135. [Google Scholar] [CrossRef]
- Glavan, A.C.; Martinez, R.V.; Maxwell, E.J.; Subramaniam, A.B.; Nunes, R.M.D.; Soh, S.; Whitesides, G.M. Rapid fabrication of pressure-driven open-channel microfluidic devices in omniphobic RF paper. Lab Chip 2013, 13, 2922–2930. [Google Scholar] [CrossRef]
- Bao, N.; Zhang, Q.; Xu, J.-J.; Chen, H.-Y. Fabrication of poly (dimethylsiloxane) microfluidic system based on masters directly printed with an office laser printer. J. Chromatogr. A 2005, 1089, 270–275. [Google Scholar] [CrossRef]
- Novak, R.; Ranu, N.; Mathies, R.A. Rapid fabrication of nickel molds for prototyping embossed plastic microfluidic devices. Lab Chip 2013, 13, 1468–1471. [Google Scholar] [CrossRef]
- Wilson, M.E.; Kota, N.; Kim, Y.T.; Wang, Y.; Stolz, D.B.; LeDuc, P.R.; Ozdoganlar, O.B. Fabrication of circular microfluidic channels by combining mechanical micromilling and soft lithography. Lab Chip 2011, 11, 1550–1555. [Google Scholar] [CrossRef]
- Singhal, J.; Pinho, D.; Lopes, R.; Sousa, P.C.; Garcia, V.; Schütte, H.; Lima, R.; Gassmann, S. Blood flow visualization and measurements in microfluidic devices fabricated by a micromilling technique. Micro Nanosyst. 2015, 7, 148–153. [Google Scholar] [CrossRef]
- Lopes, R.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Schütte, H.; Lima, R.; Gassmann, S. Low cost microfluidic device for partial cell separation: Micromilling approach. In Proceedings of the IEEE International Conference on Industrial Technology (ICIT), Seville, Spain, 17–19 March 2015; pp. 3347–3350.
- Hong, T.-F.; Ju, W.-J.; Wu, M.-C.; Tai, C.-H.; Tsai, C.-H.; Fu, L.-M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid. Nanofluid. 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Yen, M.-H.; Cheng, J.-Y.; Wei, C.-W.; Chuang, Y.-C.; Young, T.-H. Rapid cell-patterning and microfluidic chip fabrication by crack-free CO2 laser ablation on glass. J. Micromech. Microeng. 2006, 16, 1143–1153. [Google Scholar] [CrossRef]
- Nieto, D.; Couceiro, R.; Aymerich, M.; Lopez-Lopez, R.; Abal, M.; Flores-Arias, M.T. A laser-based technology for fabricating a soda-lime glass based microfluidic device for circulating tumour cell capture. Colloids Surf. B Biointerfaces 2015, 134, 363–369. [Google Scholar] [CrossRef]
- Waldbaur, A.; Carneiroand, B.; Hettich, P.; Wilhelm, E.; Rapp, B.E. Computer-aided microfluidics (CAMF): From digital 3D-CAD models to physical structures within a day. Microfluid. Nanofluid. 2013, 15, 625–635. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Simoska, O.; Pelse, I.; Weng, S.; Ingram, M. A convenient direct laser writing system for the creation of microfluidic masters. Microfluid. Nanofluid. 2015, 19, 419–426. [Google Scholar] [CrossRef]
- Itoga, K.; Kobayashi, J.; Tsuda, Y.; Yamato, M.; Okano, T. Second-generation maskless photolithography device for surface micropatterning and microfluidic channel fabrication. Anal. Chem. 2008, 80, 1323–1327. [Google Scholar] [CrossRef]
- Singh, A.; Scotti, G.; Sikanen, T.; Jokinen, V.; Franssila, S. Laser direct writing of thick hybrid polymers for microfluidic chips. Micromachines 2014, 5, 472–485. [Google Scholar] [CrossRef]
- Singh-Gasson, S.; Green, R.D.; Yue, Y.; Nelson, C.; Blattner, F.; Sussman, M.R.; Cerrina, F. Maskless fabrication of light-directed oligonucleotide microarrays using a digital micromirror array. Nat. Biotechnol. 1999, 17, 974–978. [Google Scholar] [CrossRef]
- Lee, K.-N.; Shin, D.-S.; Lee, Y.-S.; Kim, Y.-K. Protein patterning by virtual mask photolithography using a micromirror array. J. Micromech. Microeng. 2003, 13, 18–25. [Google Scholar] [CrossRef]
