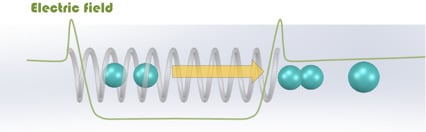
Shielding Electric Fields to Prevent Coalescence of Emulsions in Microfluidic Channels Using a 3D Metallic Coil
Abstract
:1. Introduction
2. Experimental Section
2.1. Device Fabrication and Solution Preparation

2.2. Experimental Procedure
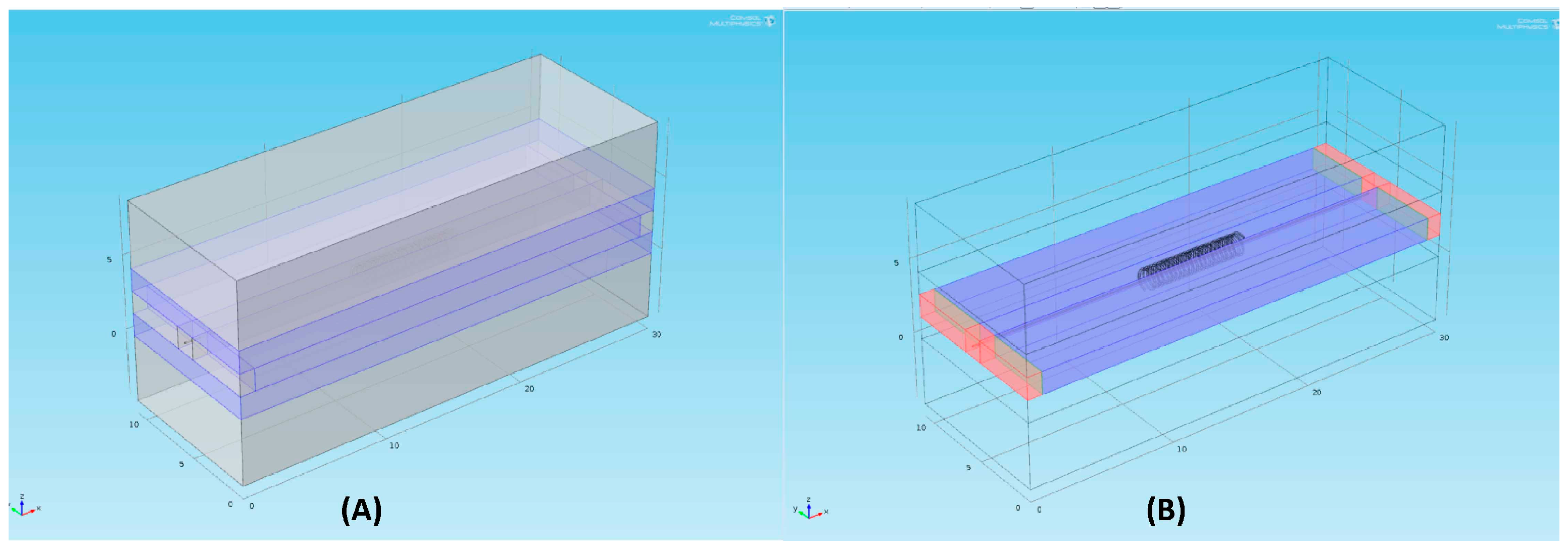
3. Numerical Model
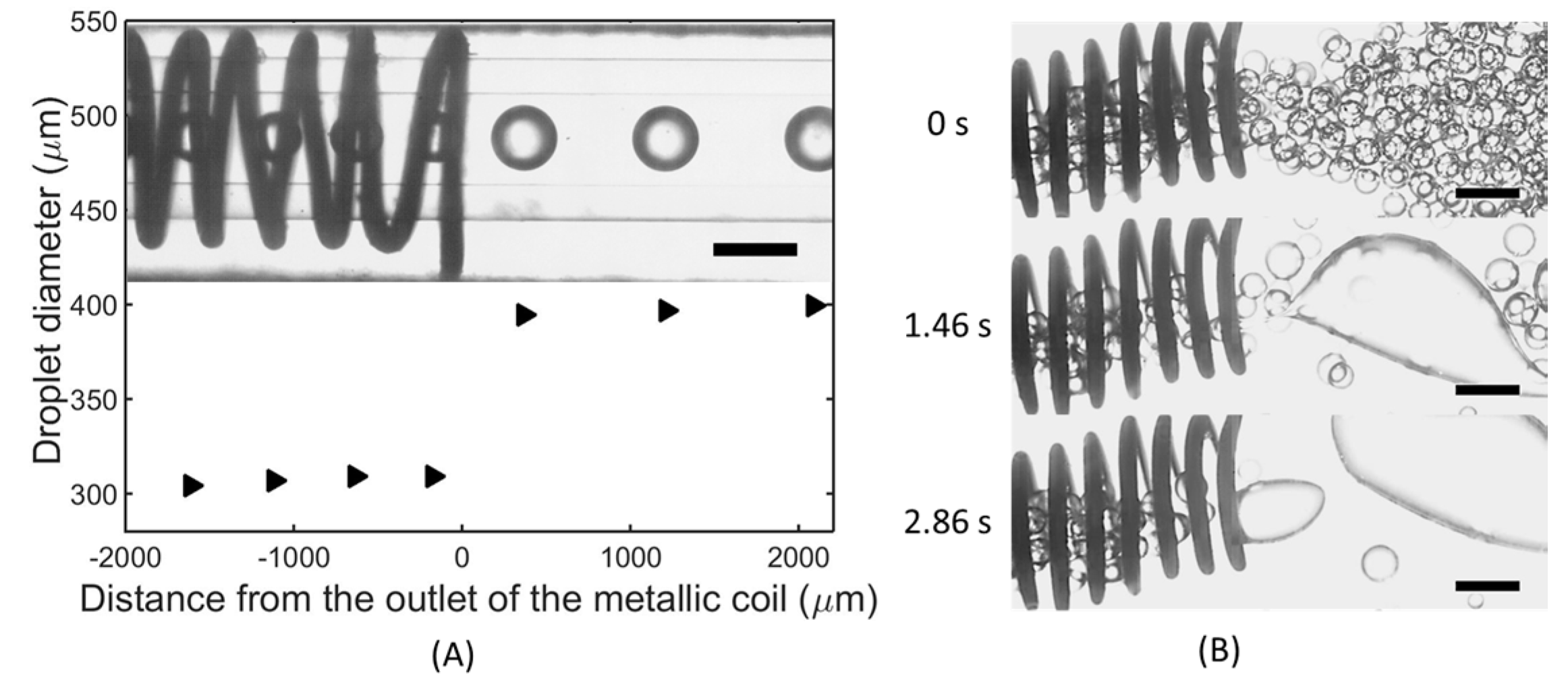
4. Results and Discussion
4.1. Dynamic Behaviors of Droplet Pairs under an Electric Field

4.2. Preventing Electro-Coalescence Using a Metallic Coil
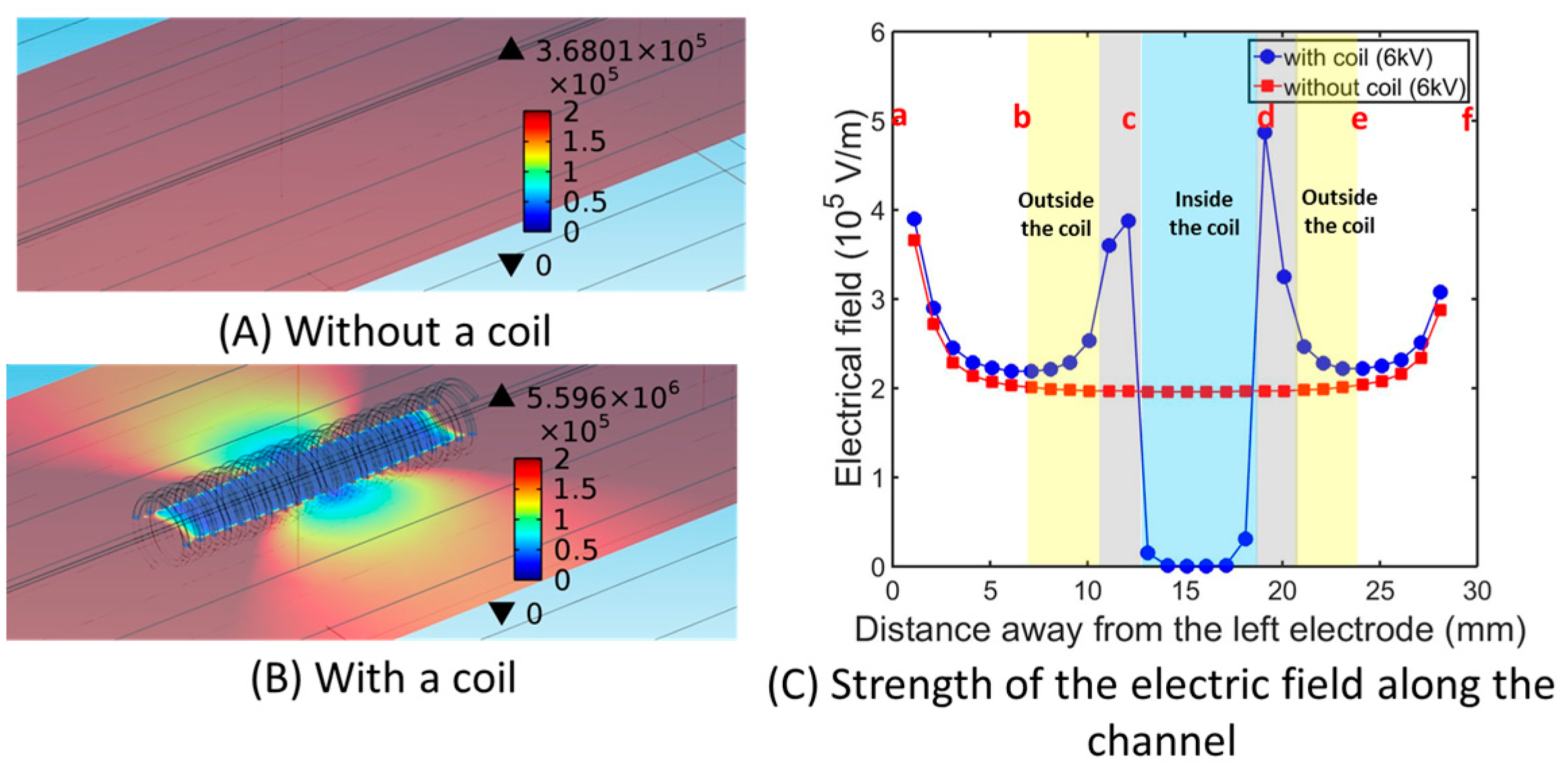
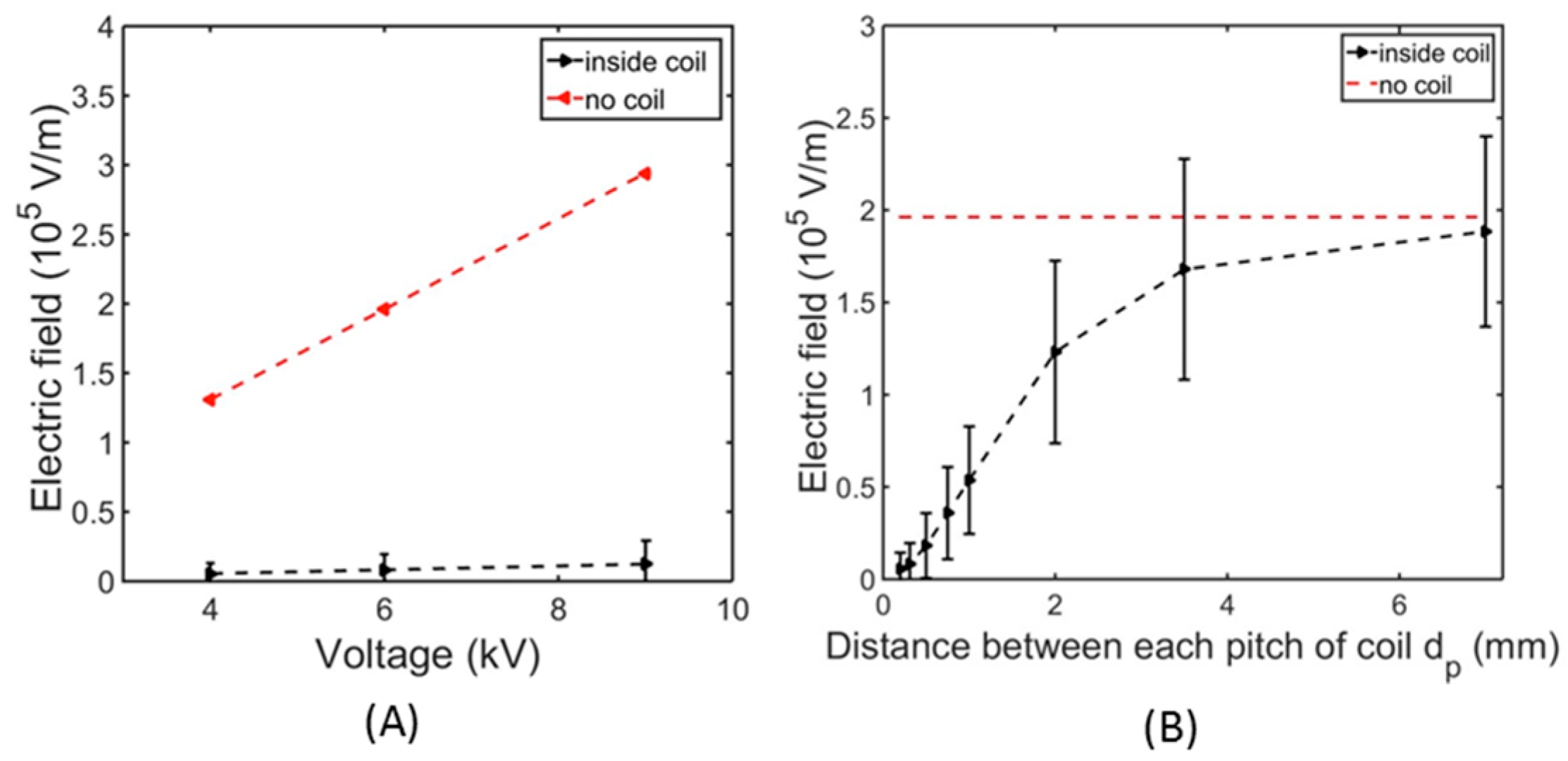
4.3. Numerical Simulation of the Electric Field Intensity Using COMSOL
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abate, A.R.; Hung, T.; Mary, P.; Agresti, J.J.; Weitz, D.A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl. Acad. Sci. USA 2010, 107, 19163–19166. [Google Scholar] [PubMed]
- Song, Y.; Chan, Y.K.; Ma, Q.; Liu, Z.; Shum, H.C. All-Aqueous Electrosprayed Emulsion for Templated Fabrication of Cytocompatible Microcapsules. ACS Appl. Mater. Interfaces 2015, 7, 13925–13933. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Gielen, F.; deMello, A.J.; Edel, J.B. Electro-Coalescence of Digitally Controlled Droplets. Anal. Chem. 2009, 81, 7321–7325. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Luo, D.; Link, D.; Weitz, D.A.; Marquez, M.; Cheng, Z. Controlled production of emulsion drops using an electric field in a flow-focusing microfluidic device. Appl. Phys. Lett. 2007, 91, 133106. