Fiber-Based, Injection-Molded Optofluidic Systems: Improvements in Assembly and Applications
Abstract
:1. Introduction
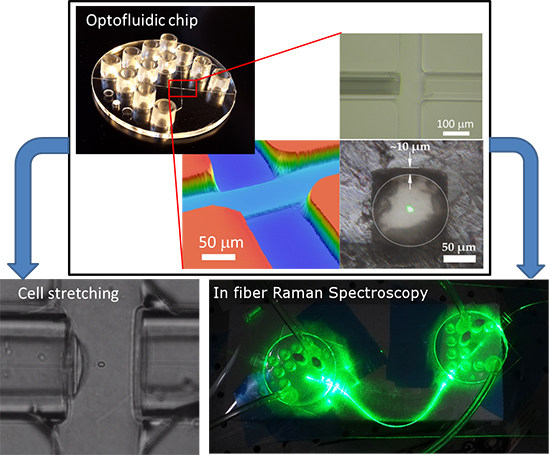
2. Results
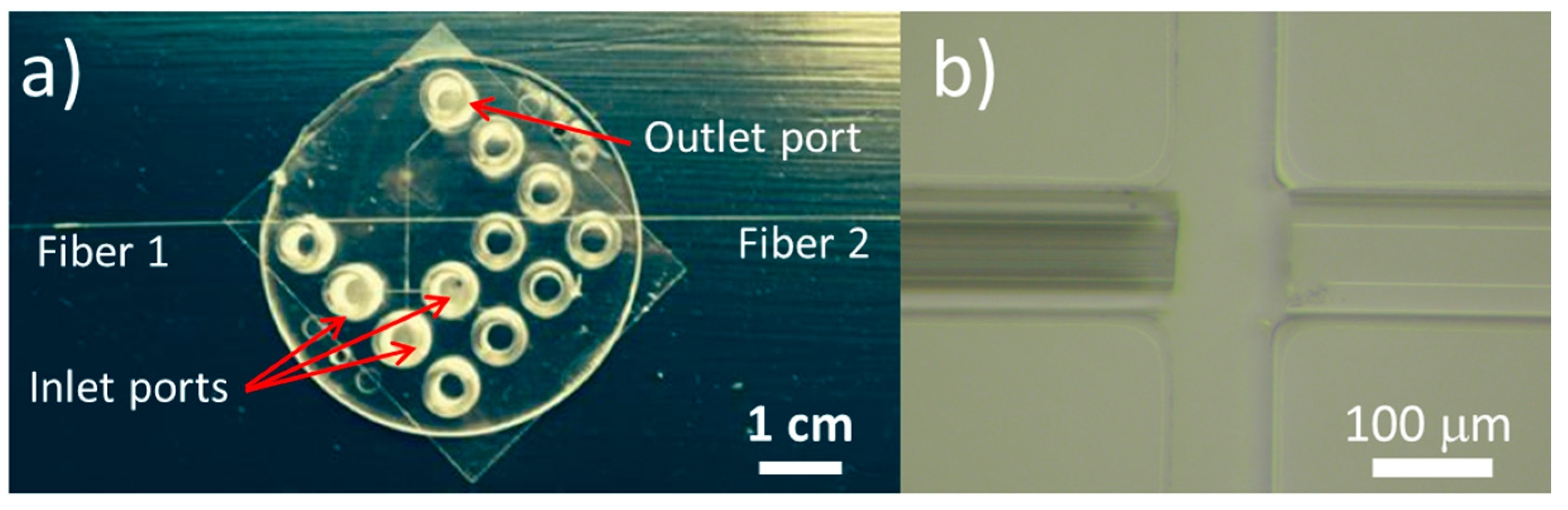
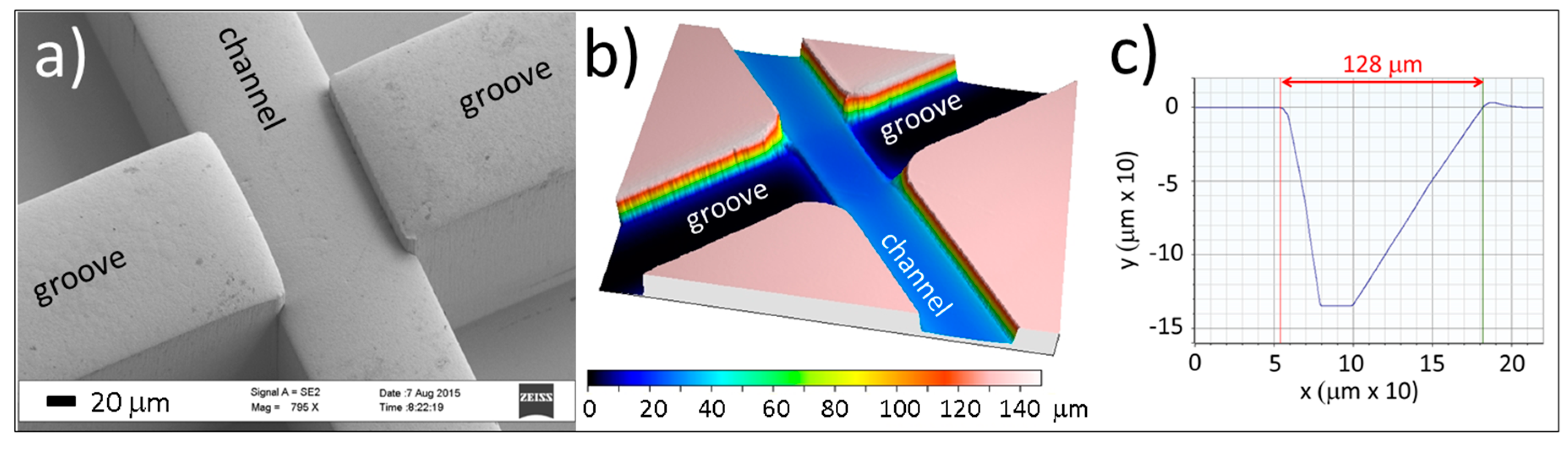
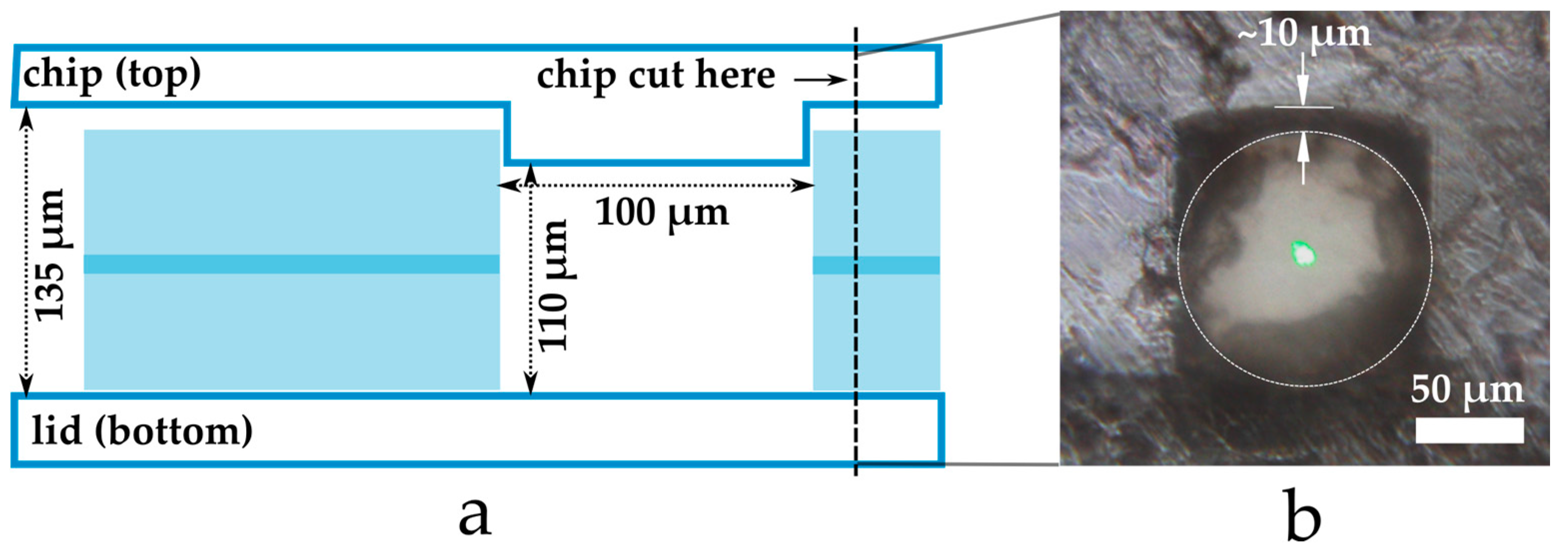
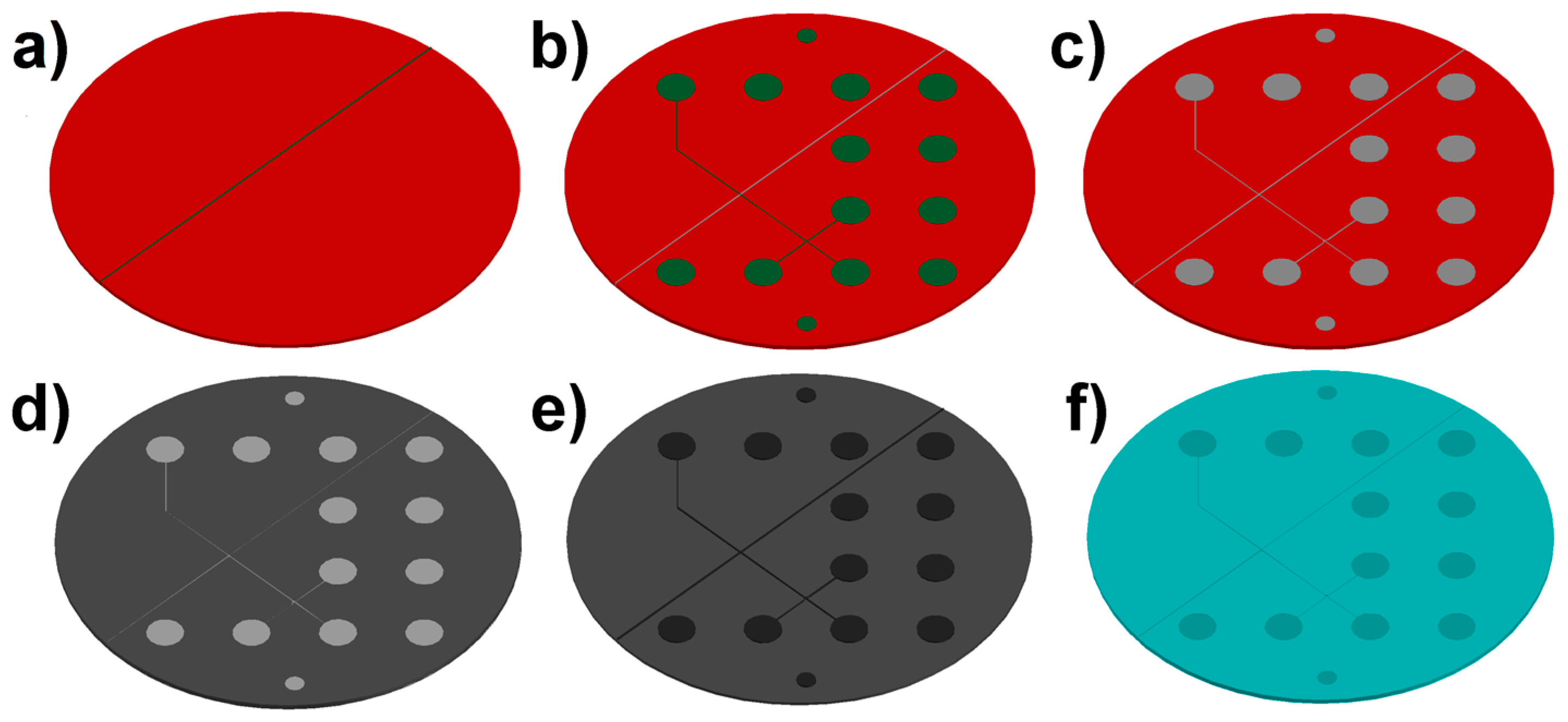
2.1. Fabrication Results
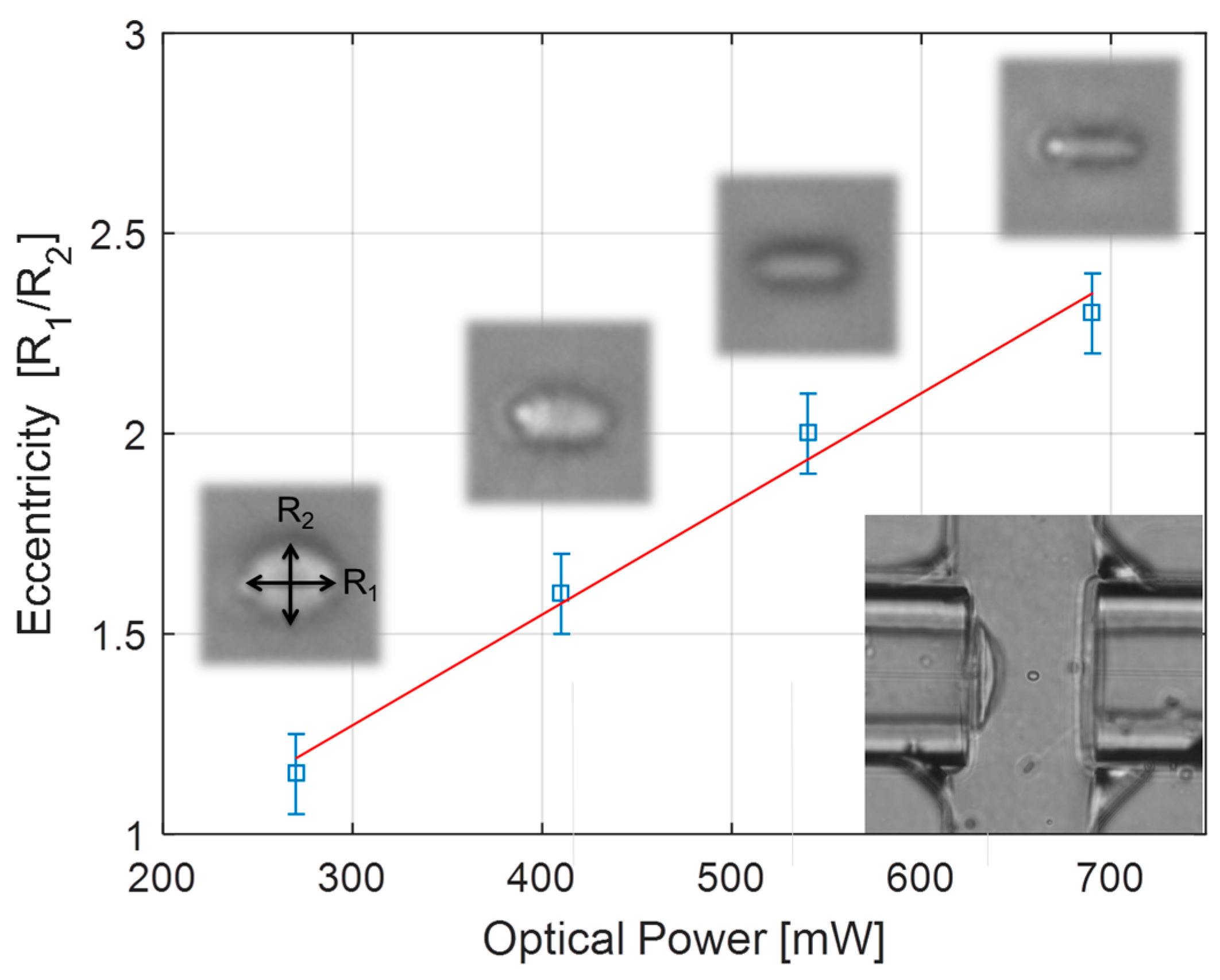
2.2. Single Chip Applied in Optical Stretching
2.3. Dual Chip Applied in Fiber-Based Raman Spectroscopy

3. Discussion
3.1. Technology Benefits for Optical Stretching
3.2. Technology Benefits for Raman Spectroscopy
4. Materials and Methods
4.1. Chip Fabrication and Assembly
4.2. Optical Alignment
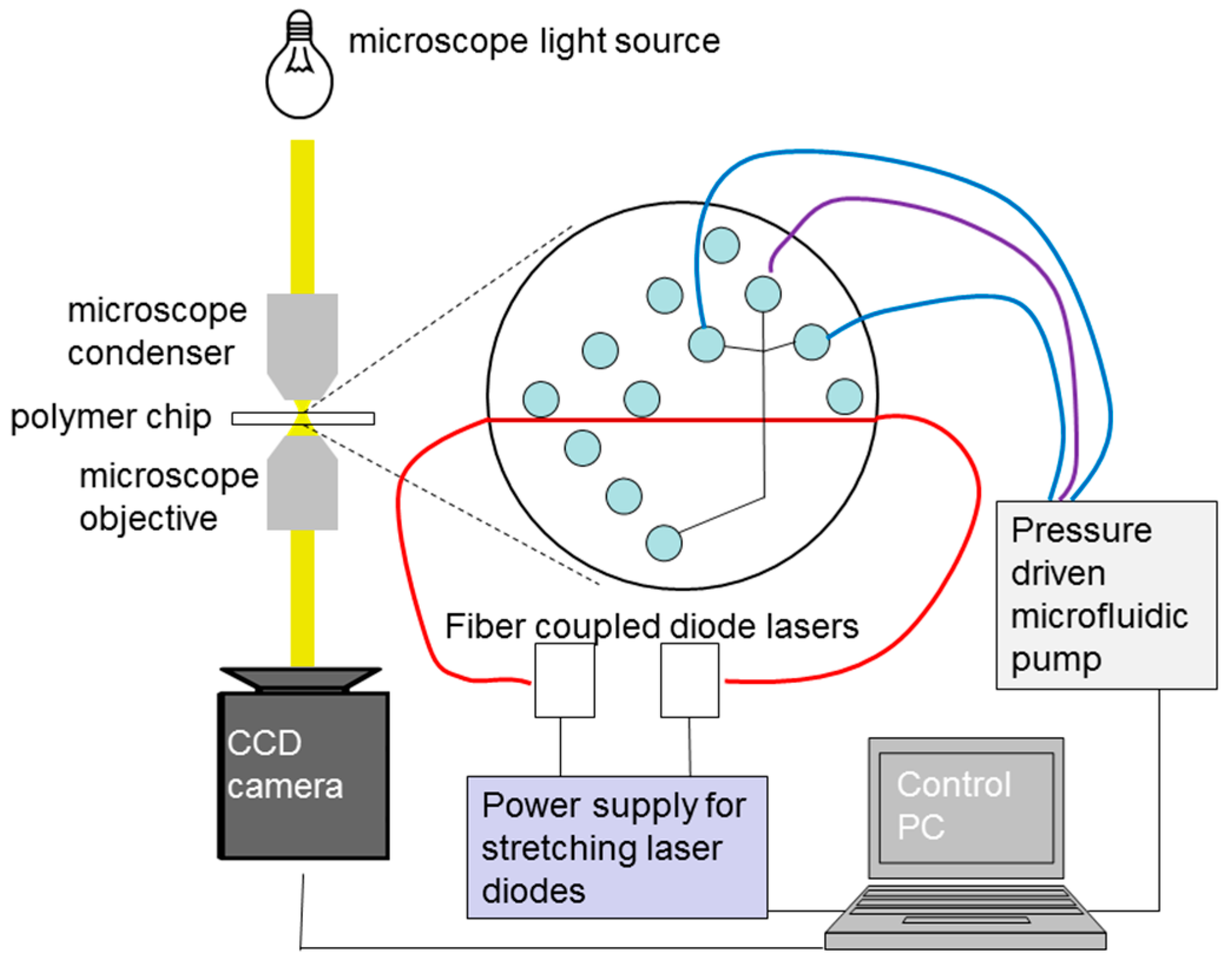
4.3. Optical Stretching Setup
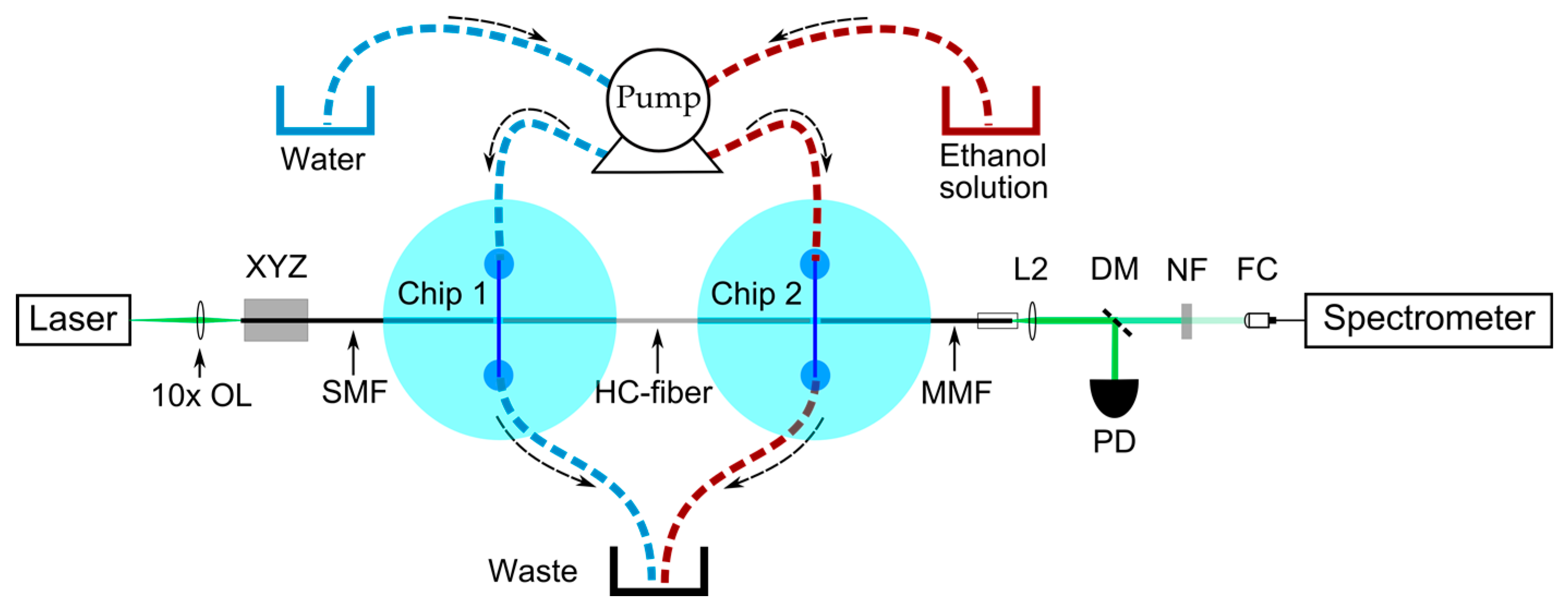
4.4. Raman Experiment Setup
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Schaap, A.; Bellouard, Y.; Rohrlack, T. Optofluidic lab-on-a-chip for rapid algae population