Miscible Organic Solvents Soak Bonding Method Use in a PMMA Multilayer Microfluidic Device
Abstract
:1. Introduction
2. Experimental Section
2.1. Equipment and Materials
2.2. Bonding Procedure
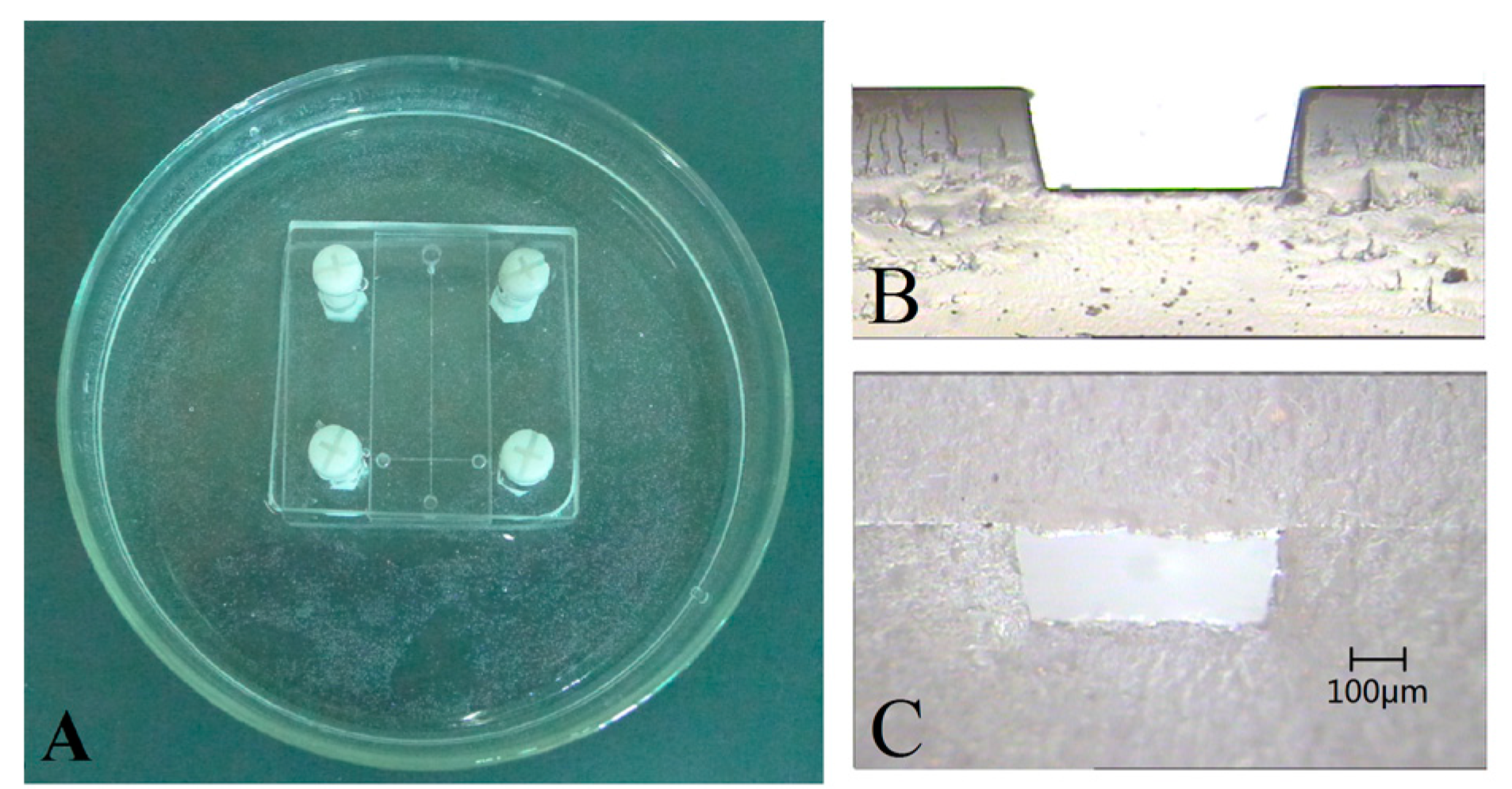
3. Results and Discussion
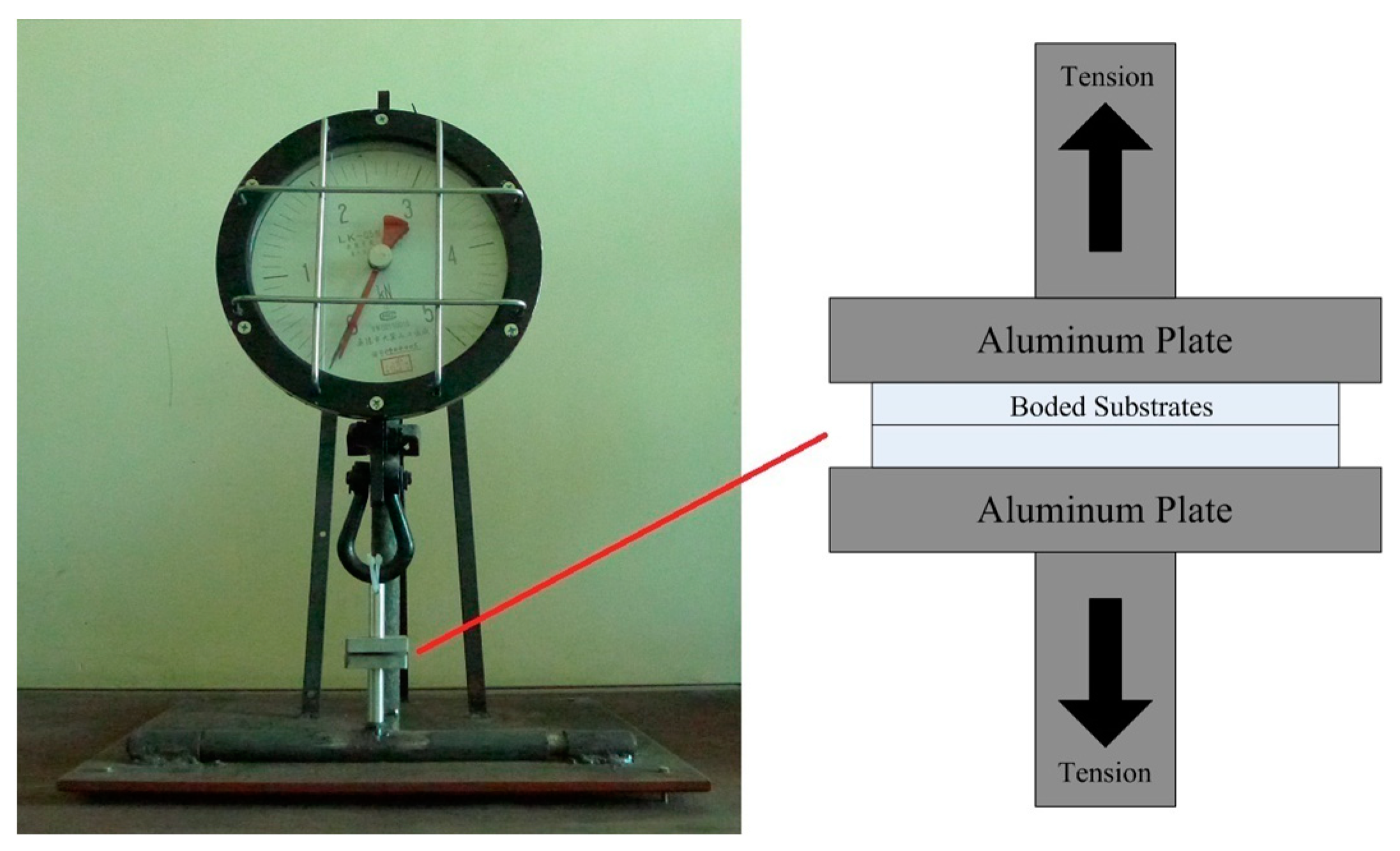
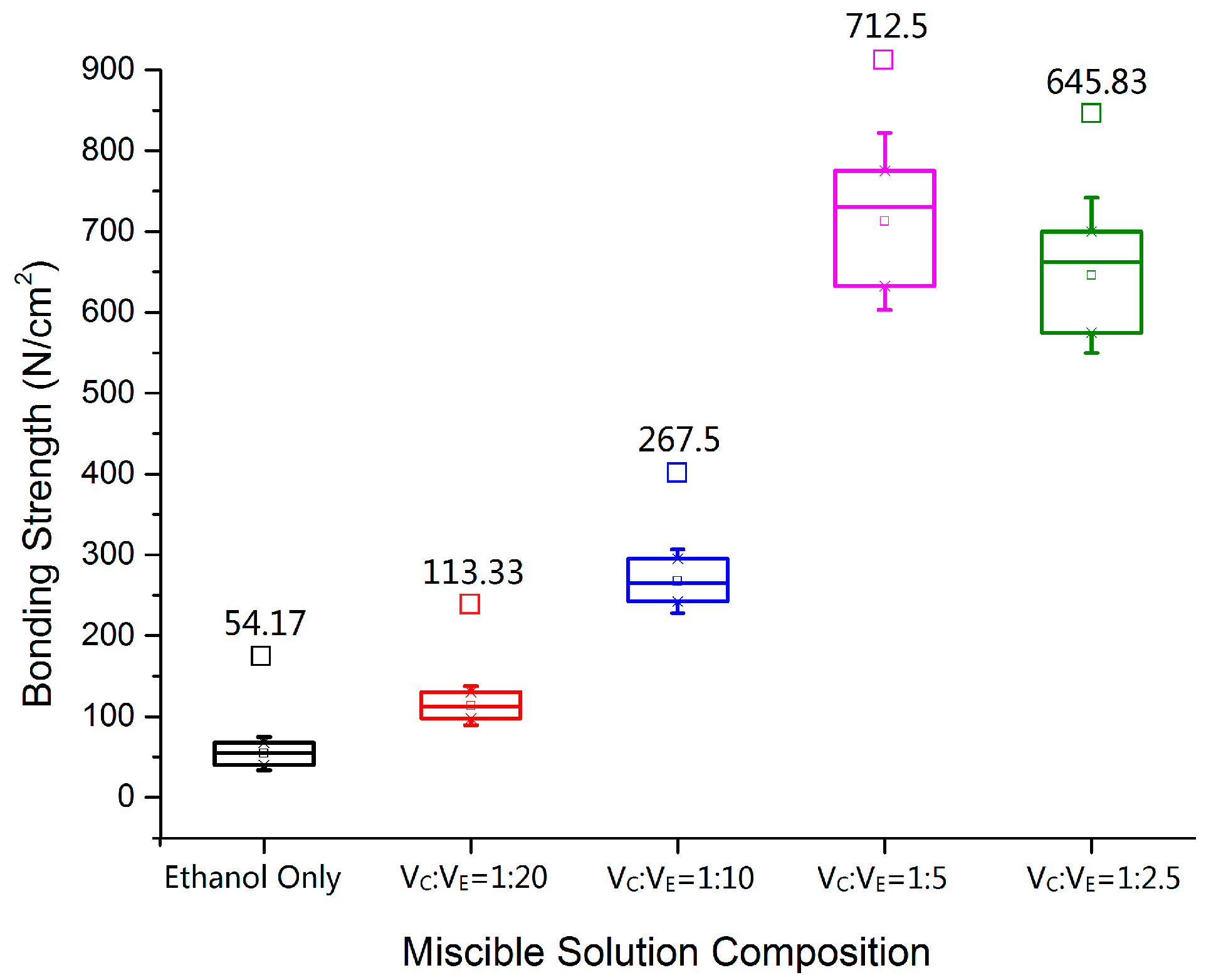
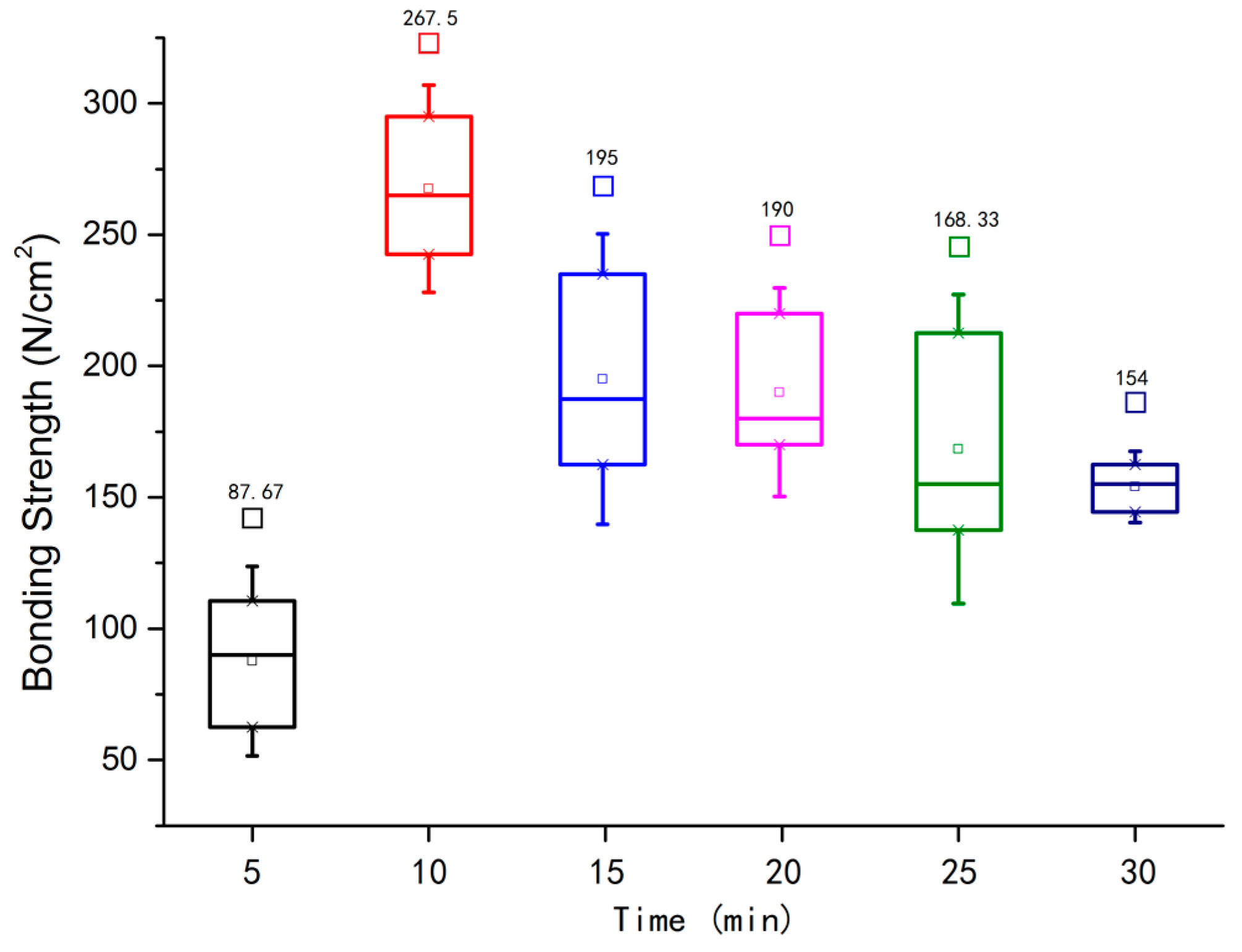
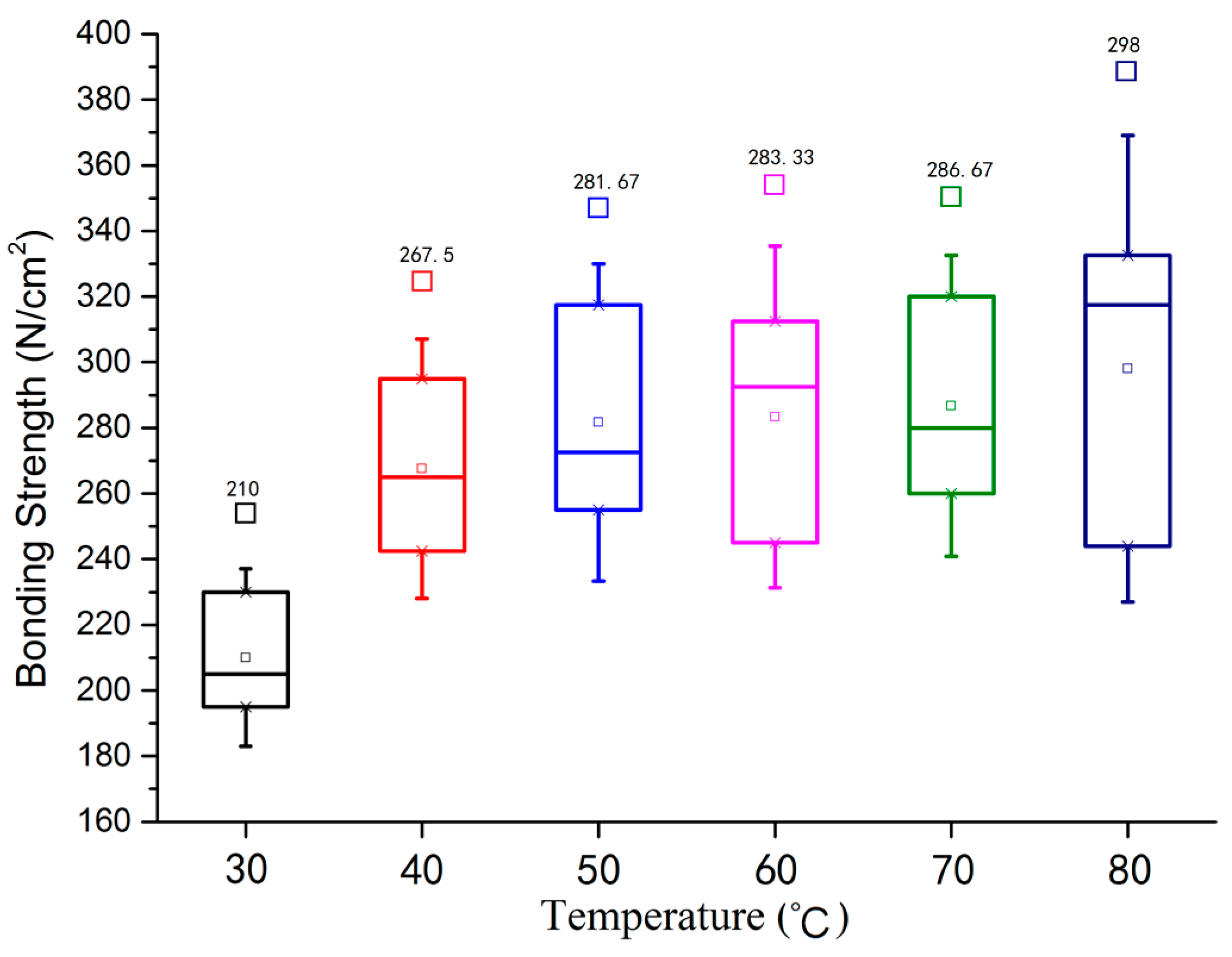
3.1. Bonding Strength
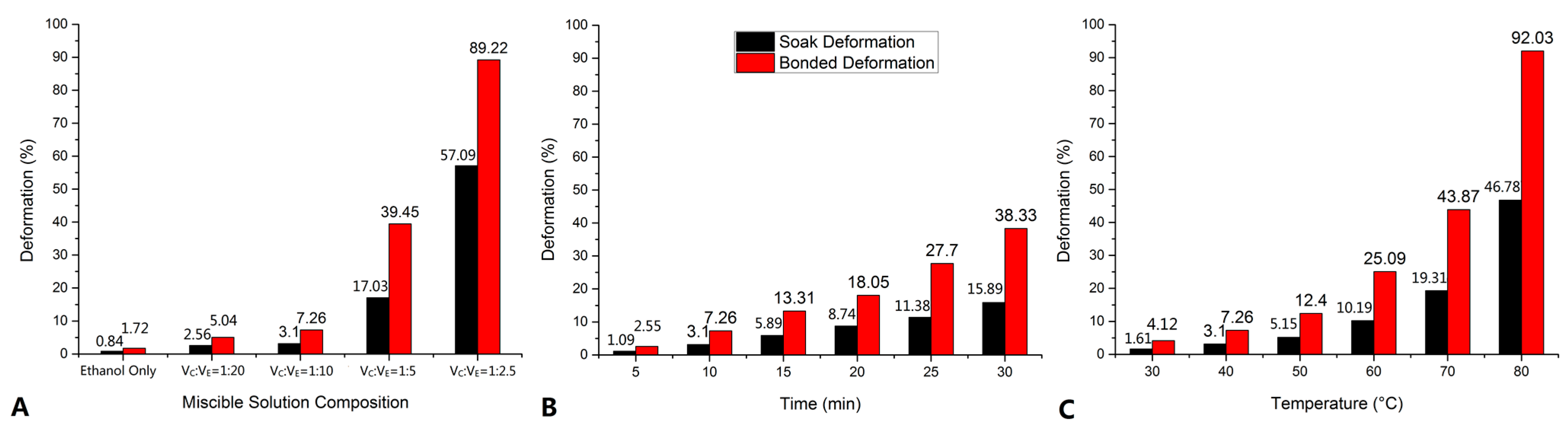
3.2. Structural Deformation
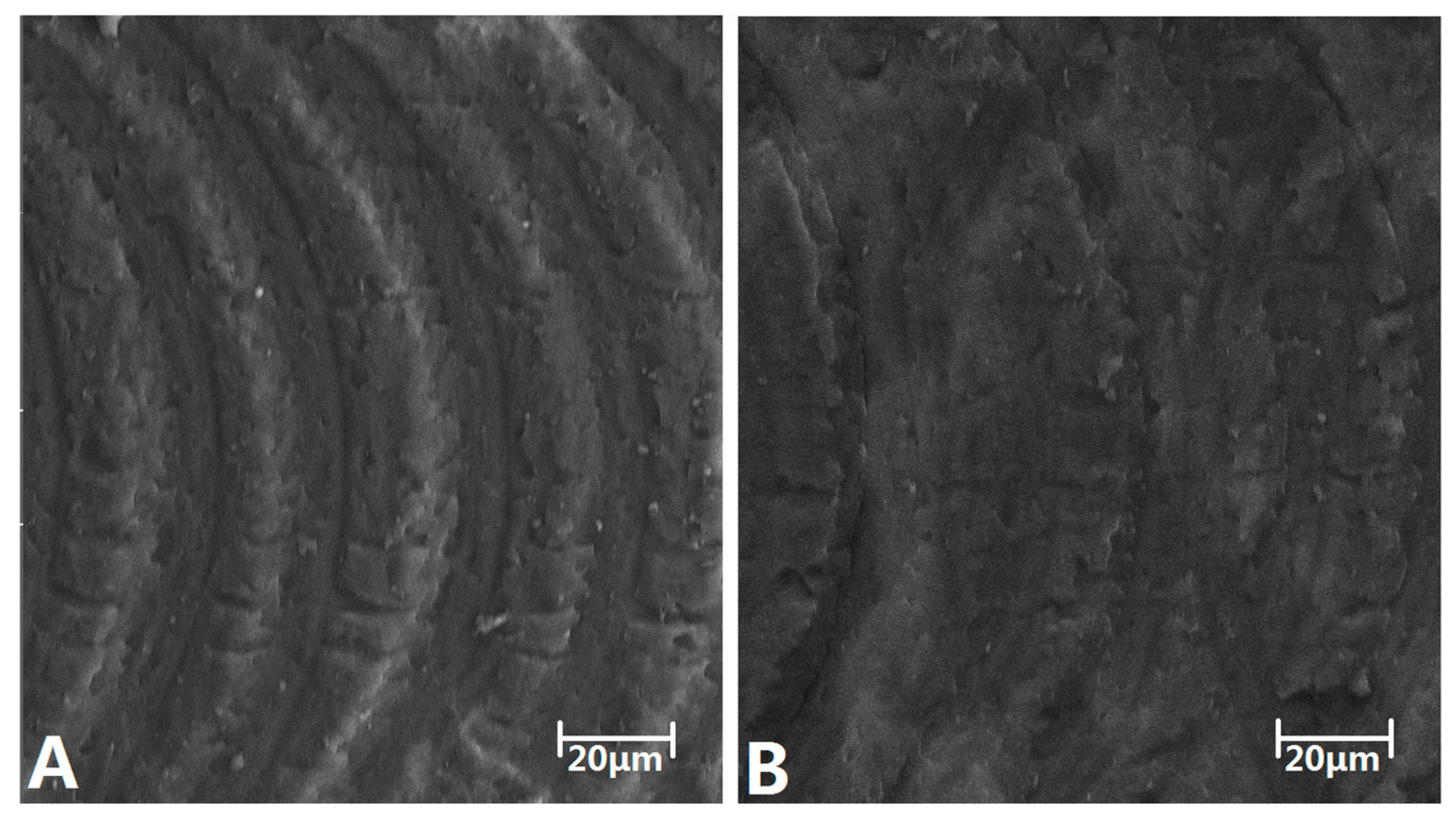
3.3. Microchannel Roughness
3.4. Comparison of Different Bonding Processes
4. Application
4.1. Mixer Design and Optimize
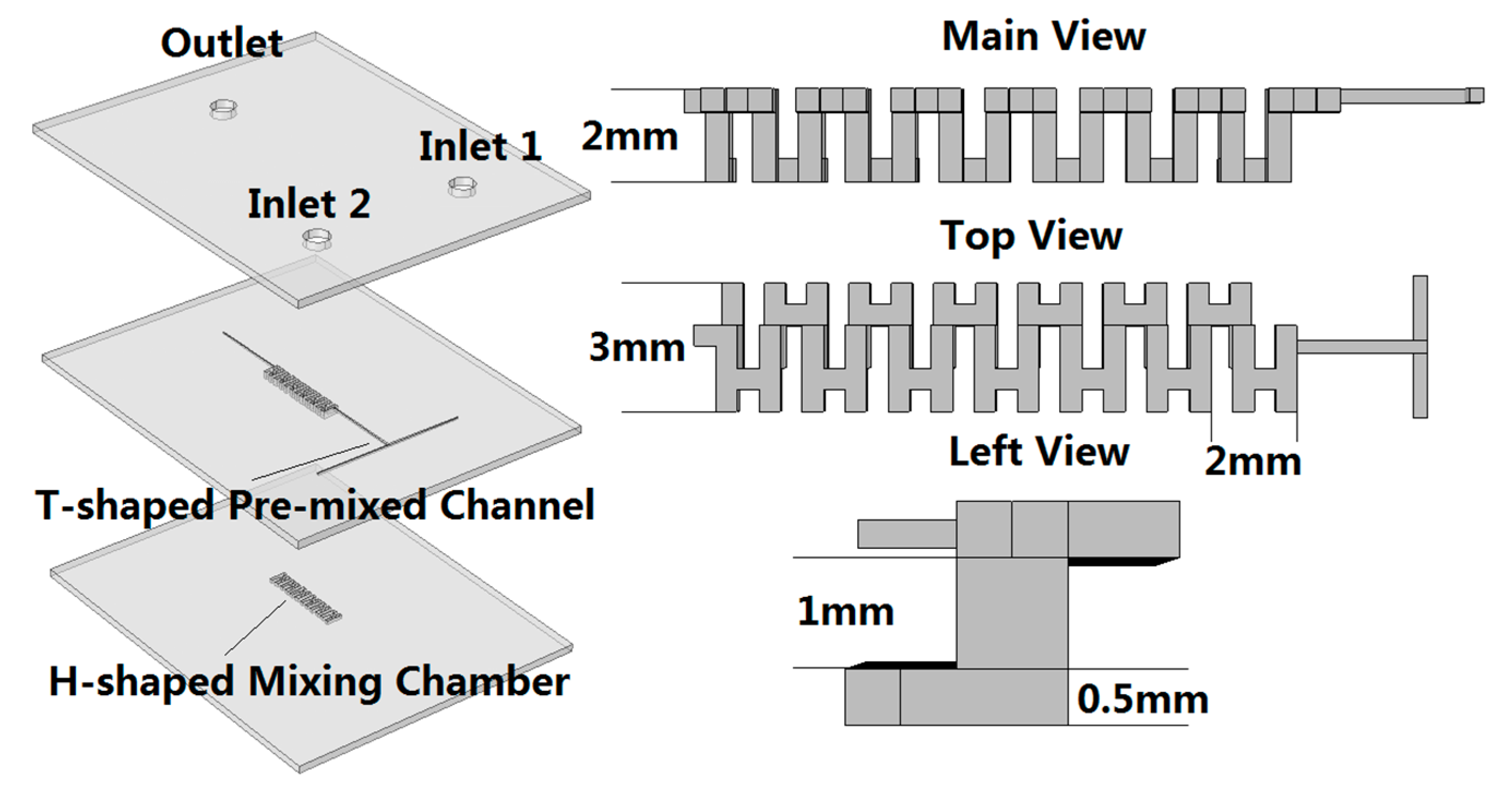
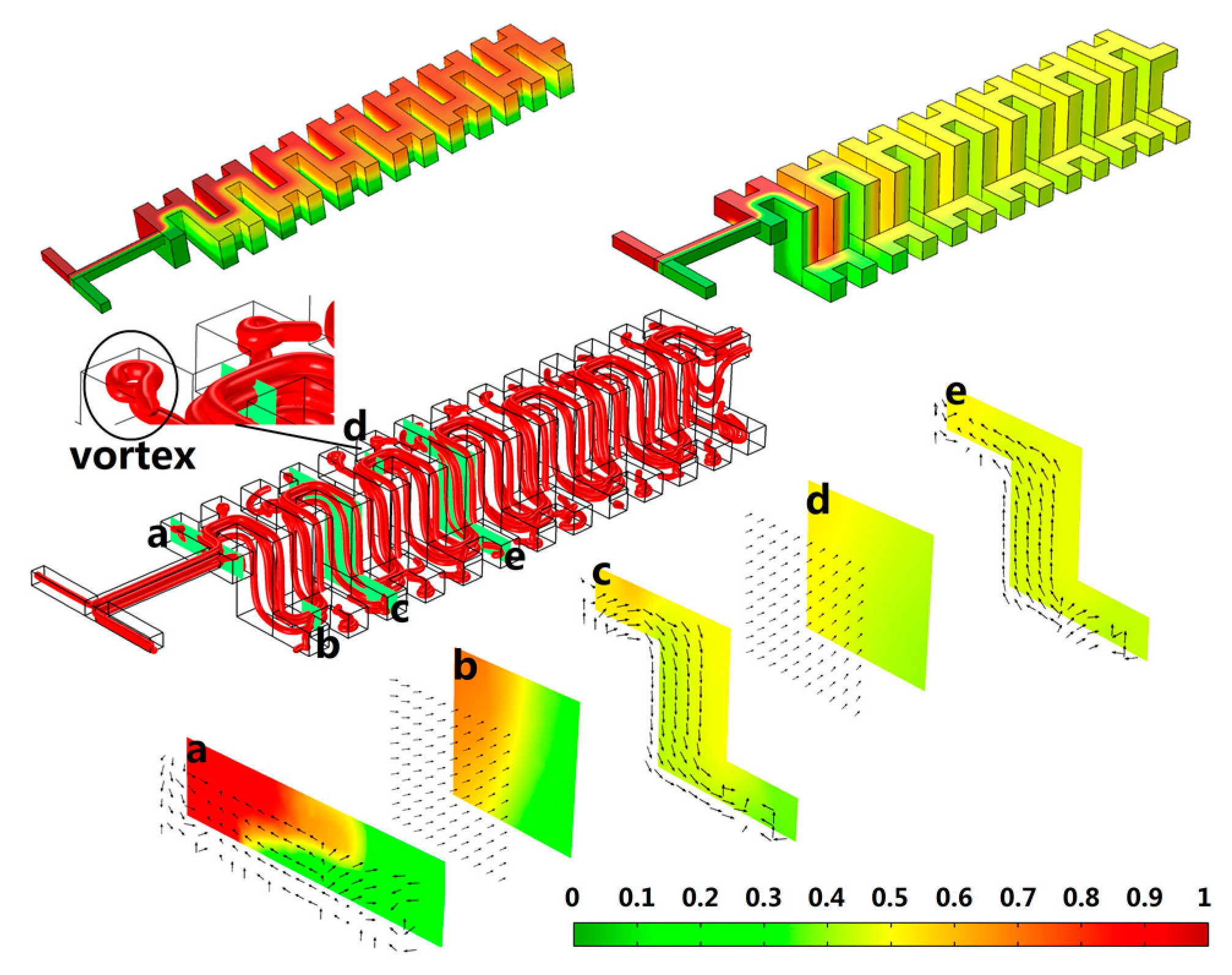
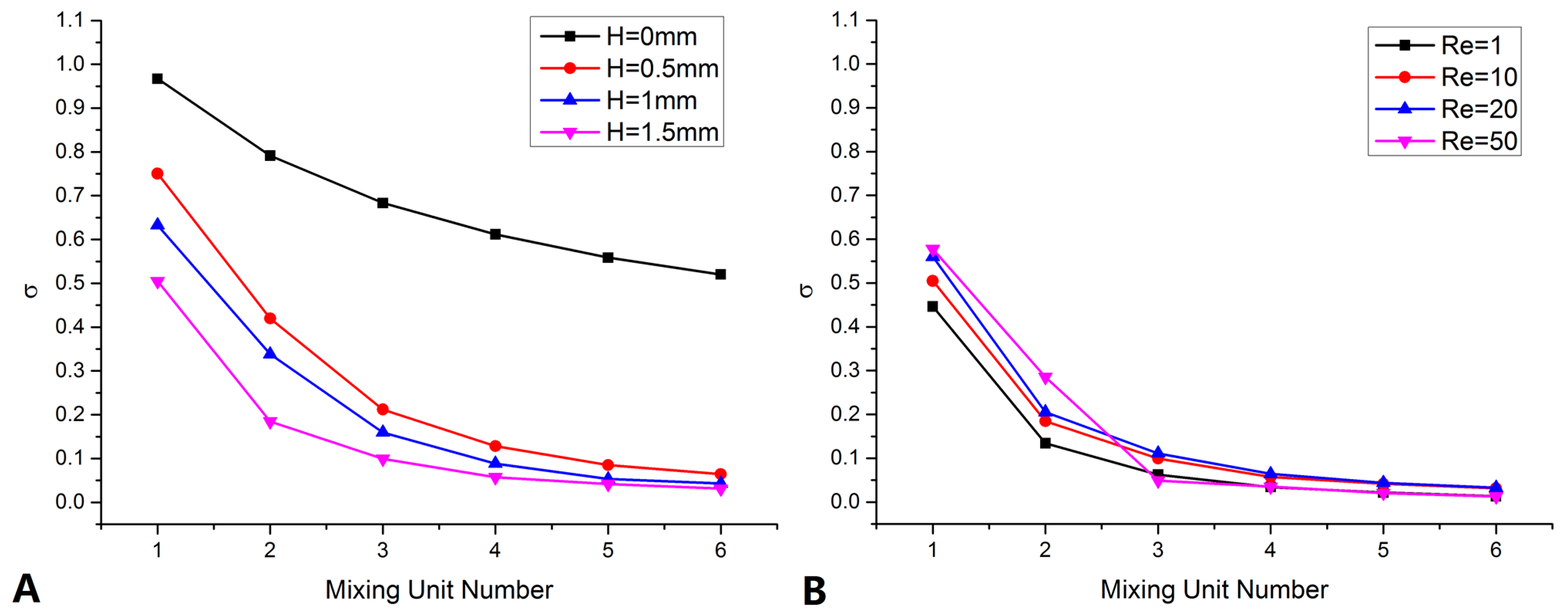
4.2. Mixer Fabrication and Test

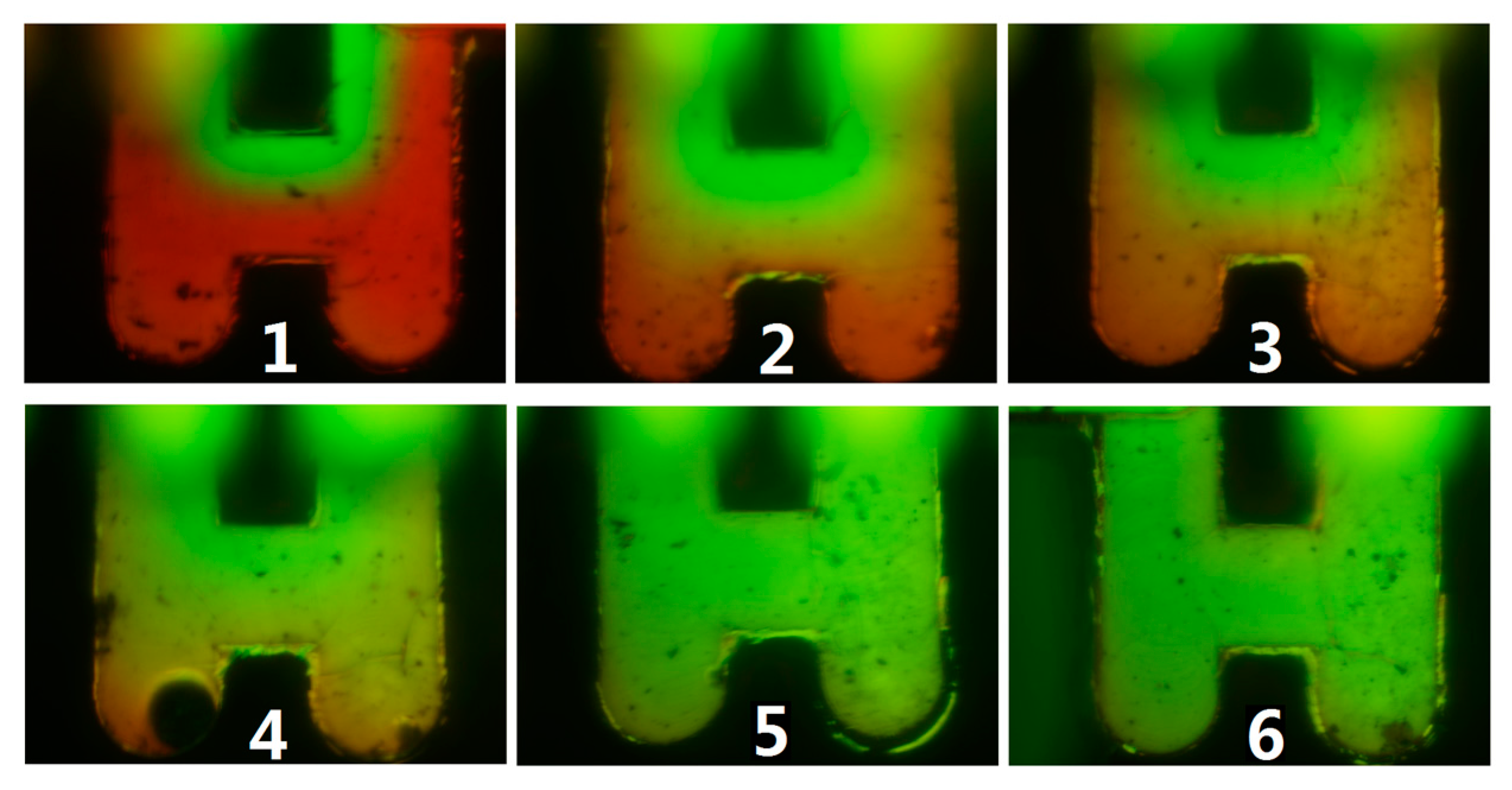
