Cell Migration According to Shape of Graphene Oxide Micropatterns
Abstract
:1. Introduction
2. Materials and Methods
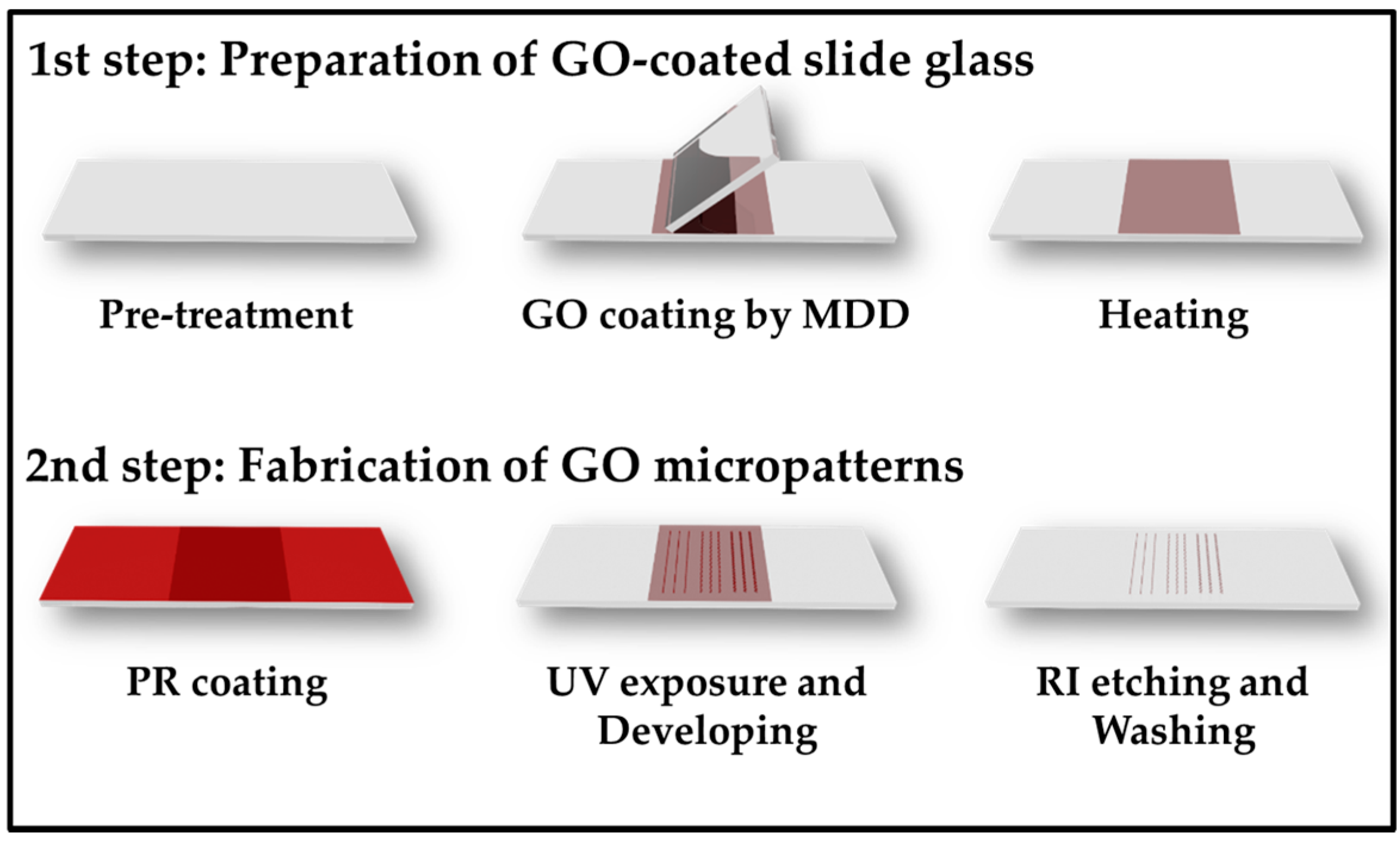
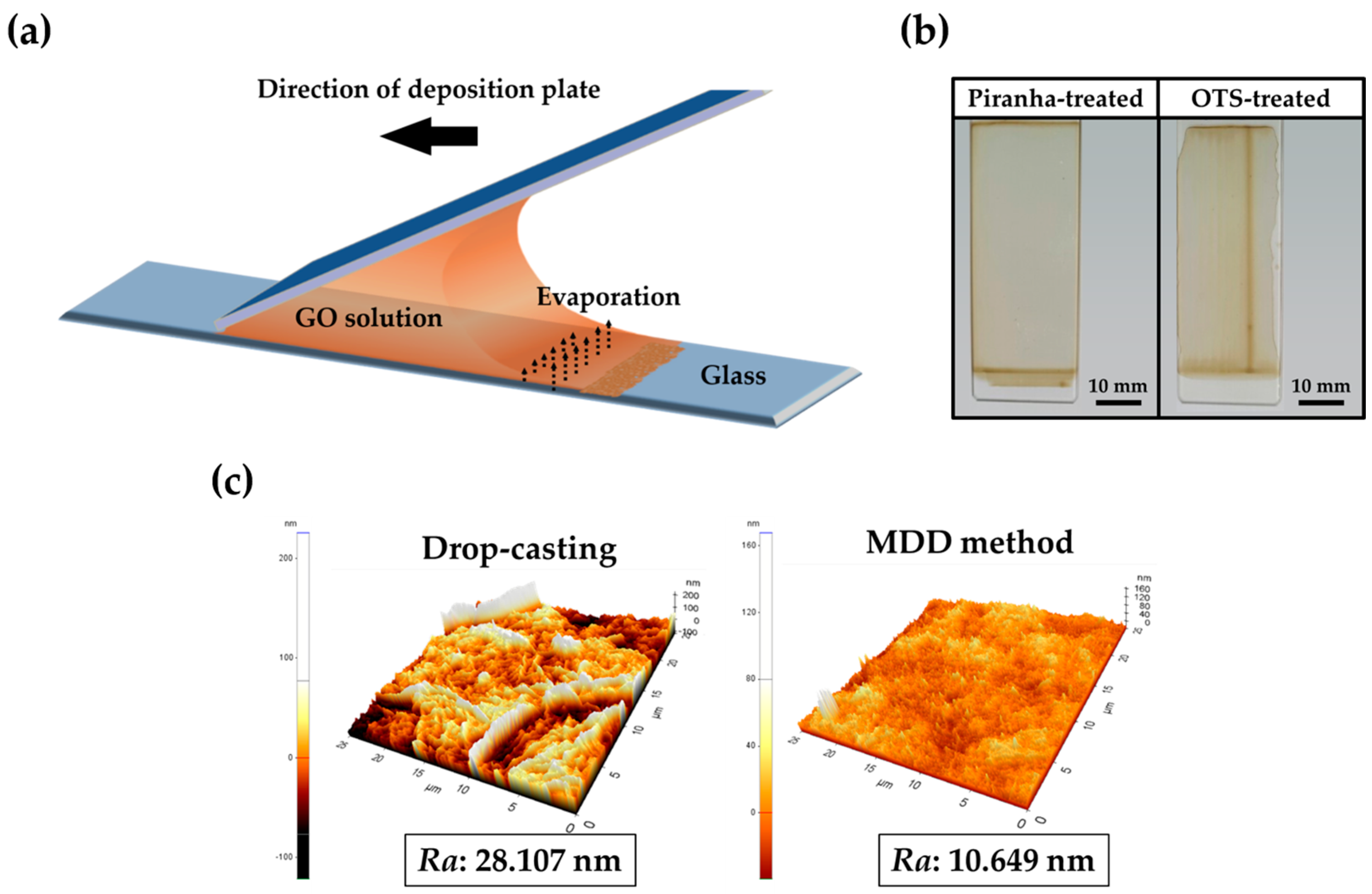
2.1. Preparation of GO-Coated Substrates and GO Micropatterns
2.2. Physicochemical Characterization of GO Micropatterns
2.3. Time-Lapse Imaging and Analysis of Cell Migration on GO Micropatterns
2.4. Statistical Analysis
3. Results and Discussion
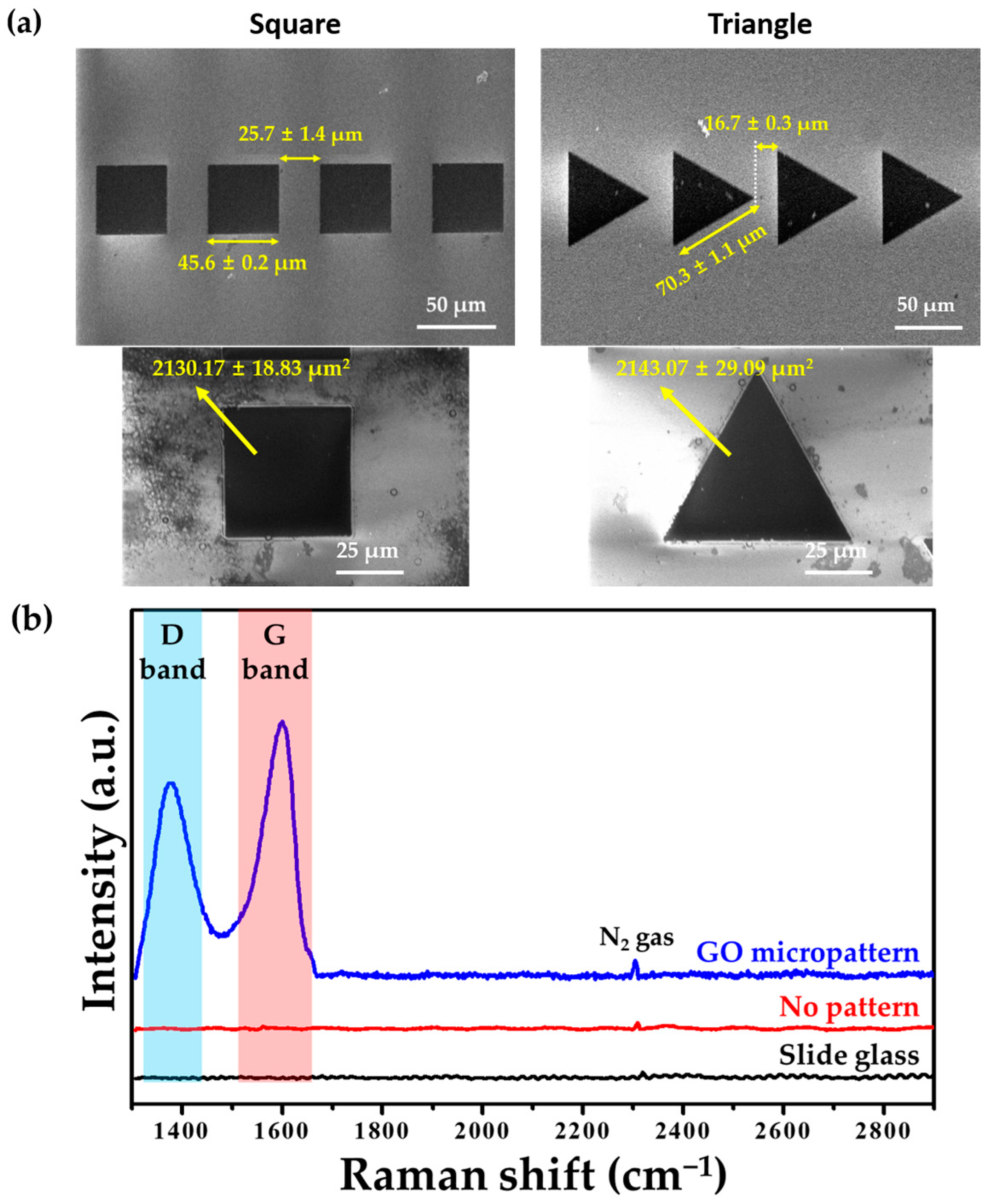
3.1. Preparation of GO-Coated Substrates and GO Micropatterns
3.2. Physicochemical Characeristics of GO Micropattenrs
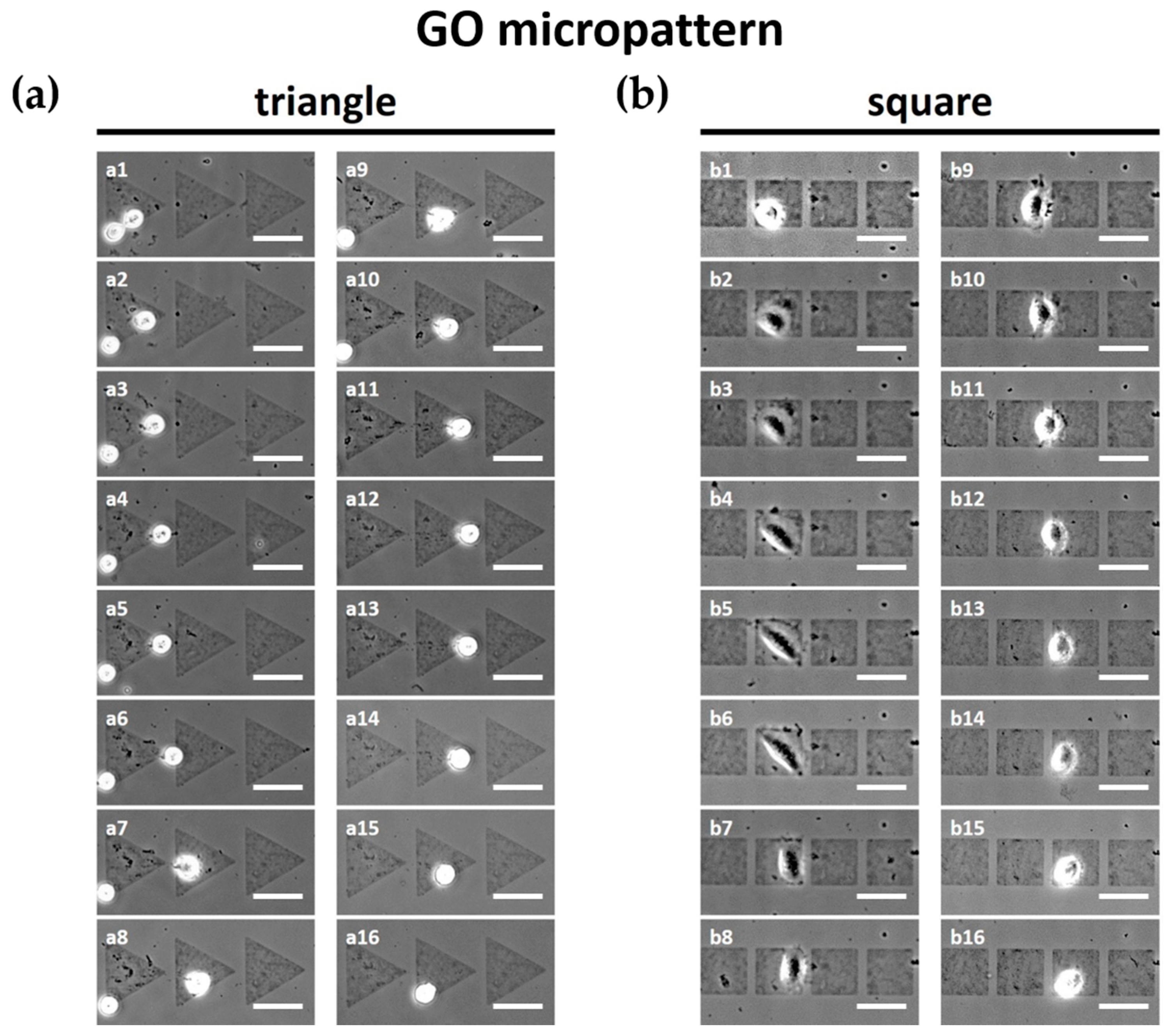
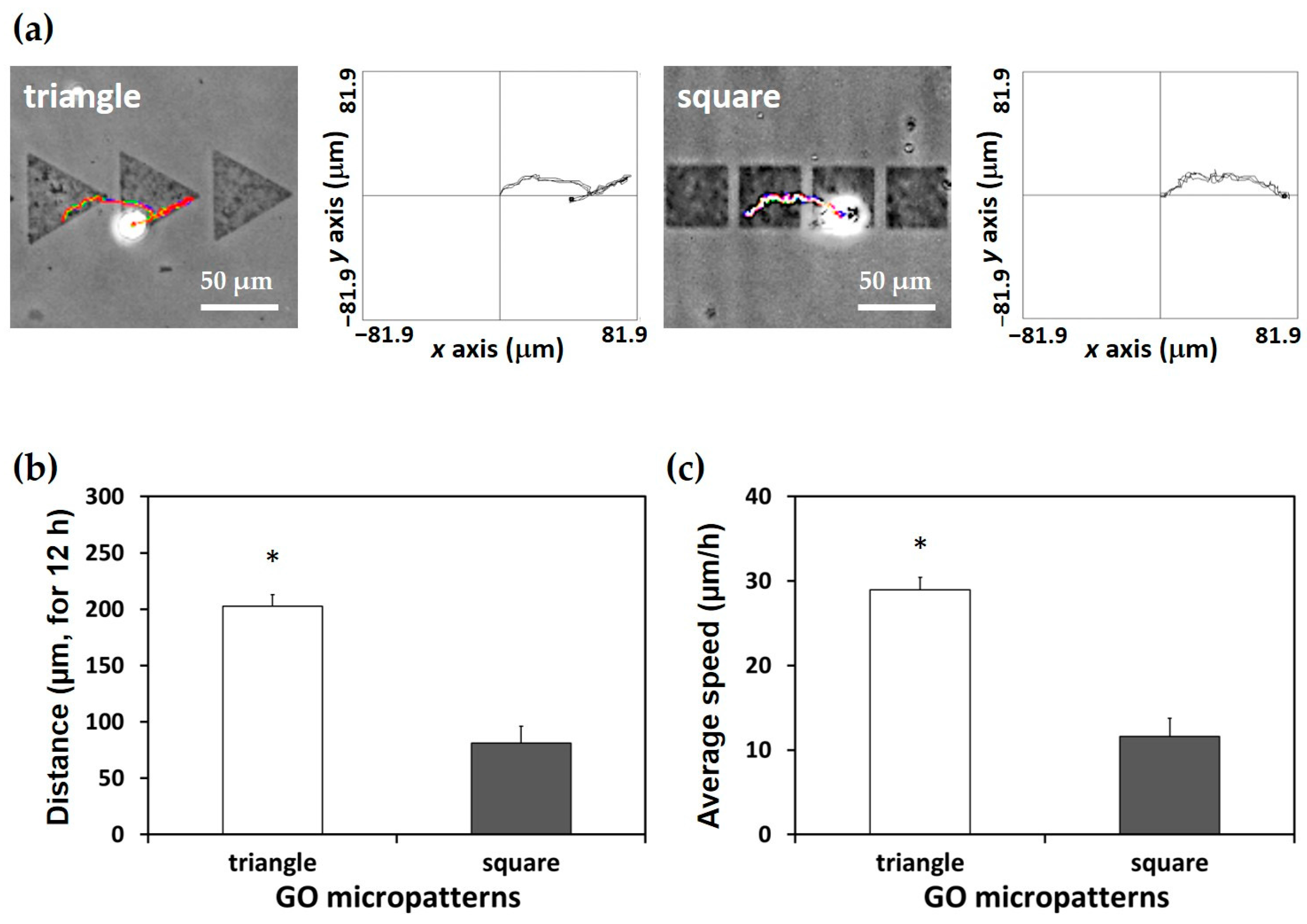
3.3. Effects of GO Micropatterns on Cell Migration
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, K.H.; Jeong, D.-W.; Jang, N.-S.; Ha, S.-H.; Kim, J.-M. Extremely stretchable conductors based on hierarchically-structured metal nanowire network. RSC Adv. 2016, 6, 56896–56902. [Google Scholar] [CrossRef]
- Voldman, J.; Gray, M.L.; Schmidt, M.A. Microfabrication in biology and medicine. Annu. Rev. Biomed. Eng. 1999, 1, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, J.H.; Shin, Y.C.; Jin, L.; Hong, S.W.; Han, D.-W.; Kim, Y.-J.; Kim, B. Stimulated myogenic differentiation of C2C12 murine myoblasts by using graphene oxide. J. Korean Phys. Soc. 2015, 67, 1910–1914. