A Rapid and Low-Cost Nonlithographic Method to Fabricate Biomedical Microdevices for Blood Flow Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geometries of the Microchannels
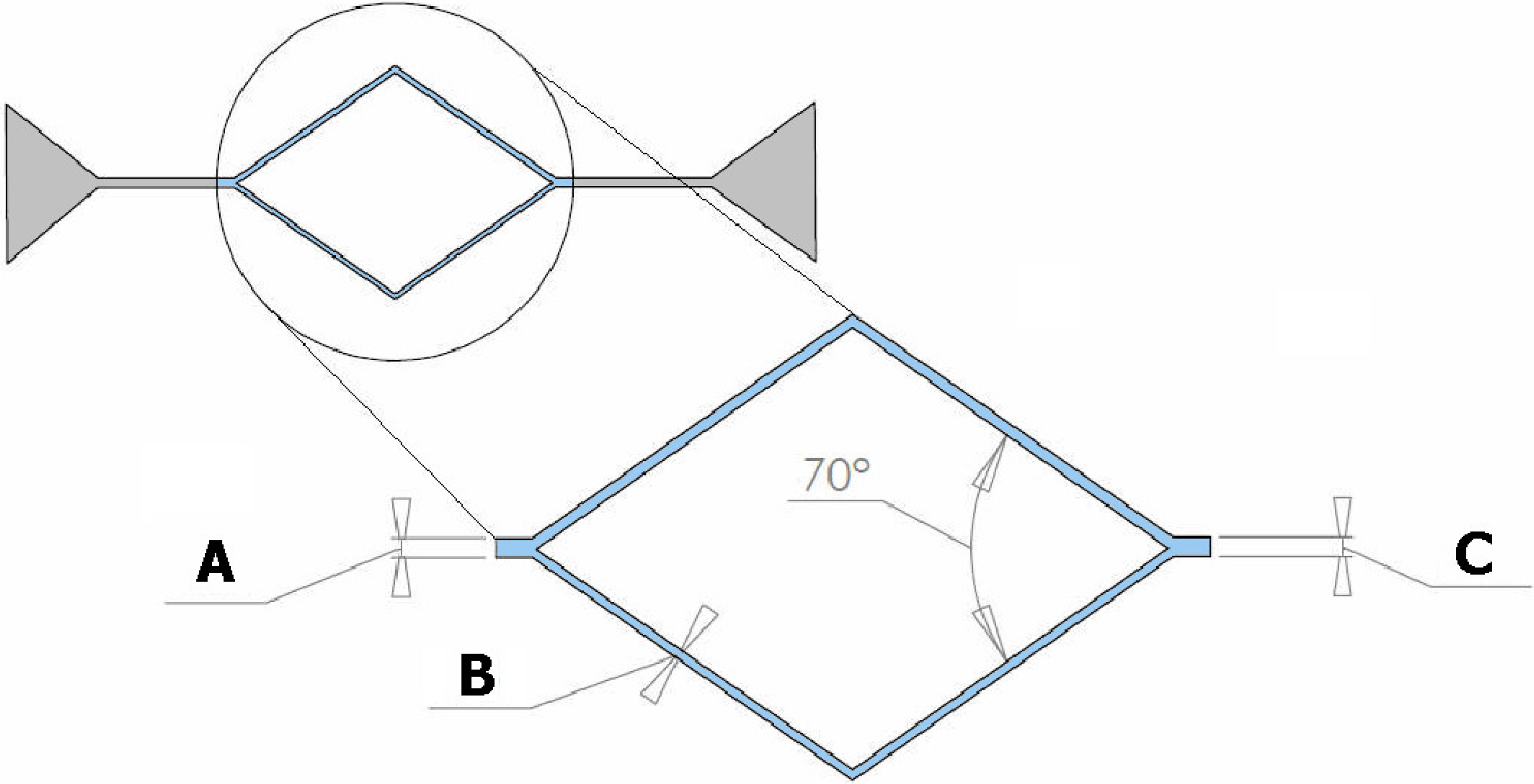
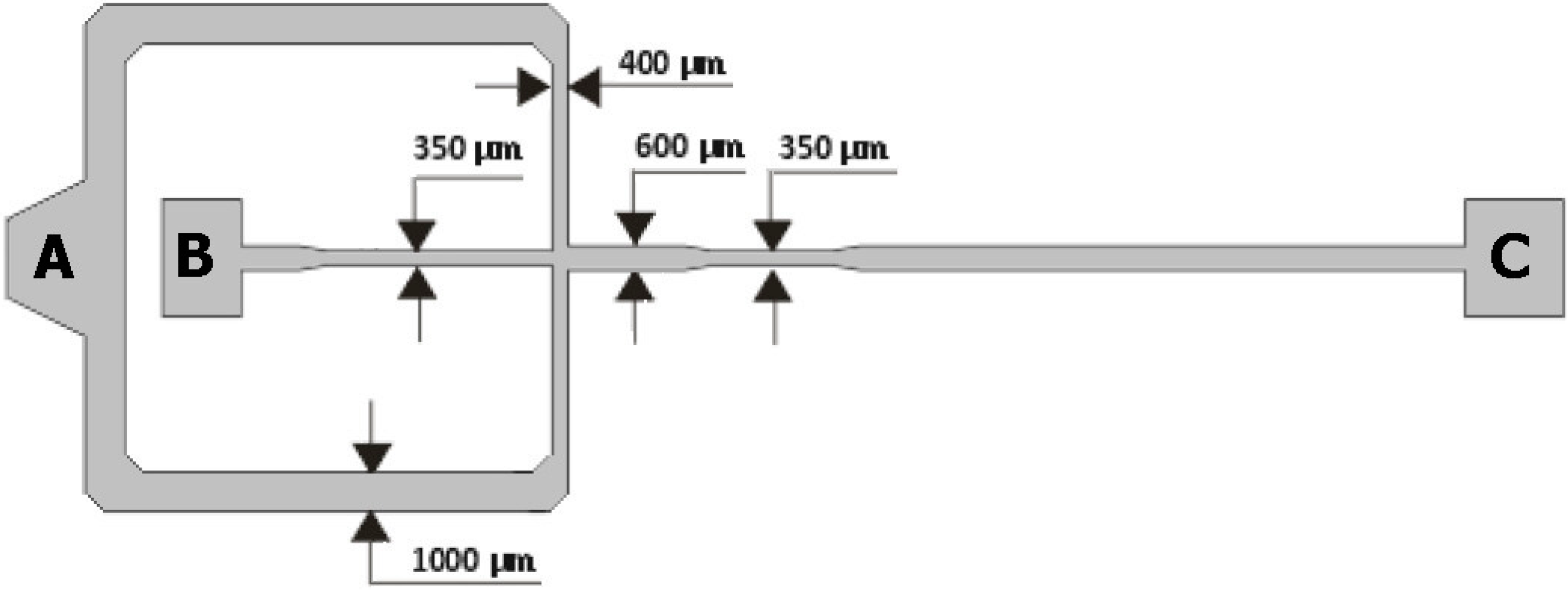