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, C.-H.; Wang, Y.-N.; Fu, L.-M. Integrated microfluidic chip for rapid DNA digestion and time-resolved capillary electrophoresis analysis. Biomicrofluidics 2012, 6, 012818. [Google Scholar] [CrossRef]
- Amin, A.M.; Thakur, R.; Madren, S.; Chuang, H.S.; Thottethodi, M.; Vijaykumar, T.N.; Jacobson, S.C. Software programmable continuous flow multi-purpose lab on a chip. Microfluid. Nanofluid. 2013, 15, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lewis, A.C.; Ródenas-García, M. Microfluidic lab-on-a-chip derivatization for gaseous carbonyl analysis. J. Chromatogr. A 2013, 1296, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.F.; Yang, J.T. A novel microreactor with 3D rotating flow to boost fluid reaction and mixing of viscous fluids. Sens. Actuators B Chem. 2009, 140, 629–642. [Google Scholar] [CrossRef]
- Chang, C.M.; Chiou, L.F.; Lin, C.C.; Shieh, D.B.; Lee, G.B. Three-dimensional microfluidic chip for the extraction of mitochondrial DNA. Microfluid. Nanofluid. 2010, 9, 489–498. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, C.; Dai, X.D.; Chen, H.H.; Liang, Y.; Sun, H.L.; Tian, H.; Ding, X.P. PMMA microfluidic devices with three-dimensional features for blood cell filtration. J. Micromech. Microeng. 2008, 18, 095021. [Google Scholar] [CrossRef]
- Land, K.J.; Mbanjwa, M.B.; Govindasamy, K.; Korvink, J.G. Low cost fabrication and assembly process for re-usable 3D polydimethylsiloxane (PDMS) microfluidic networks. Biomicrofluidics 2011, 5, 036502. [Google Scholar] [CrossRef]
- Farshchian, B.; Park, S.; Choi, J.; Amirsadeghi, A.; Lee, J.; Park, S. 3D nanomolding for lab-on-a-chip applications. Lab Chip 2012, 12, 4764–4771. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Guo, H.; Shen, Y.; Hu, Y.; Sun, Y. Determination of EOF of PMMA microfluidic chip by indirect laser-induced fluorescence detection. Sens. Actuators B Chem. 2006, 114, 1100–1107. [Google Scholar]
- Hong, T.F.; Ju, W.J.; Wu, M.C.; Tai, C.H.; Tsai, C.H.; Fu, L.M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid. Nanofluid. 