Light-Addressable Electrodeposition of Magnetically-Guided Cells Encapsulated in Alginate Hydrogels for Three-Dimensional Cell Patterning
Abstract
:1. Introduction
2. Experimental Section
2.1. Principle of Operation
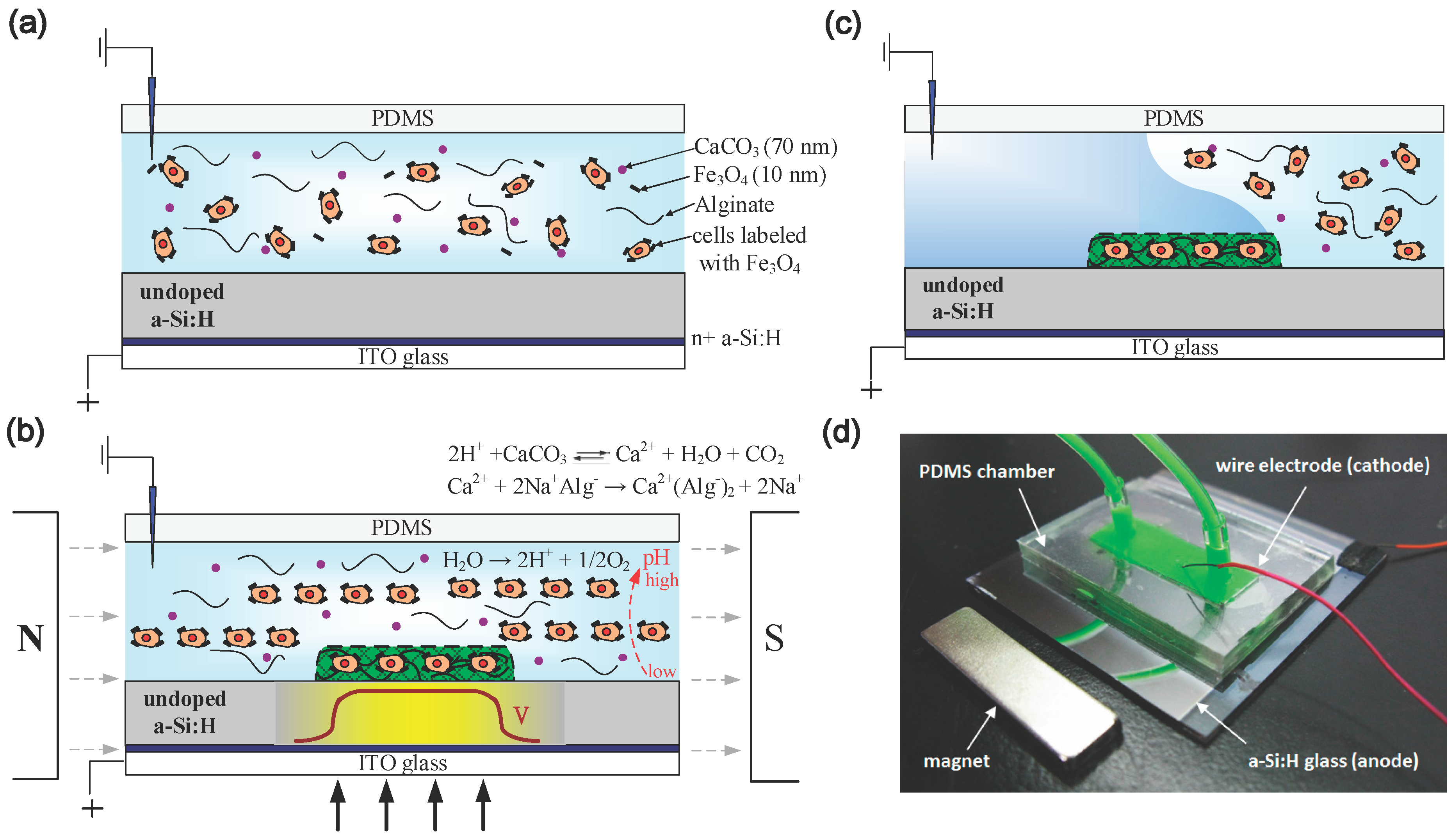
2.2. Magnetically-Labeled Cells and Chemical Compounds
2.3. Experimental Setup for a Light-Addressed Electrolytic System

3. Results and Discussion
3.1. Formation of Cell-Encapsulated Multilayer Alginate Hydrogels

| h1 (μm) | Vo (V) | t* (s) | t (s) | h2 (μm) | D/Do | Ds (μm) |
| 0 | 10 | 5 | 5 | 56 ± 1.8 | 1.05 ± 0.11 | 102 ± 2.2 |
| 30 | 280 ± 3.4 | 1.08 ± 0.16 | ||||
| 20 | 3 | 3 | 54 ± 2.5 | 1.12 ± 0.13 | ||
| 200 ± 3.6 | 10 | 34 | 34 | 63 ± 3.9 | 1.14 ± 0.17 | 155 ± 3.7 |
| 70 | 275 ± 5.8 | 1.18 ± 0.12 | Do: the illumination pattern of circular shapes.