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Kim, M.J.; Hong, S.W.; Kim, B.; Hyun, J.K.; Choi, Y.S.; Park, J.-C.; Han, D.-W. Stimulating effect of graphene oxide on myogenesis of C2C12 myoblasts on RGD peptide-decorated PLGA nanofiber matrices. J. Biol. Eng. 2015, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Lee, J.H.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Kim, B.; Park, J.-C.; Han, D.-W. Synergistic effects of reduced graphene oxide and hydroxyapatite on osteogenic differentiation of MC3T3-E1 preosteoblasts. Carbon 2015, 95, 1051–1060. [Google Scholar] [CrossRef]
- Hajjar, Z.; Rashidi, A.M.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, J.H.; Shin, Y.C.; Hwang, D.-G.; Kim, J.S.; Jin, O.S.; Jin, L.; Hong, S.W.; Han, D.-W. Graphene oxide-decorated PLGA/collagen hybrid fiber sheets for application to tissue engineering scaffolds. Biomater. Res. 2014, 18, 18–24. [Google Scholar]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.-J.; Hyun, J.K.; Jung, T.-G.; Hong, S.W.; Han, D.-W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J. Nanobiotechnol. 2015, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Park, K.O.; Lee, J.H.; Park, J.H.; Shin, Y.C.; Huh, J.B.; Bae, J.-H.; Kang, S.H.; Hong, S.W.; Kim, B.; Yang, D.J. Graphene oxide-coated guided bone regeneration membranes with enhanced osteogenesis: Spectroscopic analysis and animal study. Appl. Spectrosc. Rev. 2016, 51, 540–551. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.; Shin, Y.C.; Kim, M.J.; Park, J.H.; Hong, S.W.; Kim, B.; Oh, J.-W.; Park, K.D.; Han, D.-W. In situ forming gelatin/graphene oxide hydrogels for facilitated C2C12 myoblast differentiation. Appl. Spectrosc. Rev. 2016, 51, 527–539. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, N.H.; Lee, N.R.; Chang, S.T. Meniscus-dragging deposition of single-walled carbon nanotubes for highly uniform, large-area, transparent conductors. Carbon 2014, 77, 964–972. [Google Scholar] [CrossRef]
- Ko, Y.U.; Cho, S.-R.; Choi, K.S.; Park, Y.; Kim, S.T.; Kim, N.H.; Kim, S.Y.; Chang, S.T. Microlitre scale solution processing for controlled, rapid fabrication of chemically derived graphene thin films. J. Mater. Chem. 2012, 22, 3606–3613. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, B.J.; Ko, Y.; Cho, J.H.; Chang, S.T. Surface energy engineered, high-resolution micropatterning of solution-processed reduced graphene oxide thin films. Adv. Mater. 2013, 25, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Ko, Y.; Cheon, K.H.; Yun, H.-J.; Lee, H.-K.; Kwon, S.-K.; Kim, Y.-H.; Chang, S.T.; Chung, D.S. Wafer-scale and environmentally-friendly deposition methodology for extremely uniform, high-performance transistor arrays with an ultra-low amount of polymer semiconductors. J. Mater. Chem. C 2015, 3, 2817–2822. [Google Scholar] [CrossRef]
