Aspartate Aminotransferase and Alanine Aminotransferase Detection on Paper-Based Analytical Devices with Inkjet Printer-Sprayed Reagents
Abstract
:1. Introduction
2. Materials and Methods
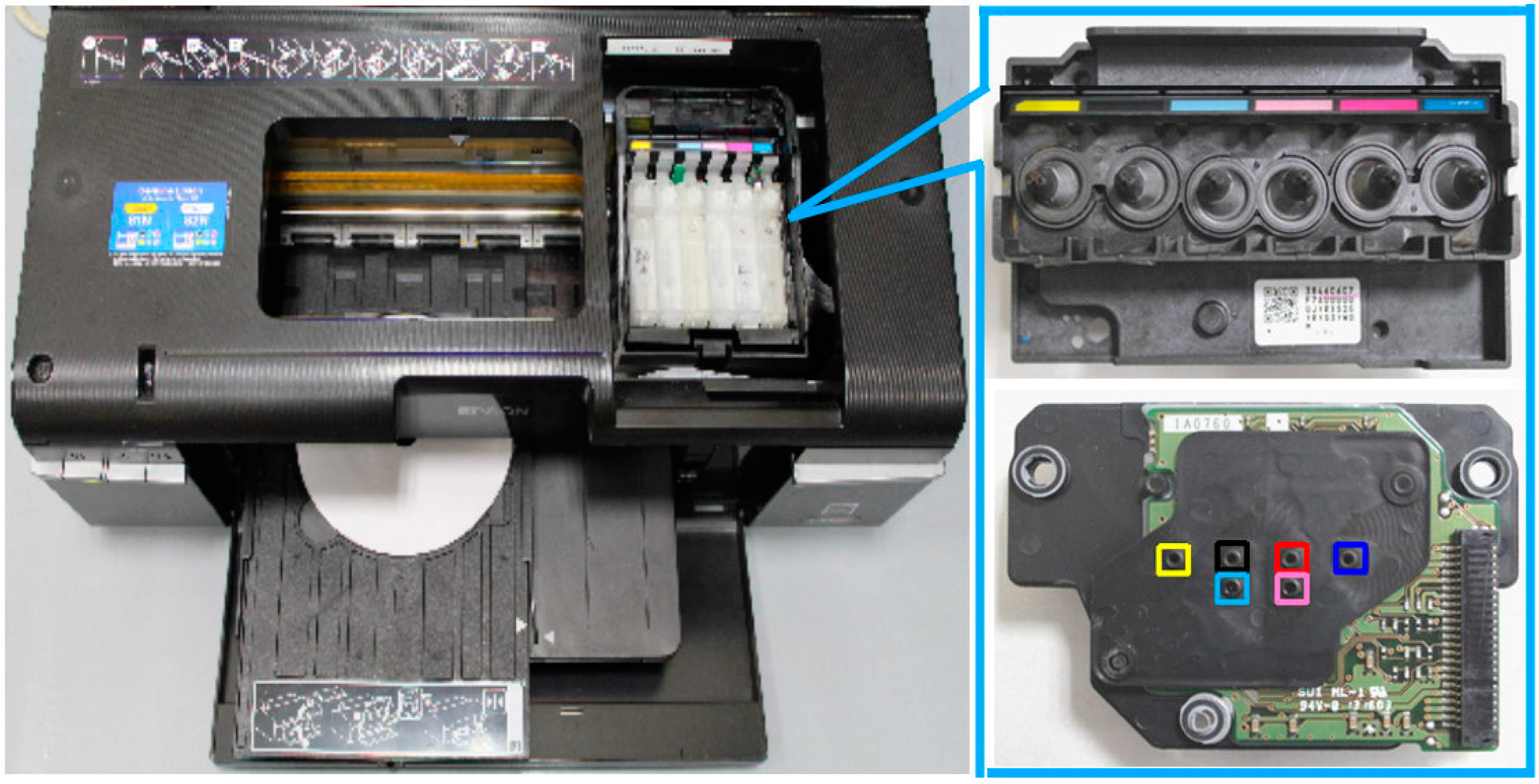
2.1. Modified Inkjet Printer
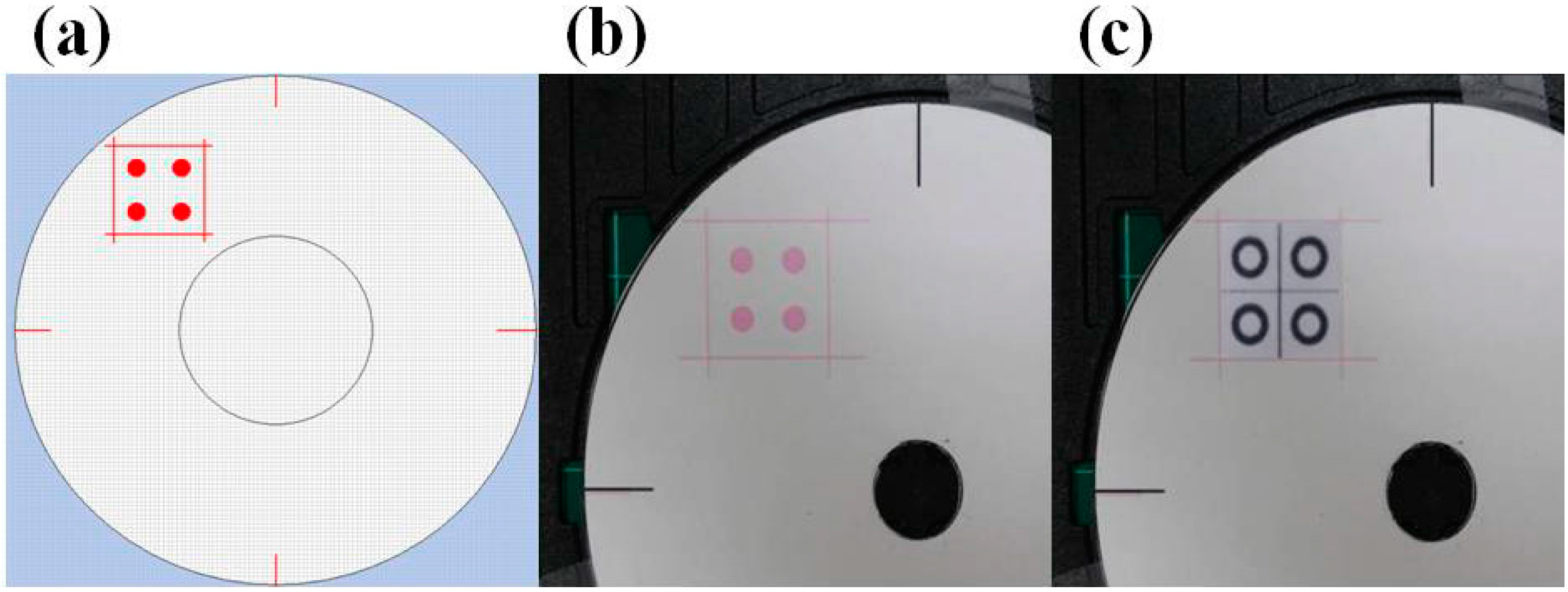
2.2. Fabrication of Paper-Based Analytical Devices
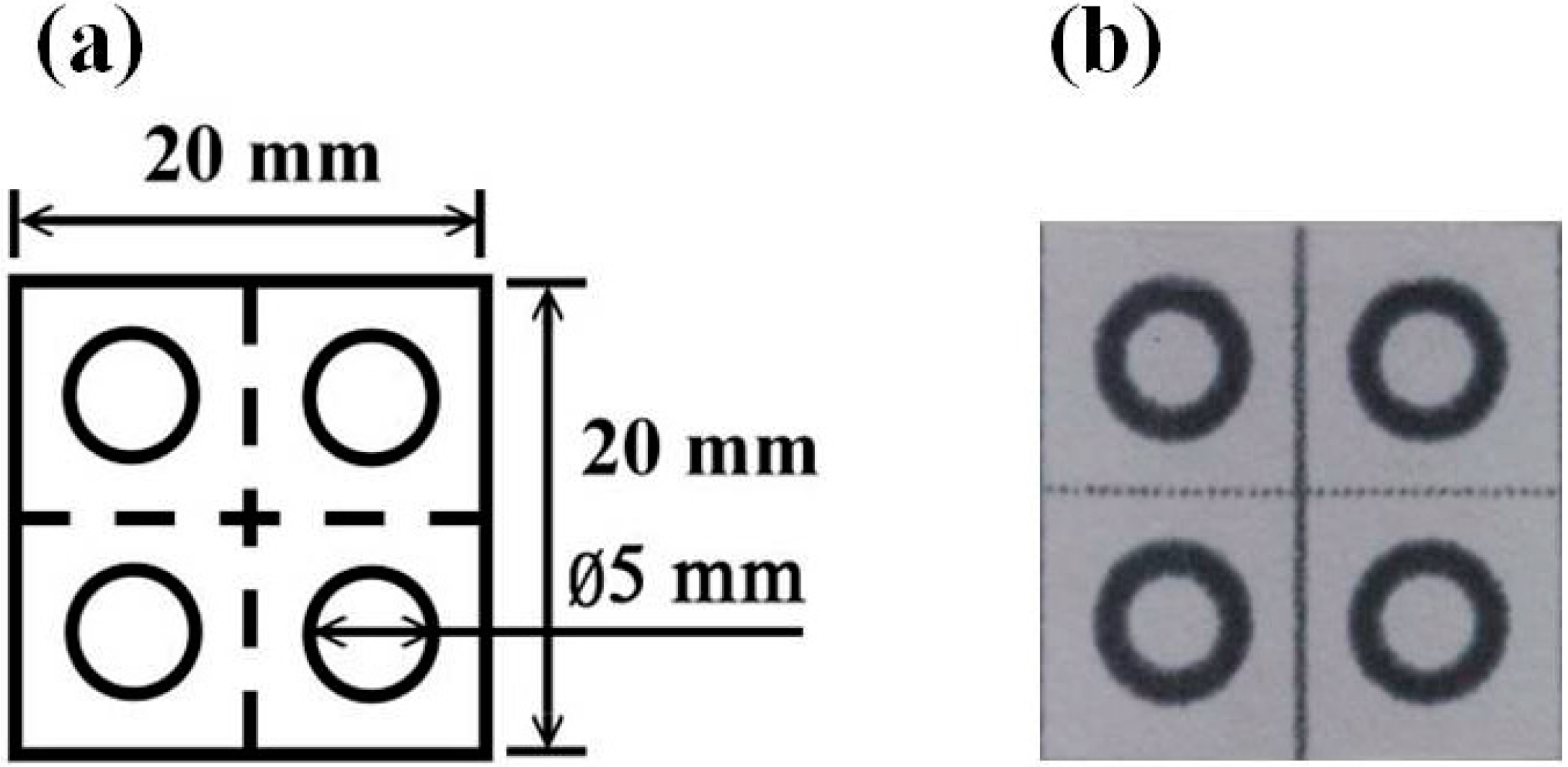
2.3. Preparation of Reagent Solutions
2.4. Reagent Spraying Process
2.5. Color Changes with Reaction Time

3. Results
3.1. Reagent Spraying Time
| Inkjet Printing Time | 4 Devices | 16 Devices | 86 Devices |
|---|---|---|---|
| Time of print 15 runs (s) | 300 | 600 | 1650 |
| Average time of print a device (s) | 75 | 37.5 | 19.18 |
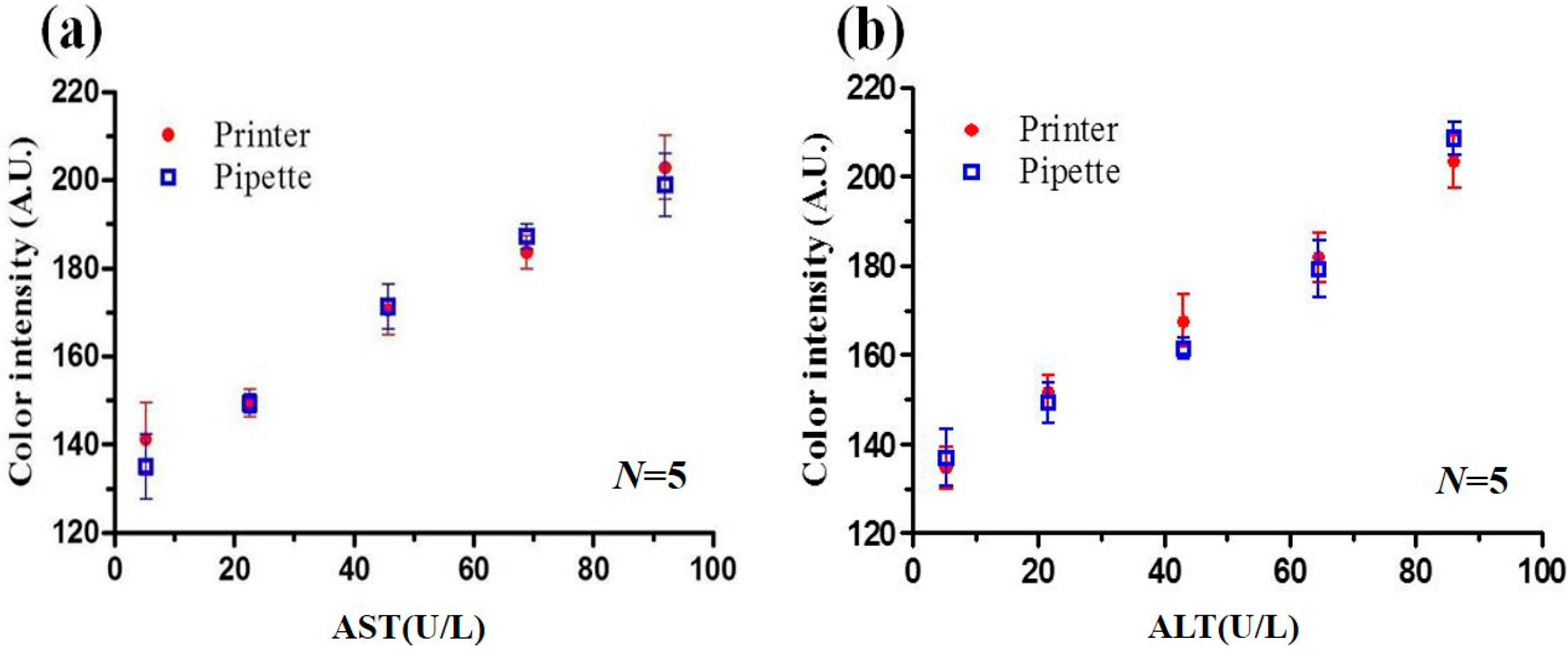