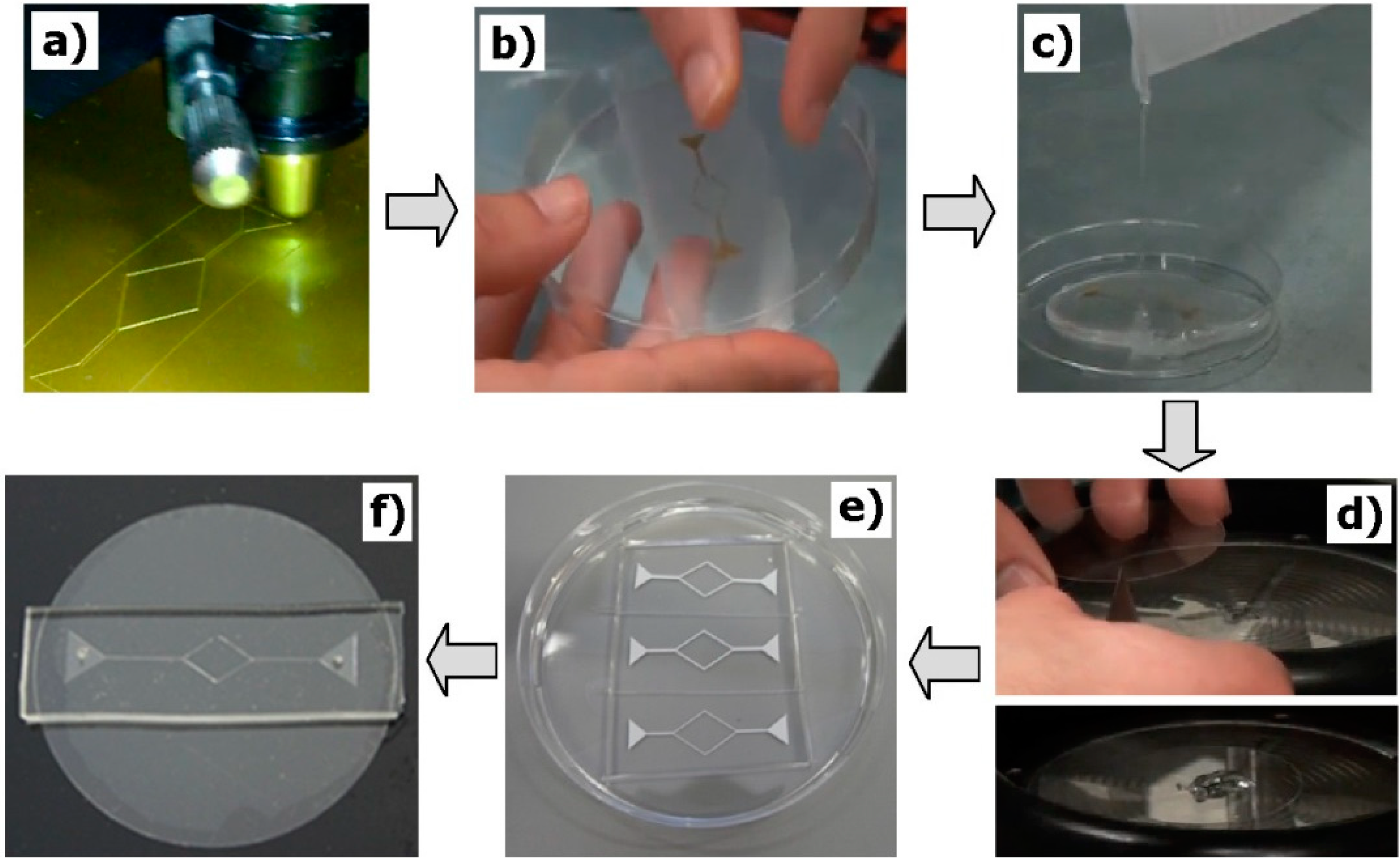
2.2. Fabrication of the Microfluidic Devices
- (a)
- The microchannel geometries were drawn using a CAD software, and then the molds were cut by using a cutting plotter Jaguar II (GCC Innovation, Capelle aan den Ijssel, The Netherlands).