D: the corresponding diameter of the alginate hydrogels produced by the illumination pattern. | |||
| 20 | 21 | 21 | 71 ± 4.2 | 1.23 ± 0.15 | ||
| 400 ± 5.9 | 10 | 82 | 82 | 69 ± 5.1 | 1.27 ± 0.14 | 213 ± 4.2 |
| 150 | 415 ± 9.4 | 1.34 ± 0.12 | ||||
| 20 | 47 | 47 | 75 ± 4.4 | 1.39 ± 0.18 |
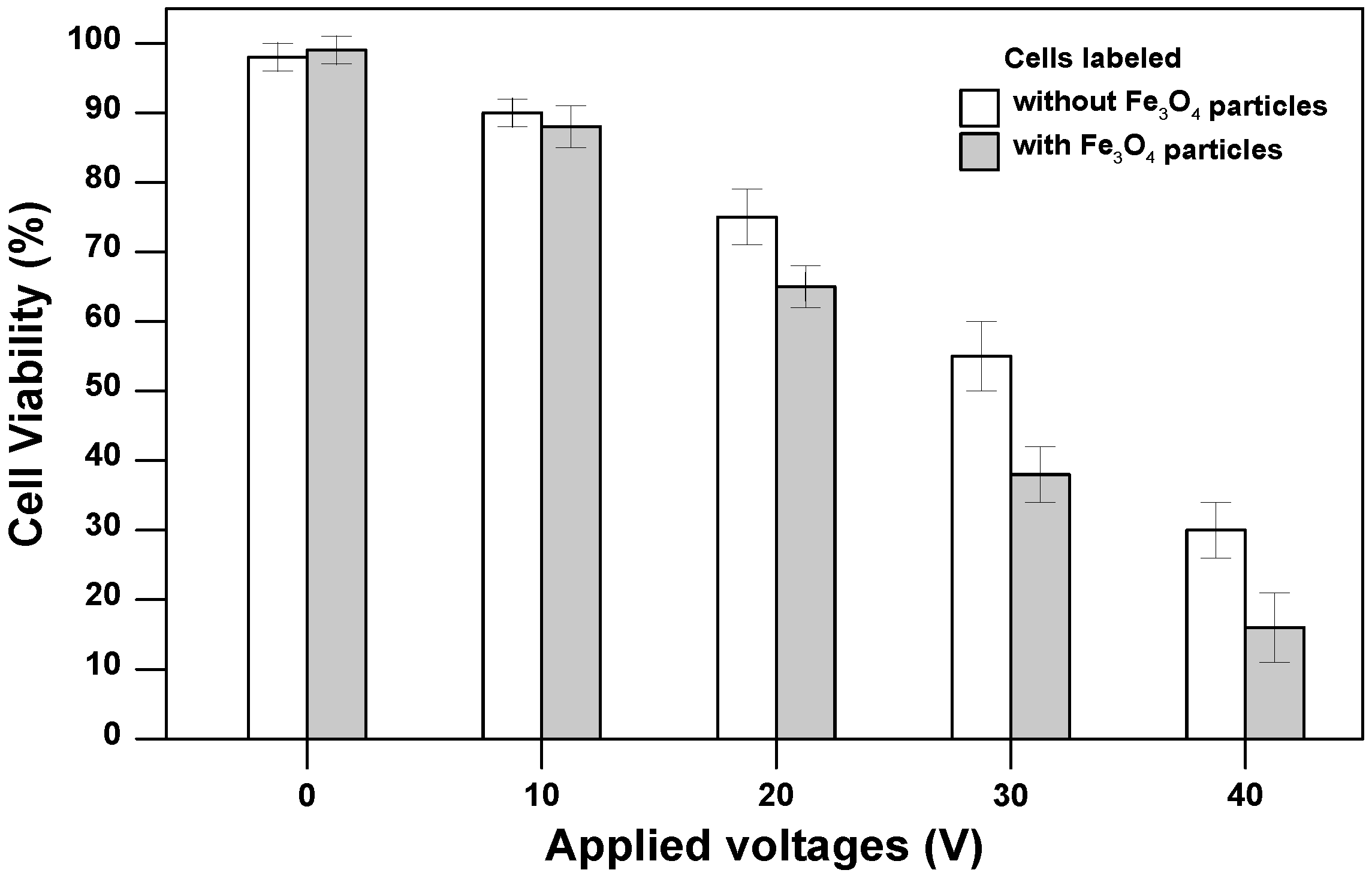
3.2. Cell Viability for Magnetically-Labeled Cells after Electrodeposition
3.3. Light-Addressable Electrodeposition of Magnetically-Guided Cells Encapsulated in Alginate Hydrogels

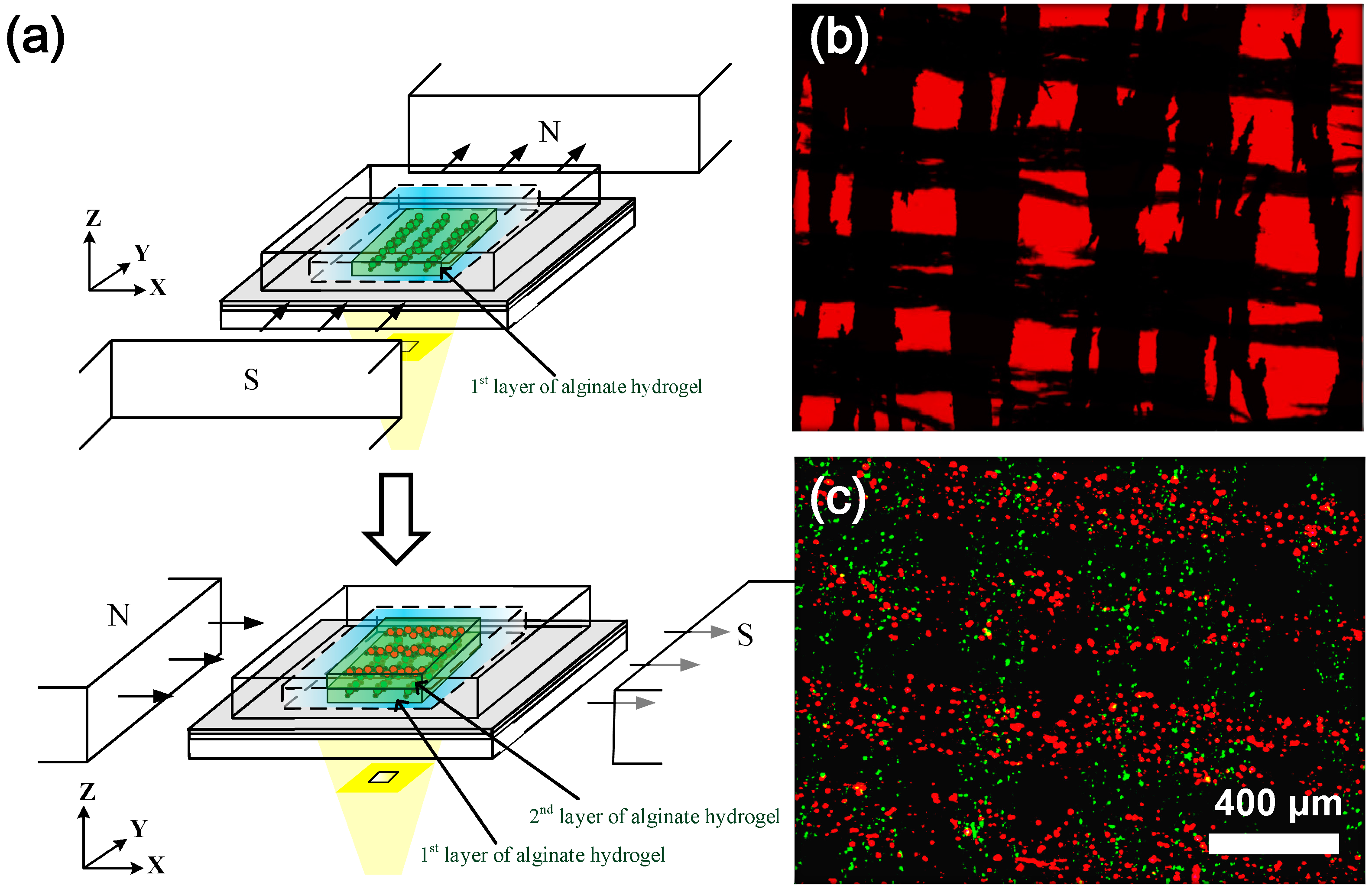
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell. Bio. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Rivron, N.C.; Rouwkema, J.; Truckenmuller, R.; Karperien, M.; De Boer, J.; van Blitterswijk, C.A. Tissue assembly and organization: Developmental mechanisms in microfabricated tissues. Biomaterials 2009, 30, 4851–4858. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.G.; Kwon, S.-J.; Bale, S.S.; Lee, M.-Y.; Diogo, M.M.; Clark, D.S.; Cabral, J.M.S.; Dordick, J.S. Three-dimensional cell culture microarray for high-throughput studies of stem cell fate. Biotechnol. Bioeng. 2010, 106, 106–118. [Google Scholar] [PubMed]
- Lee, M.-Y.; Kumar, R.A.; Sukumaran, S.M.; Hogg, M.G.; Clark, D.S.; Dordick, J.S. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc. Natl. Acad. Sci. USA 2008, 105, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Mechanism of anodic electrodeposition of calcium alginate. Soft Matter 2011, 7, 5677–5684. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Payne, G.F.; Rubloff, G.W. Biofabrication: Programmable assembly of polysaccharide hydrogels in microfluidics as biocompatible scaffolds. J. Mater. Chem. 2012, 22, 7659–7666. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.L.; Tsao, C.Y.; Wu, H.C.