- Gaio, N.; van Meer, B.; Quirós Solano, W.; Bergers, L.; van de Stolpe, A.; Mummery, C.; Sarro, P.M.; Dekker, R. Cytostretch, an organ-on-chip platform. Micromachines 2016, 7, 120. [Google Scholar] [CrossRef]
- Rao, S.; Tata, U.; Lin, V.K.; Chiao, J.-C. The migration of cancer cells in gradually varying chemical gradients and mechanical constraints. Micromachines 2014, 5, 13–26. [Google Scholar] [CrossRef]
- Malinauskas, M.; Rekštytė, S.; Lukoševičius, L.; Butkus, S.; Balčiūnas, E.; Pečiukaitytė, M.; Baltriukienė, D.; Bukelskienė, V.; Butkevičius, A.; Kucevičius, P. 3D microporous scaffolds manufactured via combination of fused filament fabrication and direct laser writing ablation. Micromachines 2014, 5, 839–858. [Google Scholar] [CrossRef]
- Parker, K.K.; Brock, A.L.; Brangwynne, C.; Mannix, R.J.; Wang, N.; Ostuni, E.; Geisse, N.A.; Adams, J.C.; Whitesides, G.M.; Ingber, D.E. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002, 16, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, G.; Campbell, C.J.; Bishop, K.J.; Komarova, Y.A.; Chaga, O.; Soh, S.; Huda, S.; Kandere-Grzybowska, K.; Grzybowski, B.A. Directing cell motions on micropatterned ratchets. Nat. Phys. 2009, 5, 606–612. [Google Scholar] [CrossRef]