- (b)
- Adhesive paper was used to transfer and place the mold master inside the petri dish.
- (c)
- The PDMS prepolymer was prepared by mixing a commercial prepolymer and a curing agent (10:1 ratio) and poured onto master mold in the petri dish and cured in an oven at 80 °C for 20 min.
- (d)
- The PDMS (20:1 ratio) was spin coated over a glass slide and cured in an oven at 80 °C for 20 min.
- (e)
- By using a blade, the microchannels were cut off and the inlet/outlet holes of the fluid were done by using a fluid-dispensing tip.
- (f)
- Finally, the channels were sealed by the PDMS covered glass slides. To have a strong adhesion of the materials, the device was placed in the oven at 80 °C for 24 h.
2.3. Working Fluids
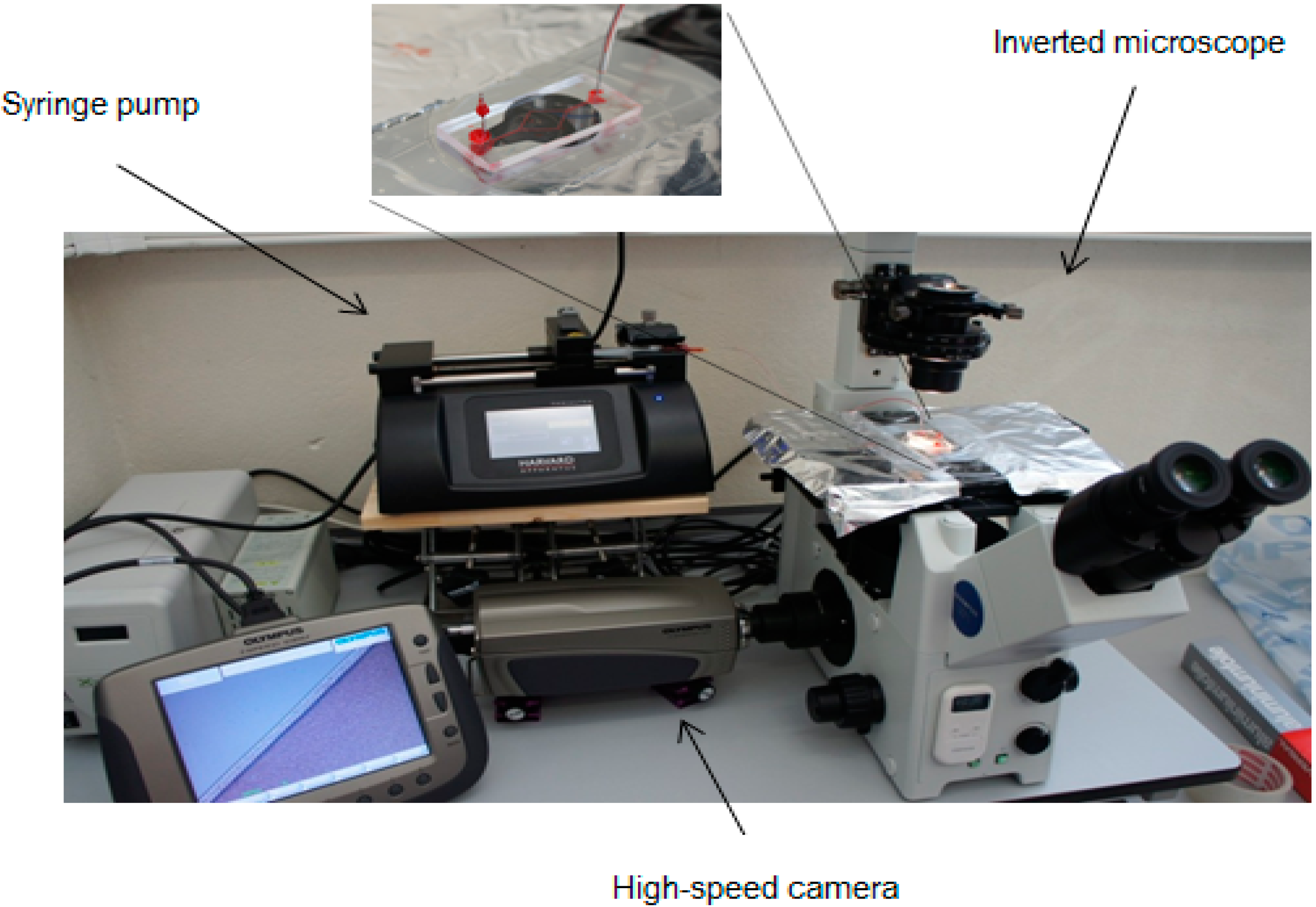
2.4. Experimental Set-Up
2.5. Image Analysis

3. Results and Discussion
3.1. Characteristics of the Microchannels

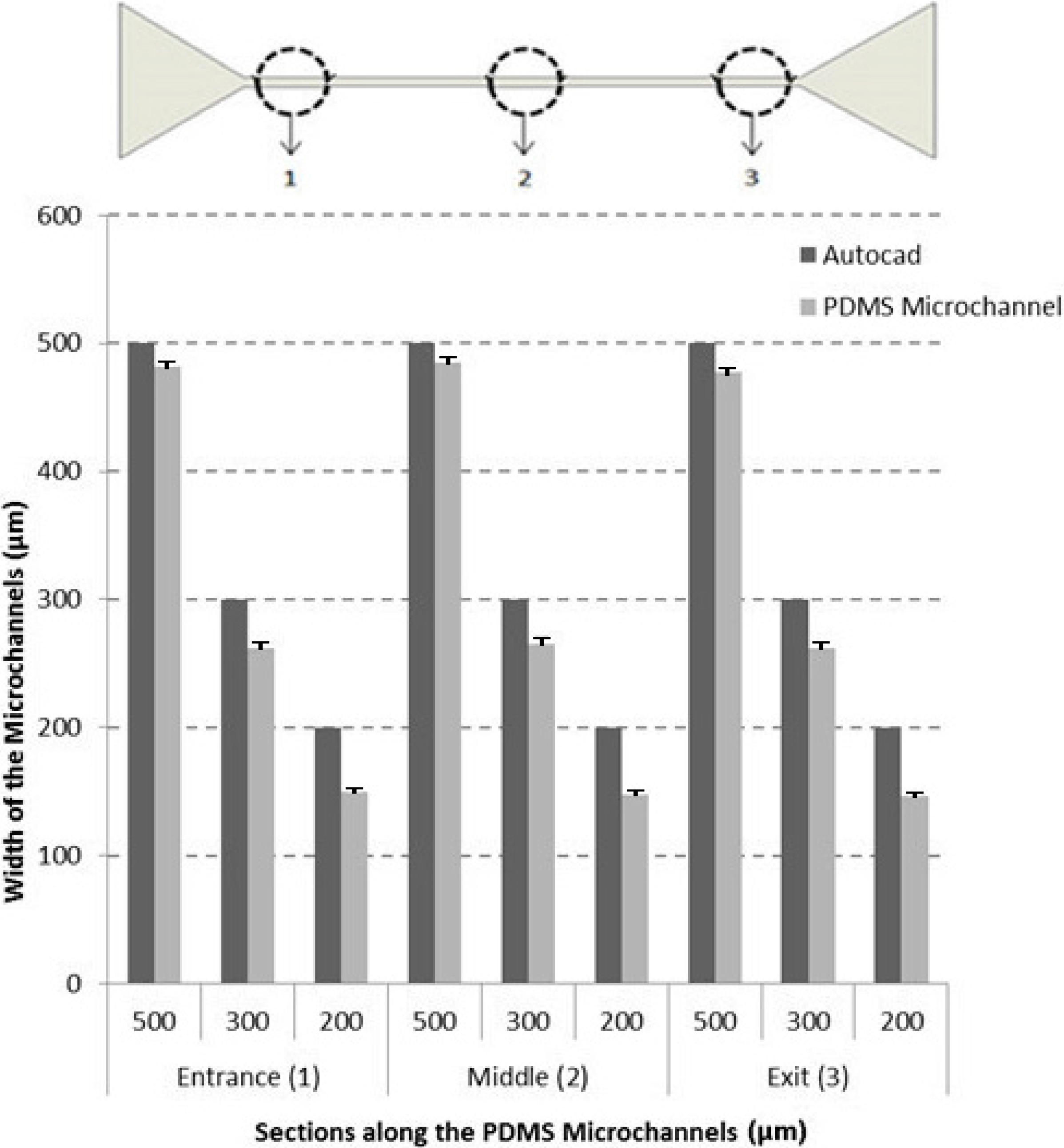
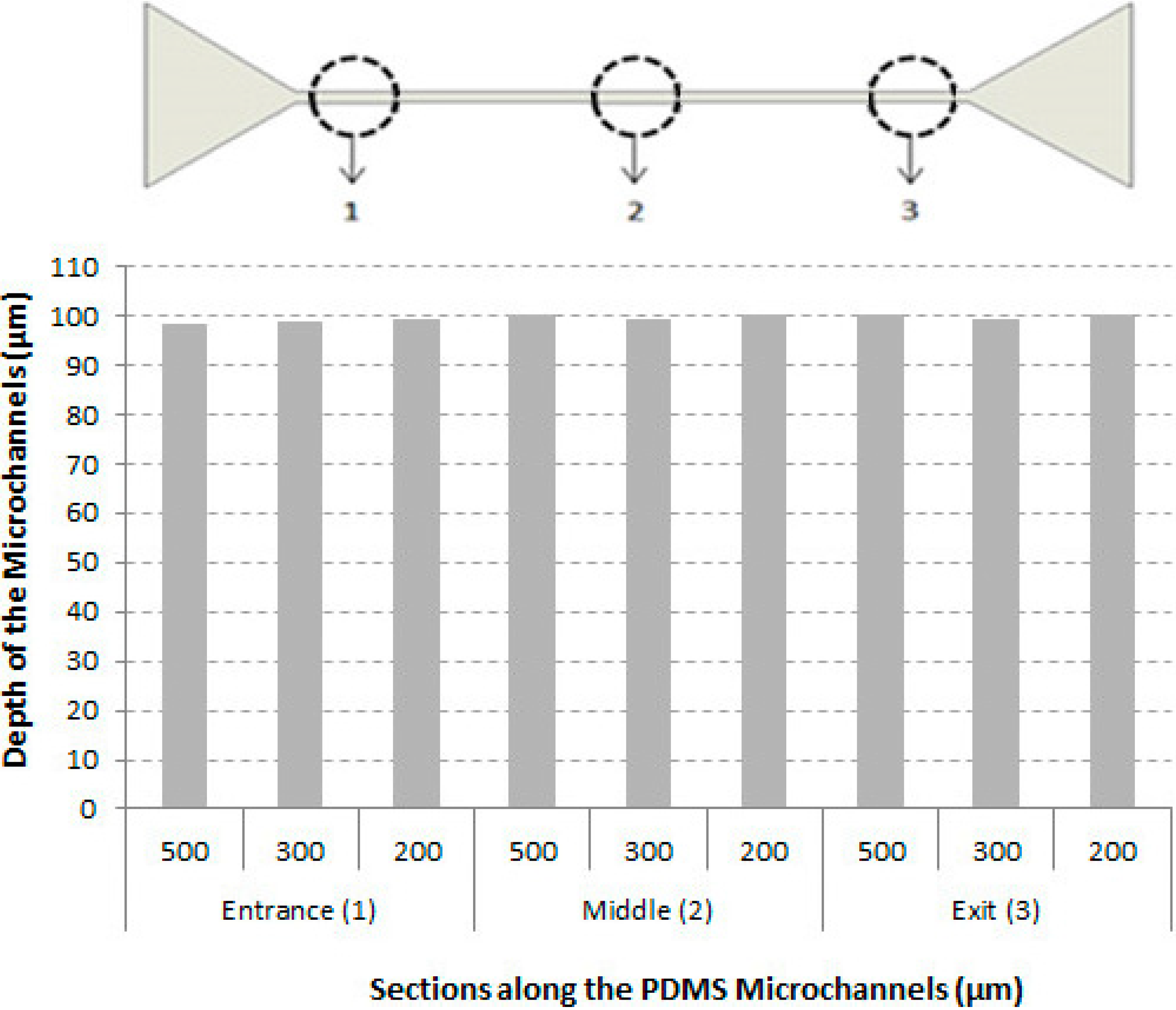
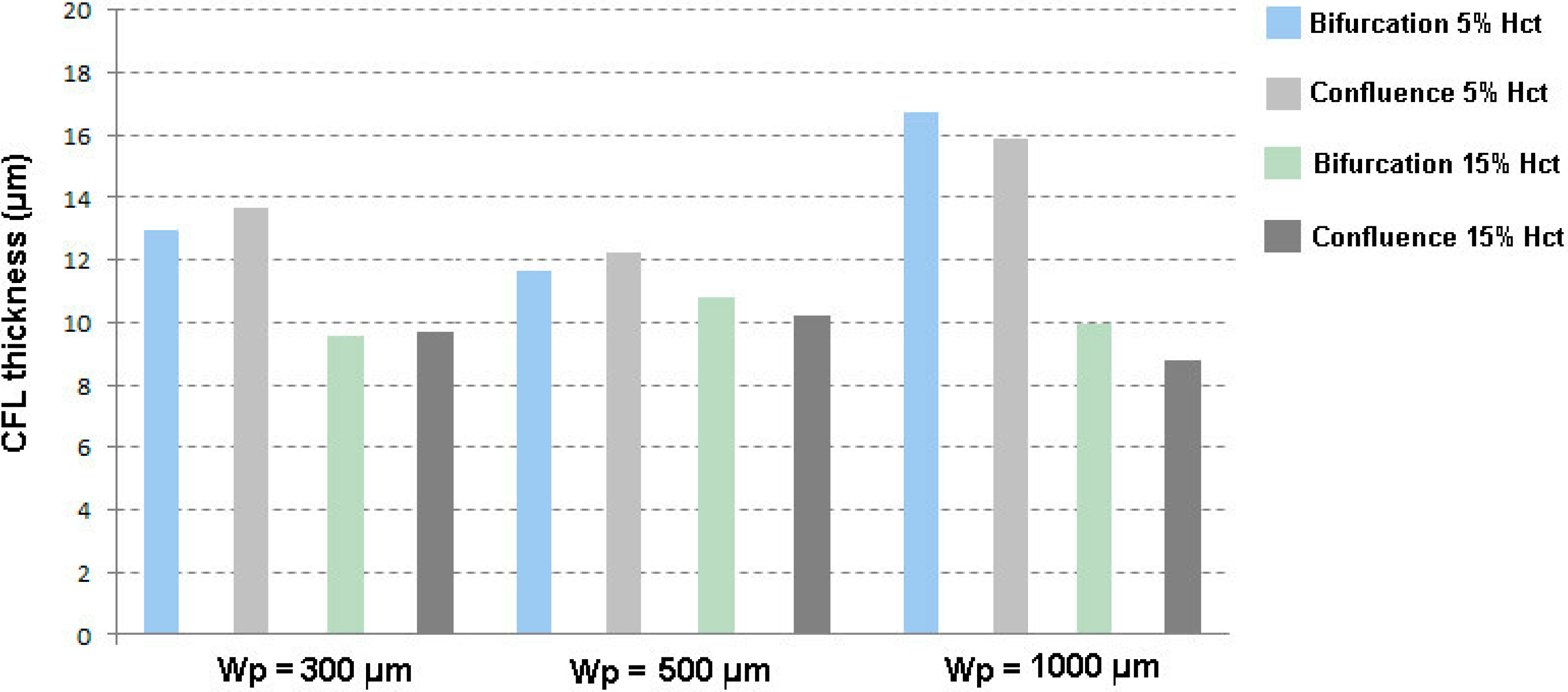
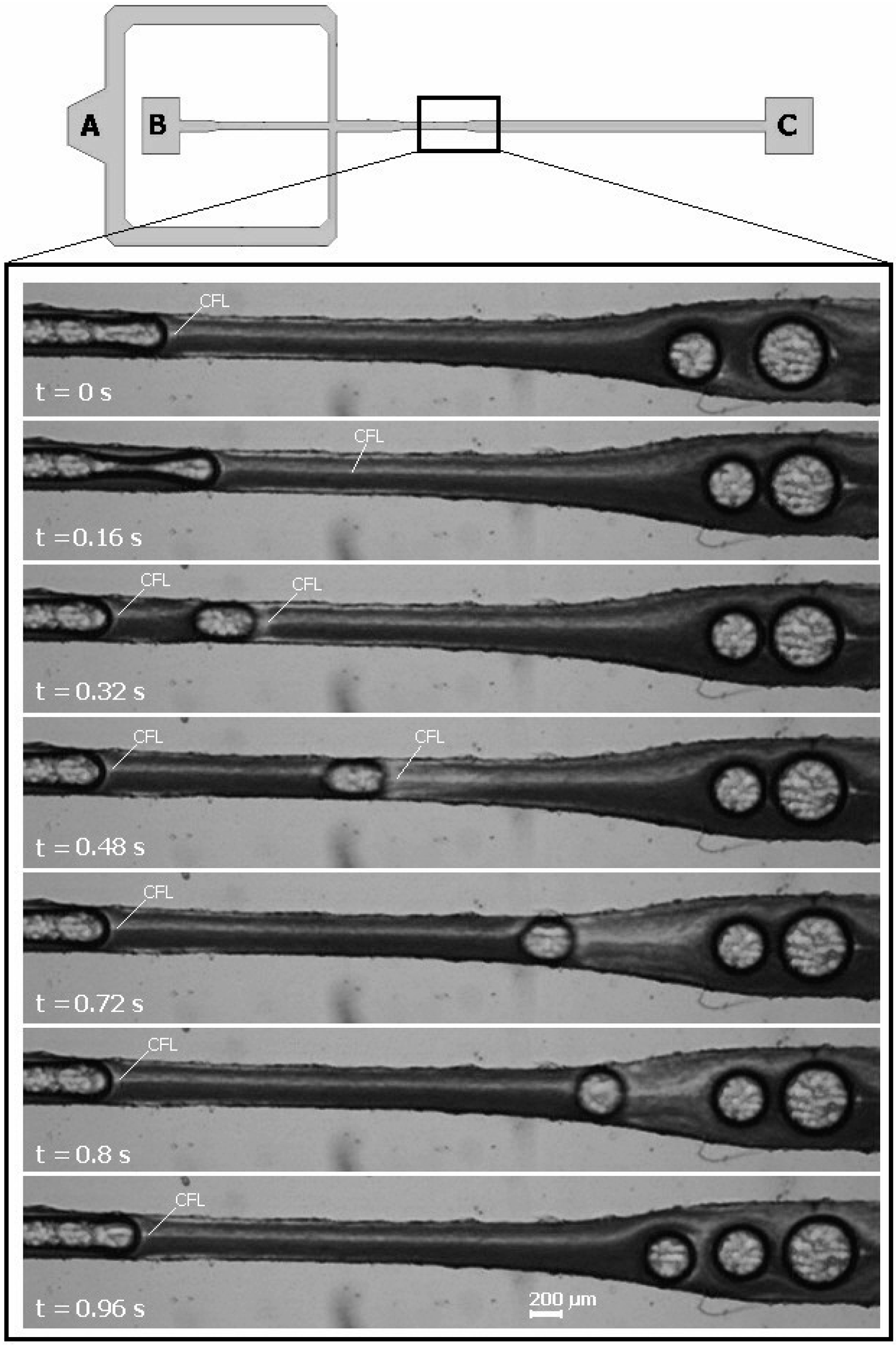
3.2. Cell-Free Layer (CFL) Measurements and Visualization


3.3. Bubbles Generation and Visualization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garcia, V.; Dias, R.; Lima, R. In vitro blood flow behaviour in microchannels with simple and complex geometries. In Applied Biological Engineering—Principles and Practice; Naik, G.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 393–416. [Google Scholar]
