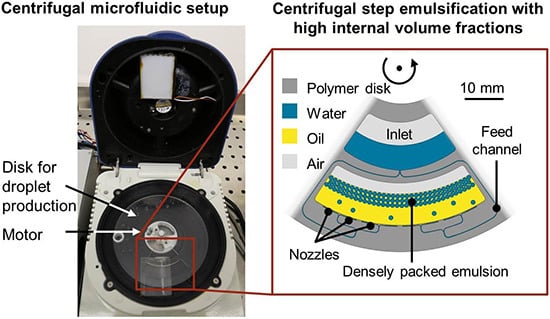
Centrifugal Step Emulsification can Produce Water in Oil Emulsions with Extremely High Internal Volume Fractions
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Working Principle
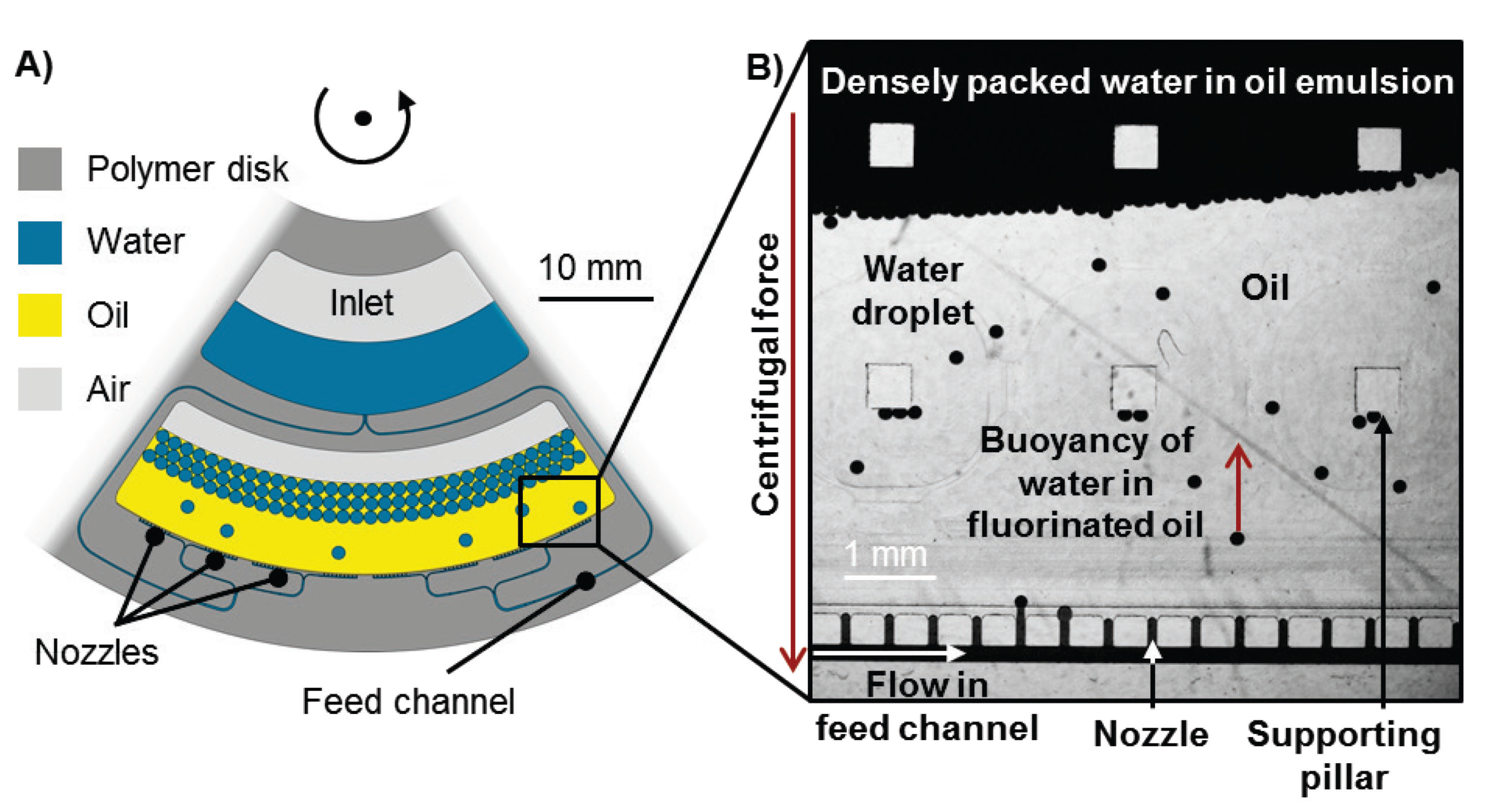
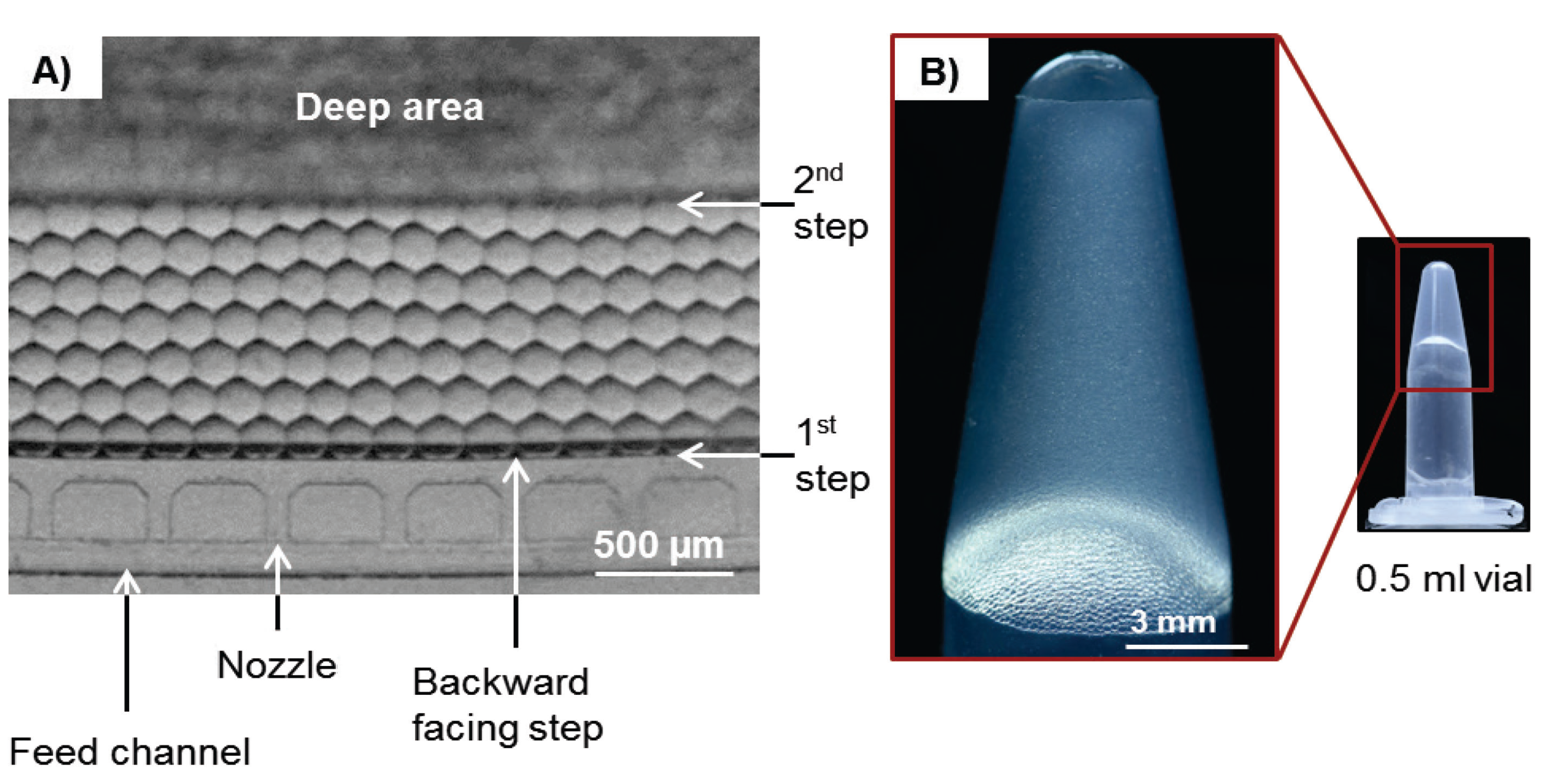
3.2. Experimental Results
| # | Aqueous Medium | Aqueous Volume | Oil Volume | Internal Volume Fraction | Spinning Frequency | Comment | Image |
|---|---|---|---|---|---|---|---|
| 1 | Water | 700 µL | 20 µL | 97.2% | 20 Hz | Functional |  |
| 2 | Water | 1000 µL | 10 µL | (99%) | 20 Hz | Merging of droplets observed | |
| 3 | Water | 700 µL | 20 µL | - | 10 Hz | Low frequency leads to accumulation of droplets at nozzles. This leads to inhomogeneous droplet production | |
| 4 | Ink Solution | 700 µL | 20 µL | 97.2% | 20 Hz | Functional |  |
| 5 | Agarose Solution | 700 µL | 50 µL | 93% | 20 Hz | Functional |  |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, Y.L.; Kim, J.W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Kang, D.K.; Ali, M.M.; Zhang, K.; Huang, S.S.; Peterson, E.; Digman, M.A.; Gratton, E.; Zhao, W. Rapid detection of single bacteria in unprocessed blood using Integrated Comprehensive Droplet Digital Detection. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Tuncer Degim, I.; Celebi, N. Controlled delivery of peptides and proteins. Curr. Pharm. Des. 2007, 13, 99–117. [Google Scholar] [CrossRef]