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation. Lab Chip 2011, 11, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tsao, C.Y.; Wu, H.C.; Luo, X.L.; Terrell, J.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Electroaddressing functionalized polysaccharides as model biofilms for interrogating cell signaling. Adv. Funct. Mater. 2012, 22, 519–528. [Google Scholar] [CrossRef]
- Luo, X.L.; Wu, H.C.; Tsao, C.Y.; Cheng, Y.; Betz, J.; Payne, G.F.; Rubloff, G.W.; Bentley, W.E. Biofabrication of stratified biofilm mimics for observation and control of bacterial signaling. Biomaterials 2012, 33, 5136–5143. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Tsao, C.Y.; Yang, X.H.; Liu, Y.; Dykstra, P.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels. Adv. Funct. Mater. 2009, 19, 2074–2080. [Google Scholar] [CrossRef]
- Shi, X.W.; Yang, X.H.; Liu, Y.; Dykstra, P.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Stimulus responsive biopolymers for protein and cell electroaddressing. In Presented at The 238th ACS National Meeting, Washington, DC, USA, 16–20 August 2009; 2009. [Google Scholar]
- Yang, X.H.; Kim, E.; Liu, Y.; Shi, X.W.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Pancer, Z.; Payne, G.F. In-film bioprocessing and immunoanalysis with electroaddressable stimuli-responsive polysaccharides. Adv. Funct. Mater. 2010, 20, 1645–1652. [Google Scholar] [CrossRef]
- Huang, S.H.; Hsueh, H.J.; Jiang, Y.L. Light-addressable electrodeposition of cell-encapsulated alginate hydrogels for a cellular microarray using a digital micromirror device. Biomicrofluidics 2011, 5, 034109. [Google Scholar] [CrossRef]
- Akiyama, H.; Ito, A.; Kawabe, Y.; Kamihira, M. Fabrication of complex three-dimensional tissue architectures using a magnetic force-based cell patterning technique. Biomed. Microdevices 2009, 11, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Ito, A.; Kawabe, Y.; Kamihira, M. Cell-patterning using poly (ethylene glycol)-modified magnetite nanoparticles. J. Biomed. Mater. Res. A 2010, 92A, 1123–1130. [Google Scholar] [PubMed]