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M.; Dávila, J.; Barrero, A. Current and droplet size in the electrospraying of liquids. Scaling laws. J. Aerosol Sci. 1997, 28, 249–275. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. In Proceedings of the Conference Record of the 1993 IEEE Industry Applications Society Annual Meeting, Toronto, ON, Canada, 2–8 October 1993; pp. 1698–1703.
- Thiam, A.R.; Bremond, N.; Bibette, J. Breaking of an Emulsion under an ac Electric Field. Phys. Rev. Lett. 2009, 102, 188304. [Google Scholar] [CrossRef] [PubMed]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O.; Williams, T.J. Electrostatic enhancement of coalescence of water droplets in oil: A review of the current understanding. Chem. Eng. J. 2001, 84, 173–192. [Google Scholar] [CrossRef]
- Taniguchi, T.; Torii, T.; Higuchi, T. Chemical reactions in microdroplets by electrostatic manipulation of droplets in liquid media. Lab Chip 2002, 2, 19–23. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, B.; Eastburn, D.J.; Abate, A.R. Electrode-free picoinjection of microfluidic drops. Lab Chip 2012, 12, 4029–4032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chan, S.T.; Faizi, H.A.; Roberts, R.C.; Shum, H.C. Droplet-based electro-coalescence for probing threshold disjoining pressure. Lab Chip 2015, 15, 2018–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.; Link, D.R.; Weitz, D. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 24104–24104. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Moon, H.; Chang-Jin, K. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Pollack, M.; Shenderov, A.; Fair, R. Electrowetting-based actuation of droplets for integrated microfluidics. Lab Chip 2002, 2, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mittal, N.; Mak, S.Y.; Song, Y.; Shum, H.C. Perturbation-induced droplets for manipulating droplet structure and configuration in microfluidics. J. Micromech. Microeng. 2015, 25, 084009. [Google Scholar] [CrossRef]
- Eggers, J.; Villermaux, E. Physics of liquid jets. Rep. Prog. Phys. 2008, 71, 036601. [Google Scholar] [CrossRef]
- Deng, N.-N.; Wang, W.; Ju, X.-J.; Xie, R.; Weitz, D.A.; Chu, L.-Y. Wetting-induced formation of controllable monodisperse multiple emulsions in microfluidics. Lab Chip 2013, 13, 4047–4052. [Google Scholar] [CrossRef] [PubMed]
- Okushima, S.; Nisisako, T.; Torii, T.; Higuchi, T. Controlled Production of Monodisperse Double Emulsions by Two-Step Droplet Breakup in Microfluidic Devices. Langmuir 2004, 20, 9905–9908. [Google Scholar] [CrossRef] [PubMed]
- Loghavi, L.; Sastry, S.K.; Yousef, A.E. Effect of moderate electric field frequency and growth stage on the cell membrane permeability of Lactobacillus acidophilus. Biotechnol. Prog. 2009, 25, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.A.; Sale, A.J.H. Effects of high electric fields on microorganisms: II. Mechanism of action of the lethal effect. Biochim. Biophys. Acta BBA General Subj. 1967, 148, 789–800. [Google Scholar] [CrossRef]