- Caballero, D.; Comelles, J.; Piel, M.; Voituriez, R.; Riveline, D. Ratchetaxis: Long-range directed cell migration by local cues. Trends Cell Biol. 2015, 25, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Ho, C.C.; Co, C.C. Guiding cell migration using one-way micropattern arrays. Adv. Mater. 2007, 19, 1084–1090. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, M.H.; Kwon, B.-J.; Koo, M.-A.; Seon, G.M.; Lee, J.H.; Han, D.-W.; Park, J.-C. Golgi polarization effects on infiltration of mesenchymal stem cells into electrospun scaffolds by fluid shear stress: Analysis by confocal microscopy and fourier transform infrared spectroscopy. Appl. Spectrosc. Rev. 2016, 51, 570–581. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, M.H.; Kwon, B.-J.; Koo, M.-A.; Seon, G.M.; Park, J.-C. Enhancement of human mesenchymal stem cell infiltration into the electrospun poly (lactic-co-glycolic acid) scaffold by fluid shear stress. Biochem. Biophys. Res. Commun. 2015, 463, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, M.H.; Kwon, B.-J.; Koo, M.-A.; Seon, G.M.; Park, J.-C. Golgi polarization plays a role in the directional migration of neonatal dermal fibroblasts induced by the direct current electric fields. Biochem. Biophys. Res. Commun. 