- Lima, R.; Nakamura, M.; Omori, T.; Ishikawa, T.; Wada, S.; Yamaguchi, T. Advances in Computational Vision and Medical Image Processing; Springer: Dordrecht, The Netherlands, 2009; pp. 203–220. [Google Scholar]
- Nguyen, N.T.; Wereley, S.T. Fundamentals and Applications of Microfluidics, 2nd ed.; Artech House: Boston, MA, USA, 2006. [Google Scholar]
- Stone, H.A.; Kim, S. Microfluidics: Basic issues, applications, and challenges. AIChE J. 2001, 47, 1250–1254. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Ann. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Leble, V.; Lima, R.; Dias, R.; Fernandes, C.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Asymmetry of red blood cell motions in a microchannel with a diverging and converging bifurcation. Biomicrofluidics 2011, 5. [Google Scholar] [CrossRef]
- Lima, R.; Wada, S.; Tanaka, S.; Takeda, M.; Ishikawa, T.; Tsubota, K.; Imai, Y.; Yamaguchi, T. In vitro blood flow in a rectangular pdms microchannel: Experimental observations using a confocal micro-piv system. Biomed. Microdevices 2008, 10, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Millare, B.; Clift, J.M.; Bao, D.; Hong, C.; Vullev, V.I. Print-and-peel fabrication for microfluidics: What's in it for biomedical applications? Ann. Biomed. Eng. 2010, 38, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Rodgers, K.; Murrihy, J.; O’Mathuna, C.; Glennon, J.D. Rapid fabrication of microfluidic devices in poly(dimethylsiloxane) by photocopying. Lab Chip 2001, 1, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Branham, M.L.; Tran-Son-Tay, R.; Schoonover, C.; Davis, P.S.; Allen, S.D.; Shyy, W. Rapid prototyping of micropatterned substrates using conventional laser printers. J. Mater. Res. 2002, 17, 1559–1562. [Google Scholar] [CrossRef]
- Bao, N.; Zhang, Q.; Xu, J.J.; Chen, H.Y. Fabrication of poly(dimethylsiloxane) microfluidic system based on masters directly printed with an office laser printer. J. Chromatogr. A 2005, 1089, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Breslauer, D.N.; Luna, J.I.; Grimes, A.; Chin, W.C.; Lee, L.P.; Khine, M. Shrinky-dink microfluidics: 3D polystyrene chips. Lab Chip 2008, 8, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Bonyár, A.; Sántha, H.; Ring, B.; Varga, M.; Gábor Kovács, J.; Harsányi, G. 3D rapid prototyping technology (rpt) as a powerful tool in microfluidic development. Procedia Eng. 2010, 5, 291–294. [Google Scholar] [CrossRef]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Klank, H.; Kutter, J.P.; Geschke, O. Co 2-laser micromachining and back-end processing for rapid production of pmma-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kaigala, G.V.; Ho, S.; Penterman, R.; Backhouse, C.J. Rapid prototyping of microfluidic devices with a wax printer. Lab Chip 2007, 7, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J. Microelectromech. Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Sundberg, S.O.; Wittwer, C.T.; Greer, J.; Pryor, R.J.; Elenitoba-Johnson, O.; Gale, B.K. Solution-phase DNA mutation scanning and snp genotyping by nanoliter melting analysis. Biomed. Microdevices 2007, 9, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Abkarian, M.; Faivre, M.; Horton, R.; Smistrup, K.; Best-Popescu, C.A.; Stone, H.A. Cellular-scale hydrodynamics. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Yaginuma, T.; Lima, R. A microfluidic device for partial cell separation and deformability assessment. Biochip J. 2013, 7, 367–374. [Google Scholar] [CrossRef]
- Yaginuma, T.; Oliveira, M.S.; Lima, R.; Ishikawa, T.; Yamaguchi, T. Human red blood cell behavior under homogeneous extensional flow in a hyperbolic-shaped microchannel. Biomicrofluidics 2013, 7. [Google Scholar] [CrossRef]
- Shevkoplyas, S.S.; Yoshida, T.; Munn, L.L.; Bitensky, M.W. Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device. Anal. Chem. 2005, 77, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.W.; Bhagat, A.A.; Chong, A.G.; Mao, P.; Tan, K.S.; Han, J.; Lim, C.T. Deformability based cell margination—A simple microfluidic design for malaria-infected erythrocyte separation. Lab Chip 2010, 10, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Faivre, M.; Abkarian, M.; Bickraj, K.; Stone, H.A. Geometrical focusing of cells in a microfluidic device: An approach to separate blood plasma. Biorheology 2006, 43, 147–159. [Google Scholar] [PubMed]