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Liu, C.; Luo, Y. Hot embossing methods for plastic microchannel fabrication. Chin. J. Mechan. Eng. Engl. Ed. 2006, 19, 223–225. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, X.W.; Peng, Z.C.; Wang, W.; Chen, Y.F. Investigation of Thermal Bonding on PMMA Capillary Electrophoresis Chip. Adv. Mater. Res. 2009, 60, 288–292. [Google Scholar] [CrossRef]
- Soper, S.A.; Hashimoto, M.; Situma, C.; Murphy, M.C.; McCarley, R.L.; Cheng, Y.W.; Barany, F. Fabrication of DNA microarrays onto polymer substrates using UV modification protocols with integration into microfluidic platforms for the sensing of low-abundant DNA point mutations. Methods 2005, 37, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Mair, D.A.; Rolandi, M.; Snauko, M.; Noroski, R.; Svec, F.; Fréchet, J.M. Room-temperature bonding for plastic high-pressure microfluidic chips. Anal. Chem. 2007, 79, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- Toh, A.G.G.; Ng, S.H.; Wang, Z. Fabrication and testing of embedded microvalves within PMMA microfluidic devices. Microsyst. Technol. 2009, 15, 1335–1342. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, H.; Liu, C.; Xu, Z.; Li, Y.; Wang, L. Plasma assisted thermal bonding for PMMA microfluidic chips with integrated metal microelectrodes. Sens. Actuators B Chem. 2009, 141, 646–651. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, C.; Liu, J.S.; Xu, Z.; Wang, L.D. Multi-layer PMMA microfluidic chips with channel networks for liquid sample operation. J. Mater. Process. Technol. 2009, 209, 5487–5493. [Google Scholar] [CrossRef]
- Gong, X.; Yi, X.; Xiao, K.; Li, S.; Kodzius, R.; Qin, J.; Wen, W. Wax-bonding 3D microfluidic chips. Lab Chip 2010, 10, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.; Ke, X.; Xu, Z.; Li, M.; Duan, Y.; Fan, Y.; Wang, L. Fabrication of a thermoplastic multilayer microfluidic chip. J. Mater. Process. Technol. 2012, 212, 2315–2320. [Google Scholar] [CrossRef]