2015, 460, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, M.H.; Kwon, B.J.; Seo, H.J.; Koo, M.A.; You, K.E.; Kim, D.; Park, J.C. Control of Neonatal Human Dermal Fibroblast Migration on Poly(lactic-co-glycolic acid)-Coated Surfaces by Electrotaxis. J. Tissue Eng. Regen. Med. 2015. Available online: http://onlinelibrary.wiley.com/doi/10.1002/term.1986/abstract;jsessionid=C98BCABAEBE4278D12956CD07DF1A1AD.f01t01 (accessed on 8 October). [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kim, F.; Tao, A.R.; Connor, S.; Yang, P. Spontaneous formation of nanoparticle stripe patterns through dewetting. Nat. Mater. 2005, 4, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Losego, M.D.; Moh, L.; Arpin, K.A.; Cahill, D.G.; Braun, P.V. Interfacial thermal conductance in spun-cast polymer films and polymer brushes. Appl. Phys. Lett. 2010, 97, 011908. [Google Scholar] [CrossRef]
- Lee, S.C.; Some, S.; Kim, S.W.; Kim, S.J.; Seo, J.; Lee, J.; Lee, T.; Ahn, J.-H.; Choi, H.-J.; Jun, S.C. Efficient direct reduction of graphene oxide by silicon substrate. Sci. Rep. 2015, 5, 12306. [Google Scholar] [PubMed]
- Ji, Z.; Shen, X.; Li, M.; Zhou, H.; Zhu, G.; Chen, K. Synthesis of reduced graphene oxide/CeO2 nanocomposites and their photocatalytic properties. Nanotechnology 2013, 24, 115603. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.-M.; Oh, Y.-S.; Yang, Y.-H.; Lim, Y.S.; Yoon, D.H.; Lee, C.; Kim, J.-Y.; Ruoff, R.S. Unoxidized graphene/alumina nanocomposite: Fracture- and wear-resistance effects of graphene on alumina matrix. Sci. Rep. 2014, 4, 5176. [Google Scholar] [CrossRef] [PubMed]