screening. Biomed. Opt. Express 2011, 2, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Vannahme, C.; Sørensen, K.T.; Kristensen, A.; Berg-Sørensen, K. Monolithic integration of DUV-induced waveguides into plastic microfluidic chip for optical manipulation. Microelectron. Eng. 2014, 121, 5–9. [Google Scholar] [CrossRef]
- Lai, C.W.; Hsiung, S.K.; Yeh, C.L.; Chiou, A.; Lee, G.B. A cell delivery and pre-positioning system utilizing microfluidic devices for dual-beam optical trap-and-stretch. Sens. Actuators B Chem. 2008, 135, 388–397. [Google Scholar] [CrossRef]
- Ferraro, J.; Nakamoto, K.; Brown, C.W. Introductory Raman Spectroscopy, 2nd ed.; Academic Press: New York, NY, USA, 2003. [Google Scholar]
- Nunes, P.S.; Ohlsson, P.D.; Ordeig, O.; Kutter, J.P. Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications. Microfluid. Nanofluid. 2010, 9, 145–161. [Google Scholar] [CrossRef]
- Khetani, A.; Riordon, J.; Tiwari, V.; Momenpour, A.; Godin, M.; Anis, H. Hollow core photonic crystal fiber as a reusable Raman biosensor. Opt. Express 2013, 21, 12340–12350. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, P.F.; Lopacinska-Jørgensen, J.; Pedersen, J.N.; Tommerup, N.; Kristensen, A.; Flyvbjerg, H.; Silahtaroglu, A.; Marie, R.; Taboryski, R. Optical mapping of single-molecule human DNA in disposable, mass-produced all-polymer devices. J. Micromech. Microeng. 2015, 25, 105002. [Google Scholar] [CrossRef]
- Kou, Q.; Yesilyurt, I.; Studer, V.; Belotti, M.; Cambril, E.; Chen, Y. On-chip optical components and microfluidic systems. Microelectron. Eng. 2004, 73–74, 876–880. [Google Scholar] [CrossRef]
- Kou, Q.; Yesilyurt, I.; Chen, Y. Collinear dual-color laser emission from a microfluidic dye laser. Appl. Phys. Lett. 2006, 88, 091101. [Google Scholar] [CrossRef]
- Constable, A.; Kim, J.; Mervis, J.; Zarinetchi, F.; Prentiss, M. Demonstration of a fiber-optical light-force trap. Opt. Lett. 1993, 18, 1867–1869. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, B.; Schinkinger, S.; Travis, K.; Wottawah, F.; Ebert, S.; Sauer, F.; Guck, J. Reconfigurable microfluidic integration of a dual-beam laser trap with biomedical applications. Biomed. Microdevices 2007, 9, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2005, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Käs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef]
- PolyNano Summer School—Course Number 33692. Available online: http://www.kurser.dtu.dk/33692.aspx?menulanguage=en-gb) (accessed on 22 October 2015).
- Cubillas, A.M.; Unterkofler, S.; Euser, T.G.; Etzold, B.J.M.; Jones, A.C.; Sadler, P.J.; Wasserscheid, P.; Russell, P.S.J. Photonic crystal fibres for chemical sensing and photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef] [PubMed]
- Maples, R.E. Petroleum Refinery Process Economics, 2nd ed.; Pennwell Pub: Nashua, NH, USA, 2000. [Google Scholar]
- Boyaci, I.H.; Genis, H.E.; Guven, B.; Tamer, U.; Alper, N. A novel method for quantification of ethanol and methanol in distilled alcoholic beverages using Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 1171–1176. [Google Scholar] [CrossRef]
- Numata, Y.; Iida, Y.; Tanaka, H. Quantitative analysis of alcohol—Water binary solutions using Raman spectroscopy. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 1043–1049. [Google Scholar] [CrossRef]
- Tanzi, S.; Østergaard, P.F.; Matteucci, M.; Christiansen, T.L.; Cech, J.; Marie, R.; Taboryski, R.J. Fabrication of combined-scale nano-and microfluidic polymer systems using a multilevel dry etching, electroplating and molding process. J. Micromech. Microeng. 2012, 22, 115008. [Google Scholar] [CrossRef]
- Matteucci, M.; Christiansen, T.L.; Tanzi, S.; Østergaard, P.F.; Larsen, S.T.; Taboryski, R. Fabrication and characterization of injection molded multi level nano and microfluidic systems. Microelectron. Eng. 2013, 111, 294–298. [Google Scholar] [CrossRef]
- Jones, D.C.; Bennett, C.R.; Smith, M.A.; Scott, A.M. High-power beam transport through a hollow-core photonic bandgap fiber. Opt. Lett. 2014, 39, 3122–3125. [Google Scholar] [CrossRef] [PubMed]