- Sollier, E.; Cubizolles, M.; Fouillet, Y.; Achard, J.L. Fast and continuous plasma extraction from whole human blood based on expanding cell-free layer devices. Biomed. Microdevices 2010, 12, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophoton. Int. 2004, 11, 36–42. [Google Scholar]
- Meijering, E.; Smal, I.; Danuser, G. Tracking in molecular bioimaging. IEEE Signal Proc. Mag. 2006, 23, 46–53. [Google Scholar] [CrossRef]
- Kim, S.; Ong, P.K.; Yalcin, O.; Intaglietta, M.; Johnson, P.C. The cell-free layer in microvascular blood flow. Biorheology 2009, 46, 181–189. [Google Scholar] [PubMed]
- Maeda, N.; Suzuki, Y.; Tanaka, J.; Tateishi, N. Erythrocyte flow and elasticity of microvessels evaluated by marginal cell-free layer and flow resistance. Am. J. Physiol. 1996, 271, 2454–2461. [Google Scholar]
- Tateishi, N.; Suzuki, Y.; Soutani, M.; Maeda, N. Flow dynamics of erythrocytes in microvessels of isolated rabbit mesentery: Cell-free layer and flow resistance. J. Biomech. 1994, 27, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Oliveira, M.S.; Ishikawa, T.; Kaji, H.; Tanaka, S.; Nishizawa, M.; Yamaguchi, T. Axisymmetric polydimethysiloxane microchannels for in vitro hemodynamic studies. Biofabrication 2009, 1. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fujiwara, H.; Matsuki, N.; Yoshimoto, T.; Imai, Y.; Ueno, H.; Yamaguchi, T. Asymmetry of blood flow and cancer cell adhesion in a microchannel with symmetric bifurcation and confluence. Biomed. Microdevices 2011, 13, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Barak, M.; Katz, Y. Microbubbles: Pathophysiology and clinical implications. Chest. J. 2005, 128, 2918–2932. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Tang, M.X.; Balestra, C.; Eckersley, R.J.; Karapantsios, T.D. Circulatory bubble dynamics: From physical to biological aspects. Adv. Colloid Interface Sci. 2014, 206, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Branger, A.B.; Eckmann, D.M. Accelerated arteriolar gas embolism reabsorption by an exogenous surfactant. Anesthesiology 2002, 96, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L. Estudo de Embolias Gasosas em Microcanais. Master’s Thesis, Instituto Politécnico de Bragança, Bragança, Portugal, 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, E.; Faustino, V.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Miranda, J.M.; Lima, R. A Rapid and Low-Cost Nonlithographic Method to Fabricate Biomedical Microdevices for Blood Flow Analysis. Micromachines 2015, 6, 121-135. https://doi.org/10.3390/mi6010121
Pinto E, Faustino V, Rodrigues RO, Pinho D, Garcia V, Miranda JM, Lima R. A Rapid and Low-Cost Nonlithographic Method to Fabricate Biomedical Microdevices for Blood Flow Analysis. Micromachines. 2015; 6(1):121-135. https://doi.org/10.3390/mi6010121
Chicago/Turabian StylePinto, Elmano, Vera Faustino, Raquel O. Rodrigues, Diana Pinho, Valdemar Garcia, João M. Miranda, and Rui Lima. 2015. "A Rapid and Low-Cost Nonlithographic Method to Fabricate Biomedical Microdevices for Blood Flow Analysis" Micromachines 6, no. 1: 121-135. https://doi.org/10.3390/mi6010121