- Poellmann, M.J.; Harrell, P.A.; King, W.P.; Johnson, A.J.W. Geometric microenvironment directs cell morphology on topographically patterned hydrogel substrates. Acta Biomater. 2010, 6, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Wasserman, M.R.; Angelini, T.E.; Millet, E.; Weitz, D.A.; Butler, J.P.; Fredberg, J.J. Physical forces during collective cell migration. Nat. Phys. 2009, 5, 426–430. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.E.; Kim, M.S.; Shin, Y.C.; Eom, S.U.; Lee, J.H.; Shin, D.-M.; Hong, S.W.; Kim, B.; Park, J.-C.; Shin, B.S.; et al. Cell Migration According to Shape of Graphene Oxide Micropatterns. Micromachines 2016, 7, 186. https://doi.org/10.3390/mi7100186
Kim SE, Kim MS, Shin YC, Eom SU, Lee JH, Shin D-M, Hong SW, Kim B, Park J-C, Shin BS, et al. Cell Migration According to Shape of Graphene Oxide Micropatterns. Micromachines. 2016; 7(10):186. https://doi.org/10.3390/mi7100186
Chicago/Turabian StyleKim, Sung Eun, Min Sung Kim, Yong Cheol Shin, Seong Un Eom, Jong Ho Lee, Dong-Myeong Shin, Suck Won Hong, Bongju Kim, Jong-Chul Park, Bo Sung Shin, and et al. 2016. "Cell Migration According to Shape of Graphene Oxide Micropatterns" Micromachines 7, no. 10: 186. https://doi.org/10.3390/mi7100186