- Ashok, P.C.; Singh, G.P.; Rendall, H.A.; Krauss, T.F.; Dholakia, K. Waveguide confined Raman spectroscopy for microfluidic interrogation. Lab Chip 2011, 11, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.O.S.; Chen, J.S.Y.; Euser, T.G.; Russell, P.S.J.; Jones, A.C. Photonic crystal fibre as an optofluidic reactor for the measurement of photochemical kinetics with sub-picomole sensitivity. Lab Chip 2012, 12, 3356–3361. [Google Scholar] [CrossRef] [PubMed]
- Utko, P.; Persson, F.; Kristensen, A.; Larsen, N.B. Injection molded nanofluidic chips: Fabrication method and functional tests using single-molecule DNA experiments. Lab Chip 2011, 11, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Mucha, J.M.; Szytuła, A.; Kwiatkowska, C.J. Magnetic properties of nickel-vanadium alloys. J. Magn. Magn. Mater. 1984, 42, 53–58. [Google Scholar] [CrossRef]
- Seryogin, G.; Golovato, S.; Smith, S.; Williams, K.; Limburn, N.; Winn, A.; Mundada, G.; Adema, G. NiV Stress Control Utilizing PVD with an Ar/N2 Gas Mixture. In Proceedings of the 45th International Symposium on Microelectronics, San Diego, CA, USA, 9–13 September 2012; pp. 73–78.
- Buck, J.A. Fundamentals of Optical Fibers, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Antonopoulos, G.; Benabid, F.; Birks, T.A.; Bird, D.M.; Knight, J.C.; Russell, P.S.J. Experimental demonstration of the frequency shift of bandgaps in photonic crystal fibers due to refractive index scaling. Opt. Express 2006, 14, 3000–3006. [Google Scholar] [CrossRef] [PubMed]
- Bland-Hawthorn, J.; Ellis, S.C.; Leon-Saval, S.G.; Haynes, R.; Roth, M.M.; Löhmannsröben, H.G.; Horton, A.J.; Cuby, J.G.; Birks, T.A.; Lawrence, J.S.; et al. A complex multi-notch astronomical filter to suppress the bright infrared sky. Nat. Commun. 2011, 2, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Ma, X.; Menon, N.V.; Li, C.M.; Zhao, Y.; Kang, Y. Dual Fluorescence-Activated Study of Tumor Cell Apoptosis by an Optofluidic System. IEEE J. Sel. Top. Quant. Electron. 2015, 21, 7100107. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, M.; Triches, M.; Nava, G.; Kristensen, A.; Pollard, M.R.; Berg-Sørensen, K.; Taboryski, R.J. Fiber-Based, Injection-Molded Optofluidic Systems: Improvements in Assembly and Applications. Micromachines 2015, 6, 1971-1983. https://doi.org/10.3390/mi6121468
Matteucci M, Triches M, Nava G, Kristensen A, Pollard MR, Berg-Sørensen K, Taboryski RJ. Fiber-Based, Injection-Molded Optofluidic Systems: Improvements in Assembly and Applications. Micromachines. 2015; 6(12):1971-1983. https://doi.org/10.3390/mi6121468
Chicago/Turabian StyleMatteucci, Marco, Marco Triches, Giovanni Nava, Anders Kristensen, Mark R. Pollard, Kirstine Berg-Sørensen, and Rafael J. Taboryski. 2015. "Fiber-Based, Injection-Molded Optofluidic Systems: Improvements in Assembly and Applications" Micromachines 6, no. 12: 1971-1983. https://doi.org/10.3390/mi6121468