3.2. Detection Results for PADs Prepared via Pipette Titration and Inkjet Spraying
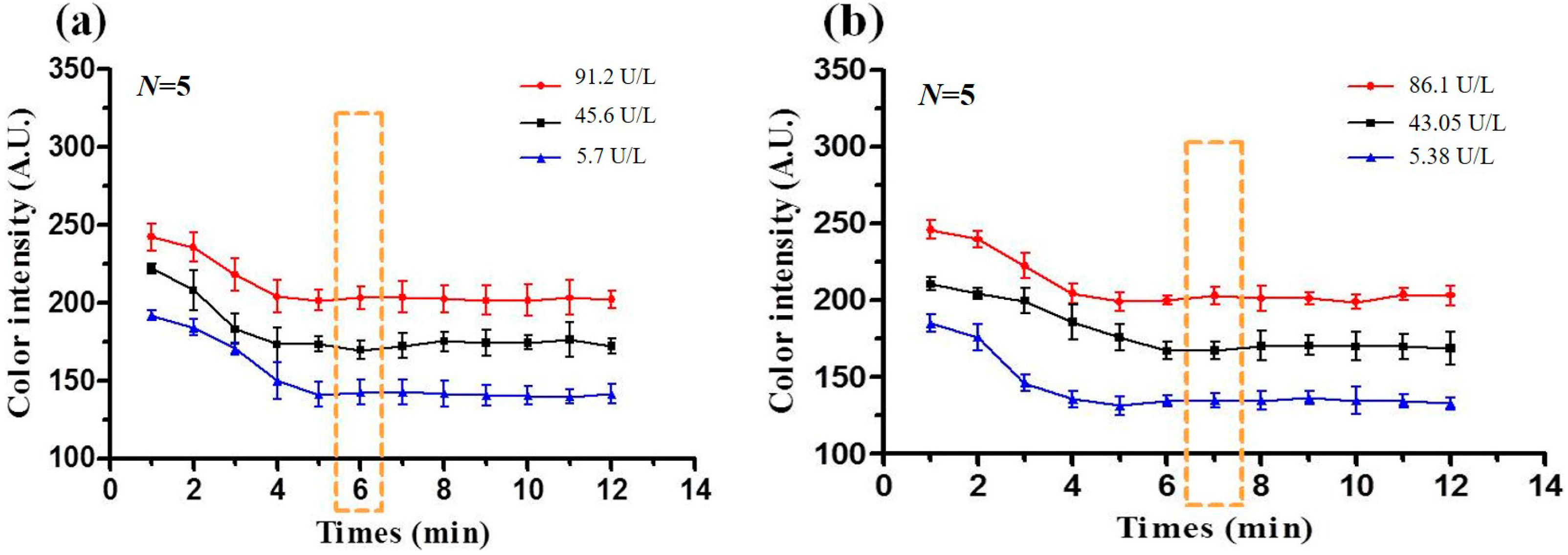
3.3. Optimal Reaction Time
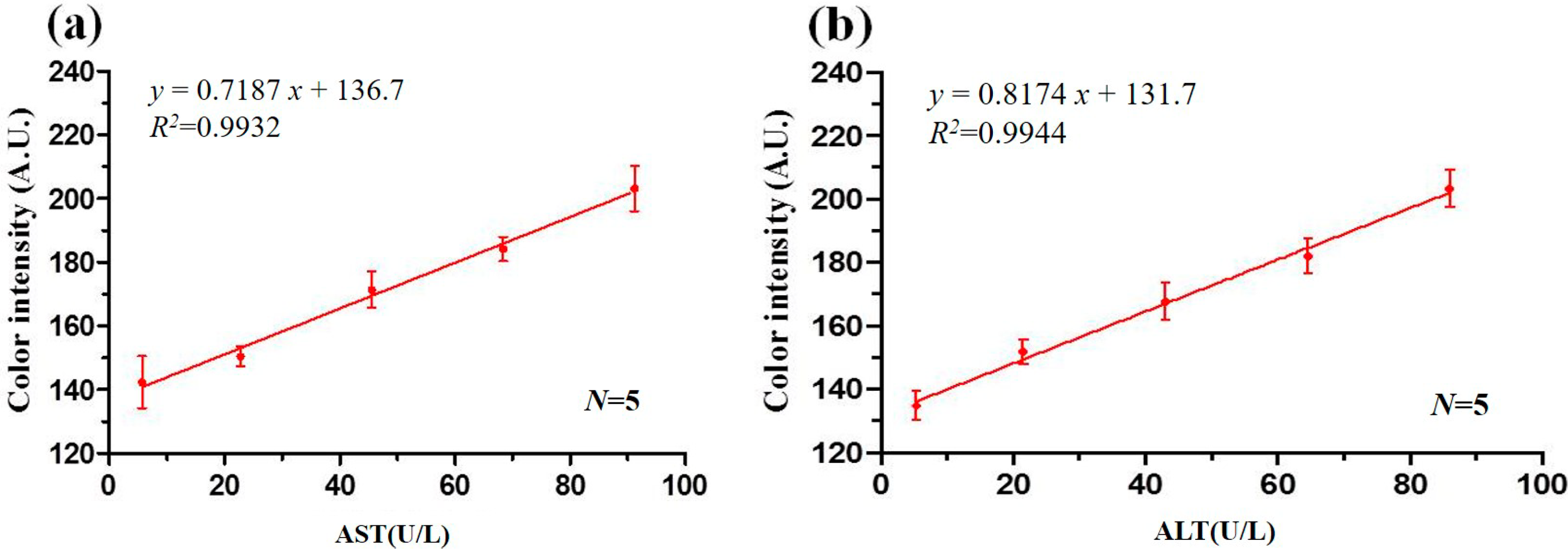
3.4. Calibration Curves
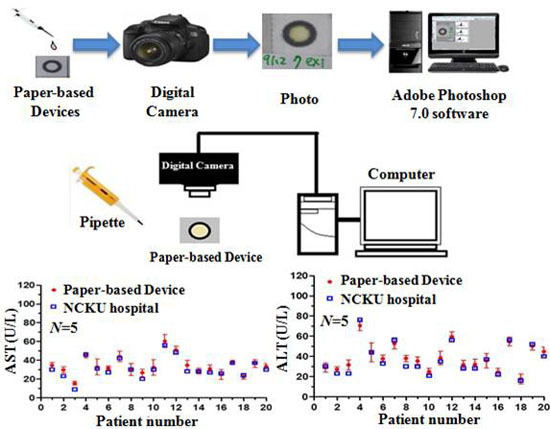
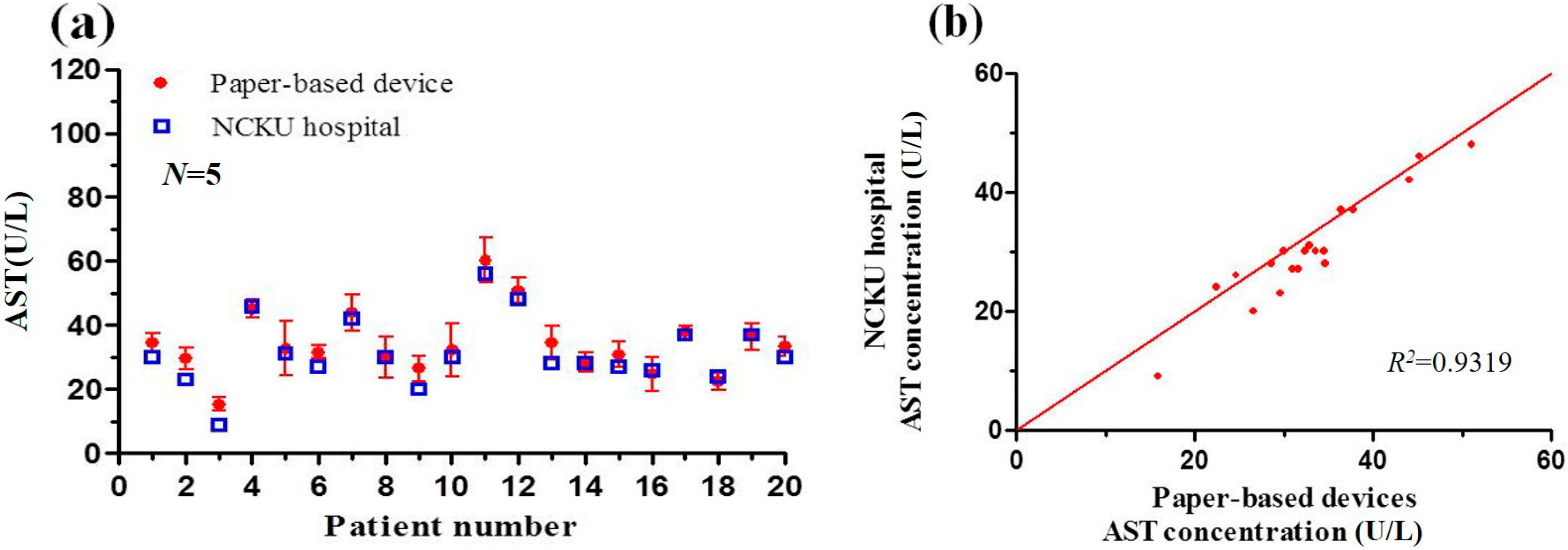
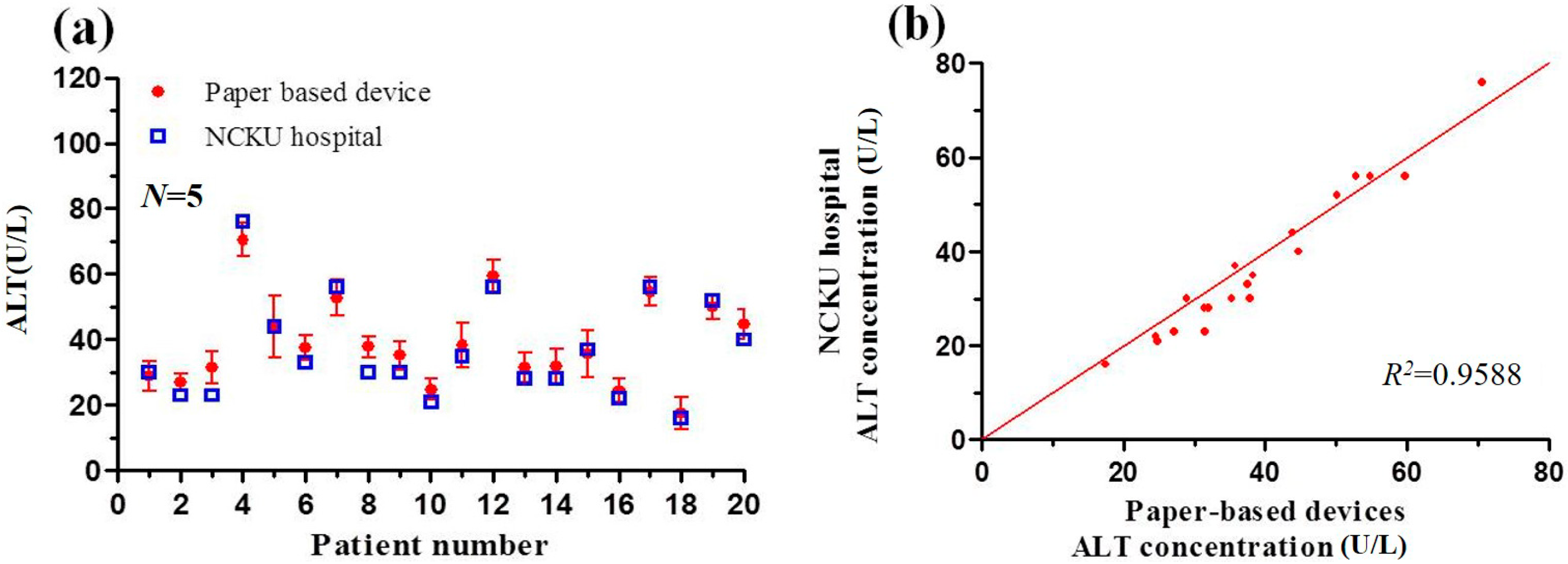
3.5. Detection Results Obtained using Paper-Based Analytical Devices and Traditional Spectrophotometric Method
| Methods | Roche | Computing and Microfluidics Lab |
|---|---|---|
| Detection method | Spectrophotometer | Paper-based devices |
| Volume of reagent | R1: 180 μL R2: 36 μL | R1 + R2: 2 μL (R1:R2 = 5:1) |
| Volume of sample | 7 μL | 2 μL |
| AST reaction time | 10 min | 6 min |