- Cerri, G.; de Leo, R.; Primiani, V.M. Theoretical and experimental evaluation of the electromagnetic radiation from apertures in shielded enclosure. IEEE Trans. Electromagn. Compat. 1992, 34, 423–432. [Google Scholar] [CrossRef]
- O’Donovan, B.; Tran, T.; Sciambi, A.; Abate, A. Picoinjection of Microfluidic Drops without Metal Electrodes. J. Vis. Exp. 2014, 2014, e50913. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Ravindran, R.E.; Varma, K. Dielectric properties of Poly (methyl methacrylate)(PMMA)/CaCu3Ti4O12 composites. In Proceedings of the 2012 IEEE 10th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Bangalore, India, 24–28 July 2012; pp. 1–4.
- Relative Permittivity-Dielectric Constant. Available online: http://www.engineeringtoolbox.com/relative-permittivity-d_1660.html (accessed on 11 August 2015).
- Lourtioz, J.-M.; Benisty, H.; Berger, V.; Gerard, J.-M.; Maystre, D.; Tchelnokov, A. Photonic Crystals: Towards Nanoscale Photonic Devices; Springer: Berlin, Germany, 2006; pp. 121–122. [Google Scholar]
- Hussien, B. The DC and AC electrical properties of (PMMA-Al2O3) composites. Eur. J. Sci. Res. 2011, 52, 236–242. [Google Scholar]
- Tables of Physical & Chemical Constants (16th Edition 1995) 2.6.3 Electrical Insulating Materials. Available online: http://www.kayelaby.npl.co.uk/general_physics/2_6/2_6_3.html (accessed on 11 August 2015).
- Electrical Resistivity and Conductivity. Available online: https://en.wikipedia.org/wiki/Electrical_resistivity_and_conductivity (accessed on 11 August 2015).