- Lei, K.F.; Ahsan, S.; Budraa, N.; Li, W.J.; Mai, J.D. Microwave bonding of polymer-based substrates for potential encapsulated micro/nanofluidic device fabrication. Sens. Actuators A Phys. 2004, 114, 340–346. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Sbarski, I.; Hayes, J.P.; Solomon, M.; Tran, N. Microwave welding of polymeric-microfluidic devices. J. Micromech. Microeng. 2005, 15, 1692–1699. [Google Scholar] [CrossRef]
- Rahbar, M.; Chhina, S.; Sameoto, D.; Parameswaran, M. Microwave-induced, thermally assisted solvent bonding for low-cost PMMA microfluidic devices. J. Micromech. Microeng. 2010, 20, 015026. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, Z.F.; de Rooij, N.F. Microfluidic connectors by ultrasonic welding. Microelectron. Eng. 2009, 86, 1354–1357. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Luo, Y.; He, S.; Wang, L. Thermal assisted ultrasonic bonding method for poly(methyl methacrylate) (PMMA) microfluidic devices. Talanta 2010, 81, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Z.; Wang, X.; Zheng, Y. Ultrasonic bonding for thermoplastic microfluidic devices without energy director. Microelectron. Eng. 2010, 87, 2429–2436. [Google Scholar] [CrossRef]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly (methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chen, T.Y. Applying Taguchi methods for solvent-assisted PMMA bonding technique for static and dynamic μ-TAS devices. Biomed. Microdevices 2007, 9, 513–522. [Google Scholar] [PubMed]
- Lin, C.H.; Chao, C.H.; Lan, C.W. Low azeotropic solvent for bonding of PMMA microfluidic devices. Sens. Actuators B Chem. 2007, 121, 698–705. [Google Scholar] [CrossRef]
- Umbrecht, F.; Müller, D.; Gattiker, F.; Boutry, C.M.; Neuenschwander, J.; Sennhauser, U.; Hierold, C. Solvent assisted bonding of polymethylmethacrylate: Characterization using the response surface methodology. Sens. Actuators A Phys. 2009, 156, 121–128. [Google Scholar] [CrossRef]