- Xiang, N.; Yi, H.; Chen, K.; Wang, S.; Ni, Z. Investigation of the maskless lithography technique for the rapid and cost-effective prototyping of microfluidic devices in laboratories. J. Micromech. Microeng. 2013, 23, 025016. [Google Scholar] [CrossRef]
- Thuo, M.M.; Martinez, R.V.; Lan, W.-J.; Liu, X.; Barber, J.; Atkinson, M.B.J.; Bandarage, D.; Bloch, J.-F.; Whitesides, G.M. Fabrication of low-cost paper-based microfluidic devices by embossing or cut-and-stack methods. Chem. Mater. 2014, 26, 4230–4237. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Wu, Z.G.; Willing, B.; Bjerketorp, J.; Jansson, J.K.; Hjort, K. Soft inertial microfluidics for high throughput separation of bacteria from human blood cells. Lab Chip 2009, 9, 1193–1199. [Google Scholar] [CrossRef]
| Process Steps | Time (min) |
|---|---|
| Enter cleanroom | 10 |
| Lithographic patterning 1 | 115 |
| Development | 2 |
| Rinsing and drying | 5 |
| Etching | 10 |
| Photoresist removing | 5 |
| Mask drying | 3 |
| Total | 155 |
| Process Steps | Photolithography (min) | Our Method (min) |
|---|---|---|
| Wafer cleaning | 30 | 30 |
| Dehydration bake (200 °C) | 5 | 5 |
| Cooling time | 5 | 5 |
| Spin-coating photoresist (SU-8) | 2 | 2 |
| Soft bake | 20 | 20 |
| Cooling time | 10 | 10 |
| Exposure | 3 | 8 |
| Post-exposure bake | 6 | 6 |
| Development | 10 | 10 |
| Hard bake | 15 | 15 |
| Total | 106 | 111 |
| Process Steps | Time |
|---|---|
| Coating master 1 | 4 h |
| Mixing PDMS 2 | 5 min |
| Degassing 3 | 20 min |
| Curing | 20 min |
| Punching hole and cleaning | 5 min |
| Bonding | 10 min |
| Total | 4 h 35 min |
| Method | Idea to Pattern | Pattern to Master | Master to Device | Total |
|---|---|---|---|---|
| Soft lithography | 1 h | Mask fabrication: 155 min | 4 h 35 min | 596 min |
| Master fabrication: 106 min | ||||
| Our method | 1 h | 111 min | 4 h 35 min | 446 min |
| Method | Resolution | Roughly Estimated Prototyping Time (Dependence on Pattern) | Main Instruments | Advantages | Disadvantages |
|---|---|---|---|---|---|
| This method | 25 µm | 3 h | UV laser machine | Flexible, good accuracy | Planar structure |
| Dry photoresist [40] | 20 µm | 2 h | UV oven | Fast, convenience | Mask-based necessary |
| Xurography [21] | 100 µm | 1.5 h | Cutting plotter | Fast, convenience | Limited minimum dimension and materials |
| Laser printer [43] | 50 µm | 3 h | Laser printer | Fast, convenience | Rough edge |
| Micromilling [45] | 30 µm | 4 h | Micromilling machine | Semicircular channel, durable master | High-cost initialization and easily tool breakage |
| CO2 laser [22] | 50 µm | 3 h | CO2 laser machine | Fast, convenience | Rough edge and bottom |
| Maskless photolithography [51] | 1.5 µm | 6 h | Direct laser writing machine | High precision | High-cost initialization |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xu, W.; Hou, Z.; Wu, Z. A Rapid Prototyping Technique for Microfluidics with High Robustness and Flexibility. Micromachines 2016, 7, 201. https://doi.org/10.3390/mi7110201
Liu Z, Xu W, Hou Z, Wu Z. A Rapid Prototyping Technique for Microfluidics with High Robustness and Flexibility. Micromachines. 2016; 7(11):201. https://doi.org/10.3390/mi7110201
Chicago/Turabian StyleLiu, Zhenhua, Wenchao Xu, Zining Hou, and Zhigang Wu. 2016. "A Rapid Prototyping Technique for Microfluidics with High Robustness and Flexibility" Micromachines 7, no. 11: 201. https://doi.org/10.3390/mi7110201