- Vasiljevic, D.; Parojcic, J.; Primorac, M.; Vuleta, G. An investigation into the characteristics and drug release properties of multiple W/O/W emulsion systems containing low concentration of lipophilic polymeric emulsifier. Int. J. Pharm. 2006, 309, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Leal-Calderon, F.; Thivilliers, F.; Schmitt, V. Structured emulsions. Curr. Opin. Colloid Interface Sci. 2007, 12, 206–212. [Google Scholar] [CrossRef]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Shah, R.K.; Kim, J.W.; Agresti, J.J.; Weitz, D.A.; Chu, L.Y. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter 2008, 4, 2303–2309. [Google Scholar] [CrossRef]
- Kim, J.W.; Utada, A.S.; Fernández-Nieves, A.; Hu, Z.; Weitz, D.A. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. 2007, 119, 1851–1854. [Google Scholar] [CrossRef]
- Pulko, I.; Krajnc, P. High internal phase emulsion templating—A path to hierarchically porous functional polymers. Macromol. Rapid Commun. 2012, 33, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef]
- Solans, C.; Esquena, J.; Azemar, N. Highly concentrated (gel) emulsions, versatile reaction media. Curr. Opin. Colloid Interface Sci. 2003, 8, 156–163. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M. Generation of steady liquid microthreads and micron-sized monodisperse sprays in gas streams. Phys. Rev. Lett. 1998, 80, 285–288. [Google Scholar] [CrossRef]
- Umbanhowar, P.B.; Prasad, V.; Weitz, D.A. Monodisperse emulsion generation via drop break off in a coflowing stream. Langmuir 2000, 16, 347–351. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40. [Google Scholar] [CrossRef]
- Guillot, P.; Colin, A. Stability of parallel flows in a microchannel after a T junction. Phys. Rev. E 2005, 72, 066301. [Google Scholar] [CrossRef]