- Tran, H.H.; Wu, W.; Lee, N.Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators B Chem. 2013, 181, 955–962. [Google Scholar] [CrossRef]
- De Marco, C.; Eaton, S.M.; Martinez-Vazquez, R.; Rampini, S.; Cerullo, G.; Levi, M.; Turri, S.; Osellame, R. Solvent vapor treatment controls surface wettability in PMMA femtosecond-laser-ablated microchannels. Microfluid. Nanofluid. 2013, 14, 171–176. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, X.; Li, T.; Han, X. Miscible Organic Solvents Soak Bonding Method Use in a PMMA Multilayer Microfluidic Device. Micromachines 2014, 5, 1416-1428. https://doi.org/10.3390/mi5041416
Zhang H, Liu X, Li T, Han X. Miscible Organic Solvents Soak Bonding Method Use in a PMMA Multilayer Microfluidic Device. Micromachines. 2014; 5(4):1416-1428. https://doi.org/10.3390/mi5041416
Chicago/Turabian StyleZhang, He, Xiaowei Liu, Tian Li, and Xiaowei Han. 2014. "Miscible Organic Solvents Soak Bonding Method Use in a PMMA Multilayer Microfluidic Device" Micromachines 5, no. 4: 1416-1428. https://doi.org/10.3390/mi5041416