| ALT reaction time | 10 min | 7 min |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mukai, M.; Ozasa, K.; Hayashi, K.; Kawai, K. Various S-GOT/S-GPT ratios in nonviral liver disorders and related physical conditions and life-style. Dig. Dis. Sci. 2002, 47, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Mussoa, G.; Gambinob, R.; Cassaderb, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Choi, Y.K.; Im, H.S.; Yarimaga, O.; Yoon, E.; Kim, H.S. Aspartate aminotransferase (AST/GOT) and Alanine aminotransferase (ALT/GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Karmen, A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J. Clin. Investig. 1955, 34, 131–133. [Google Scholar] [PubMed]
- Itoh, H.; Srere, P.A. A new assay for glutamate-oxaloacetate transaminase. Anal. Biochem. 1970, 35, 405–410. [Google Scholar] [CrossRef]
- Janasek, D.; Spohn, U. Chemiluminometric flow injection analysis procedures for the enzymatic determination of l-alanine, α-ketoglutarate and l-glutamate. Biosens. Bioelectron. 1999, 14, 123–129. [Google Scholar] [CrossRef]
- Babson, A.L.; Shapiro, P.O.; Williams, P.A.R.; Phillips, G.E. The use of a diazonium salt for the determination of glutamic-oxalacetic transaminase in serum. Clin. Chim. Acta 1962, 7, 199–205. [Google Scholar] [CrossRef]
- Lippi, U.; Guidi, G. A new colorimetric ultramicromethod for serum glutamicoxalacetic and glutamic-pyruvic transaminase determination. Clin. Chim. Acta 1970, 28, 431–437. [Google Scholar] [CrossRef]