- Malsch, D.; Gleichmann, N.; Kielpinski, M.; Mayer, G.; Henkel, T.; Mueller, D.; van Steijn, V.; Kleijn, C.R.; Kreutzer, M.T. Dynamics of droplet formation at T-shaped nozzles with elastic feed lines. Microfluid. Nanofluid. 2010, 8, 497–507. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, S.W.; Tan, J.; Luo, G.S. Correlations of droplet formation in T-junction microfluidic devices: From squeezing to dripping. Microfluid. Nanofluid. 2008, 5, 711–717. [Google Scholar] [CrossRef]
- Zeng, W.; Jacobi, I.; Beck, D.J.; Li, S.; Stone, H.A. Characterization of syringe-pump-driven induced pressure fluctuations in elastic microchannels. Lab Chip 2014, 15, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Korczyk, P.M.; Cybulski, O.; Makulska, S.; Garstecki, P. Effects of unsteadiness of the rates of flow on the dynamics of formation of droplets in microfluidic systems. Lab Chip 2011, 11, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Nisisako, T.; Torii, T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 2008, 8, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Young, E.W.K.; Seo, M.; Nie, Z.; Garstecki, P.; Simmons, C.A.; Kumacheva, E. Simultaneous generation of droplets with different dimensions in parallel integrated microfluidic droplet generators. Soft Matter 2008, 4, 258–262. [Google Scholar] [CrossRef]
- Priest, C.; Herminghaus, S.; Seemann, R. Generation of monodisperse gel emulsions in a microfluidic device. Appl. Phys. Lett. 2006, 88, 024106. [Google Scholar] [CrossRef]
- Mittal, N.; Cohen, C.; Bibette, J.; Bremond, N. Dynamics of step-emulsification: From a single to a collection of emulsion droplet generators. Phys. Fluid. 2014, 26, 082109. [Google Scholar] [CrossRef]