- Mollet, H.; Grubenmann, A. Formulation Technology: Emulsions, Suspensions, Solid Forms; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 60. [Google Scholar]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Stubenrauch, C.; von Klitzing, R. Disjoining pressure in thin liquid foam and emulsion films—New concepts and perspectives. J. Phys. Condens. Matter 2003, 15, R1197. [Google Scholar]
- Liu, Z.; Wyss, H.M.; Fernandez-Nieves, A.; Shum, H.C. Dynamics of oppositely charged emulsion droplets. Phys. Fluids 2015, 27, 082003. [Google Scholar]
- Allan, R.; Mason, S. Particle behaviour in shear and electric fields. I. Deformation and burst of fluid drops. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1962, 267, 45–61. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, Z.; Huang, H.; Shum, H.C. Shielding Electric Fields to Prevent Coalescence of Emulsions in Microfluidic Channels Using a 3D Metallic Coil. Micromachines 2015, 6, 1459-1468. https://doi.org/10.3390/mi6101430
Li J, Liu Z, Huang H, Shum HC. Shielding Electric Fields to Prevent Coalescence of Emulsions in Microfluidic Channels Using a 3D Metallic Coil. Micromachines. 2015; 6(10):1459-1468. https://doi.org/10.3390/mi6101430
Chicago/Turabian StyleLi, Jingmei, Zhou Liu, Haibo Huang, and Ho Cheung Shum. 2015. "Shielding Electric Fields to Prevent Coalescence of Emulsions in Microfluidic Channels Using a 3D Metallic Coil" Micromachines 6, no. 10: 1459-1468. https://doi.org/10.3390/mi6101430