Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
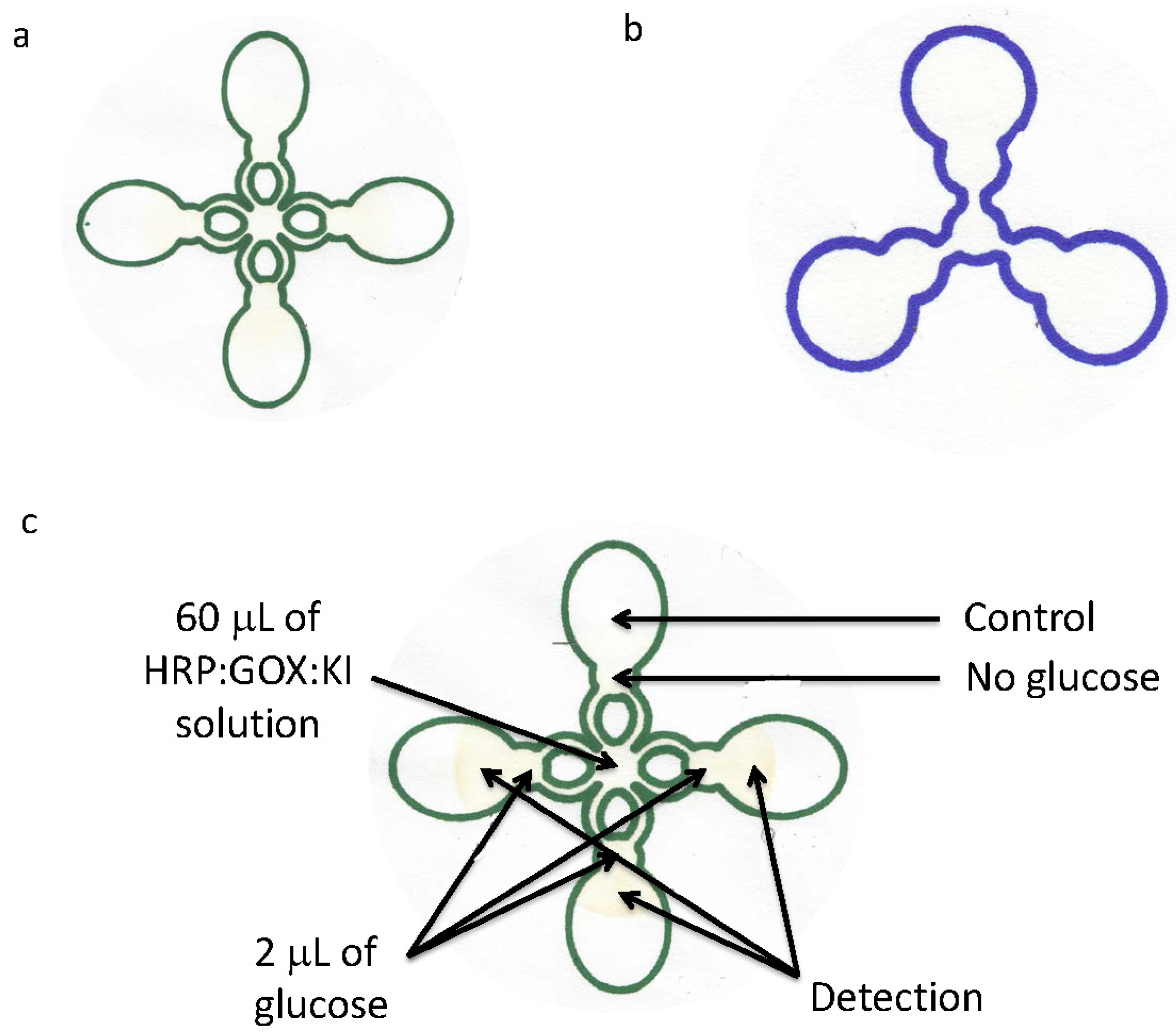
2.2. Device Fabrication
3. Results and Discussion
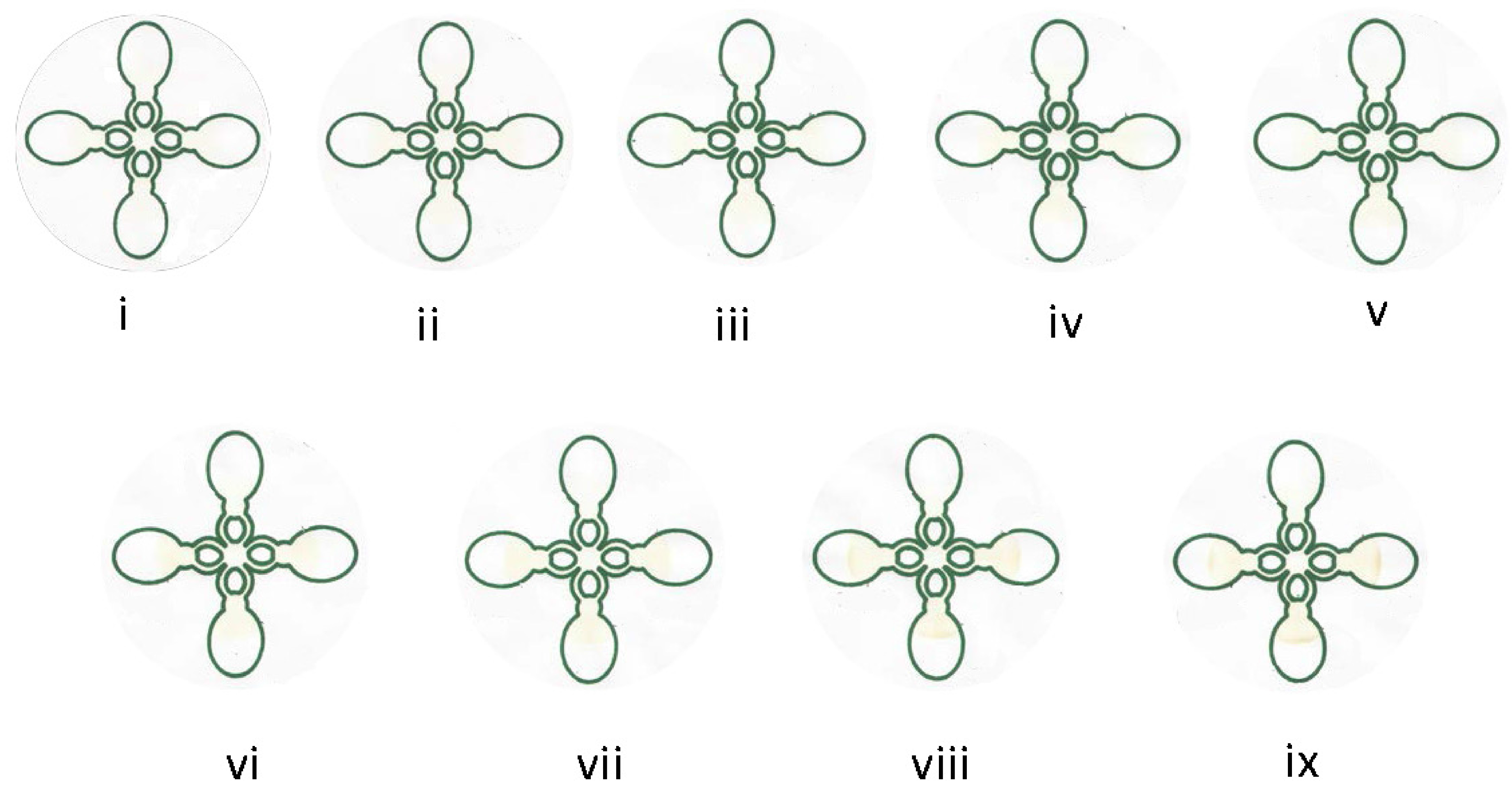


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jung, W.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Weaver, W.; Kittur, H.; Dhar, M.; di Carlo, D. Research highlights: Microfluidic point-of-care diagnostics. Lab Chip 2014, 14, 1962–1965. [Google Scholar] [CrossRef]
- Khanna, P.; Walt, D.R. Salivary diagnostics using a portable point-of-service platform: A review. Clin. Ther. 2015, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Puchberger-Enengl, D.; Krutzler, C.; Keplinger, F.; Vellekoop, M.J. Single-step design of hydrogel-based microfluidic assays for rapid diagnostics. Lab Chip 2014, 14, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Gao, X.; Jiang, L.; Qin, J. Microfluidic platform towards point-of-care diagnostics in infectious diseases. J. Chromatogr. A 2015, 1377, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.A. Paper microfluidics in bioanalysis. Bioanalysis 2014, 6, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W., III; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-cost printing of poly(dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Phillips, S.T.; Vella, S.J.; Martinez, A.W.; Whitesides, G.M. Paper microzone plates. Anal. Chem. 2009, 81, 5990–5998. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Lepore, A.L.; Kurian, J.A.; Martinez, A.W. Fully enclosed microfluidic paper-based analytical devices. Anal. Chem. 2012, 84, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based ELISA. Angew. Chem. 2010, 122, 4881–4884. [Google Scholar] [CrossRef]
- Nie, J.; Liang, Y.; Zhang, Y.; Shangwang, L.; Li, D.; Zhang, S. One-step patterning of hollow microstructures in paper by laser cutting to create microfluidic analytical devices. Analyst 2013, 138, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.G.; Shan, X.; Pan, Z.Q.; Xu, J.J.; Lu, C.; Bao, N.; Gu, H.Y. Quantum dot (QD)-modified carbon tape electrodes for reproducible electrochemiluminescence (ECL) emission on a paper-based platform. Anal. Chem. 2012, 84, 3033–3038. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Koehne, J.; Ricco, A.J.; Crooks, R.M. Three-dimensional wax patterning of paper fluidic devices. Langmuir 2014, 30, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Song, S. Facile and precise flow control for a paper-based microfluidic device through varying paper permeability. Lab Chip 2015, 15, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.-J.; Wei, J.-F.; Xu, J.-R.; Wang, Y.-H.; Zheng, G.-X. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.M.; Valadez, H.; Estala, L.; Gomez, F.A. Paper microfluidic-based enzyme catalyzed double microreactor. Electrophoresis 2014, 35, 2417–2419. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.L.; Hogan, C.F.; Tian, J.F.; Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 2011, 83, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Paper-based microfluidic devices by plasma treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, G.; Ding, Z.W.; Chang, C.L.; Savranacde, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Fenton, E.M.; Mascareñas, M.R.; Lopez, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-cost fabrication of paper-based microfluidic devices by one-step plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, C.; Nie, J.; Le, S.; Qin, Q.; Liu, F.; Li, Y.; Li, J. Equipment-free quantitative measurement for microfluidic paper-based analytical devices fabricated using the principles of moveable-type printing. Anal. Chem. 2014, 86, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.T.; Cardoso, T.M.G.; Garcia, C.D.; Carrilho, E.; Coltro, W.K.T. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. RSC Adv. 2014, 4, 37637–37644. [Google Scholar] [CrossRef]
- Cardoso, T.M.G.; Garcia, P.T.; Coltro, W.K.T. Colorimetric determination of nitrite in clinical, food, and environmental samples using microfluidic devices stampled in paper platforms. Anal. Methods 2015, 7, 7311–7317. [Google Scholar] [CrossRef]
- Dornelas, K.L.; Dossi, N.; Piccin, E. A simple method for patterning poly(dimethylsiloxane) barriers in paper using contact-printing with low-cost rubber stamps. Anal. Chim. Acta 2015, 858, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Mazzeo, A.D.; Gong, J.L.; Martinez, A.W.; Phillips, S.T.; Jain, N.; Whitesides, G.M. Millimeter-scale contact printing of aqueous solutions using a stamp made out of paper and tape. Lab Chip 2010, 10, 3201–3205. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Xiao, X.; Fu, J.Z.; Xue, G.H. A low-cost and rapid microfluidic paper-based analytical device fabrication method: Flash foam stamp lithography. RSC Adv. 2014, 4, 63860–63865. [Google Scholar] [CrossRef]
- Weaver, A.A.; Reiser, H.; Barstis, T.; Benvenuti, M.; Ghosh, D.; Hunckler, M.; Joy, B.; Koenig, L.; Raddell, K.; Lieberman, M. Paper analytical devices for fast field screening of beta lactam antibiotics and antituberculosis pharmaceuticals. Anal. Chem. 2013, 85, 6453–6460. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Li, B.; Ellington, A.D.; Crooks, R.M. DNA detection using origami paper analytical devices. Anal. Chem. 2013, 85, 9713–9720. [Google Scholar] [CrossRef] [PubMed]
- Deiss, F.; Mazzeo, A.; Hong, E.; Ingber, D.E.; Derda, R.; Whitesides, G.M. Platform for high-throughput testing of the effect of soluble compounds on 3D cell cultures. Anal. Chem. 2013, 85, 8085–8094. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Li, X.; Fosdick, S.E.; Crooks, R.M. Hollow-channel paper analytical devices. Anal. Chem. 2013, 85, 7976–7979. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Crooks, R.M. Paper-based slipPAD for high-throughput chemical sensing. Anal. Chem. 2013, 85, 4263–4267. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ge, L.; Ge, S.; Yu, J.; Yan, M.; Huang, J. A paper-based phtoelectrochemical immunoassay for low-cost and multiplexed point-of-care testing. Chem. Commun. 2013, 49, 3294–3296. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallibu, C.; Gallibu, C.; Avoundjian, A.; Gomez, F.A. Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays. Micromachines 2016, 7, 6. https://doi.org/10.3390/mi7010006
Gallibu C, Gallibu C, Avoundjian A, Gomez FA. Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays. Micromachines. 2016; 7(1):6. https://doi.org/10.3390/mi7010006
Chicago/Turabian StyleGallibu, Coreen, Chrisha Gallibu, Ani Avoundjian, and Frank A. Gomez. 2016. "Easily Fabricated Microfluidic Devices Using Permanent Marker Inks for Enzyme Assays" Micromachines 7, no. 1: 6. https://doi.org/10.3390/mi7010006