- Li, Z.; Leshansky, A.M.; Pismen, L.M.; Tabeling, P. Step-emulsification in a microfluidic device. Lab Chip 2015, 15, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Dangla, R.; Kayi, S.C.; Baroud, C.N. Droplet microfluidics driven by gradients of confinement. Proc. Natl. Acad. Sci. USA 2013, 110, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Nakajima, M.; Iwamoto, S.; Seki, M. Interfacial tension driven monodispersed droplet formation from microfabricated channel array. Langmuir 2001, 17, 5562–5566. [Google Scholar] [CrossRef]
- Schuler, F.; Schwemmer, F.; Trotter, M.; Wadle, S.; Zengerle, R.; von Stetten, F.; Paust, N. Centrifugal step emulsification applied for absolute quantification of nucleic acids by digital droplet RPA. Lab Chip 2015, 15, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M. Lab-on-a-Chip Design + Foundry-Service. Available online: http://www.hahn-schickard.de/fertigung/lab-on-a-chip-design-foundry-service/ (accessed on 1 July 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuler, F.; Paust, N.; Zengerle, R.; Von Stetten, F. Centrifugal Step Emulsification can Produce Water in Oil Emulsions with Extremely High Internal Volume Fractions. Micromachines 2015, 6, 1180-1188. https://doi.org/10.3390/mi6081180
Schuler F, Paust N, Zengerle R, Von Stetten F. Centrifugal Step Emulsification can Produce Water in Oil Emulsions with Extremely High Internal Volume Fractions. Micromachines. 2015; 6(8):1180-1188. https://doi.org/10.3390/mi6081180
Chicago/Turabian StyleSchuler, Friedrich, Nils Paust, Roland Zengerle, and Felix Von Stetten. 2015. "Centrifugal Step Emulsification can Produce Water in Oil Emulsions with Extremely High Internal Volume Fractions" Micromachines 6, no. 8: 1180-1188. https://doi.org/10.3390/mi6081180