- Canepari, S.; Carunchio, V.; Girelli, A.M.; Messina, A. Determination of aspartate aminotransferase activity by high-performance liquid chromatography. J. Chromatogr. B 1994, 656, 191–195. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-cost printing of poly(dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Rolland, J.P.; Whitesides, G.M. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012, 4, 152ra129. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Kim, Y.G.; Chung, B.G.; Demirci, U.; Khademhosseini, A. Nano/microfluidics for diagnosis of infectious diseases in developing countries. Adv. Drug Deliv. Rev. 2010, 62, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Liang, Y.; Zhang, Y.; Le, S.; Lia, D.; Zhang, S. One-step patterning of hollow microstructures in paper by laser cutting to create microfluidic analytical devices. Analyst 2013, 138, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, L.; Zheng, Z. Multiplex microfluidic paper-based immunoassay for the diagnosis of hepatitis C virus infection. Anal. Chem. 2014, 86, 5338–5344. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Johnson, A. Measurement of total antioxidant capacity in sub-μL blood samples using craft paper-based analytical devices. RSC Adv. 2015, 5, 55633–55639. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Qin, J.; Lin, B. Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal. Chem. 2010, 82, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.W.; Wang, Z.P.; Huang, G.X.D. Investigation of wax and paper materials for the fabrication of paper-based microfluidic devices. Microsyst. Technol. 2012, 18, 649–659. [Google Scholar] [CrossRef]
- Renault, C.; Koehne, J.; Ricco, A.J.; Crooks, R.M. Three-dimensional wax patterning of paper fluidic devices. Langmuir 2014, 30, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ali, M.M.; Filipe, C.D.M.; Li, Y.; Pelton, R. Microgel-based inks for paper-suppor biosensing applications. Biomacromolecules 2008, 9, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.H.; Chu, C.H.; Yang, R.J. Bio-sample detection on paper-based devices with inkjet printer-sprayed reagents. Talanta 2015, 145, 6–11. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-L.; Chu, C.-H.; Tsai, S.-J.; Yang, R.-J. Aspartate Aminotransferase and Alanine Aminotransferase Detection on Paper-Based Analytical Devices with Inkjet Printer-Sprayed Reagents. Micromachines 2016, 7, 9. https://doi.org/10.3390/mi7010009
Wang H-L, Chu C-H, Tsai S-J, Yang R-J. Aspartate Aminotransferase and Alanine Aminotransferase Detection on Paper-Based Analytical Devices with Inkjet Printer-Sprayed Reagents. Micromachines. 2016; 7(1):9. https://doi.org/10.3390/mi7010009
Chicago/Turabian StyleWang, Hsiang-Li, Chien-Hung Chu, Sing-Jyun Tsai, and Ruey-Jen Yang. 2016. "Aspartate Aminotransferase and Alanine Aminotransferase Detection on Paper-Based Analytical Devices with Inkjet Printer-Sprayed Reagents" Micromachines 7, no. 1: 9. https://doi.org/10.3390/mi7010009