- Fu, C.Y.; Lin, C.Y.; Chu, W.C.; Chang, H.Y. A simple cell patterning method using magnetic particle-containing photosensitive poly (ethylene glycol) hydrogel blocks: A technical note. Tissue Eng. Part. C Methods 2011, 17, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Ito, A.; Honda, H. Cell patterning using magnetite nanoparticles and magnetic force. Biotechnol. Bioeng. 2007, 97, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Okochi, M.; Konishi, N.; Nakatochi, M.; Imai, R.; Shikida, M.; Ito, A.; Honda, H. Cell culture arrays using magnetic force-based cell patterning for dynamic single cell analysis. Lab Chip 2008, 8, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Pauli, C.; Chen, P.; Du, J.; Chung, C.B.; Kong, S.D.; Colwell, C.W.; Lotz, M.K.; Jin, S.G.; D’Lima, D.D. In situ tissue engineering using magnetically guided three-dimensional cell patterning. Tissue Eng. Part C Methods 2012, 18, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Wei, L.S.; Chu, H.T.; Jiang, Y.L. Light-addressed electrodeposition of enzyme-entrapped chitosan membranes for multiplexed enzyme-based bioassays using a digital micromirror device. Sens. Basel 2013, 13, 10711–10724. [Google Scholar] [CrossRef]
- Dobson, J.; Bowtell, R.; Garcia-Prieto, A.; Pankhurst, Q. Safety implications of high-field MRI: Actuation of endogenous magnetic iron oxides in the human body. PLoS One 2009, 4, e5431. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Oliveira, T.R.; Mamani, J.B.; Malheiros, S.M.F.; Malavolta, L.; Pavon, L.F.; Sibov, T.T.; Amaro, E.; Tannus, A.; Vidoto, E.L.G.; Martins, M.J.; Santos, R.S.; Gamarra, L.F. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomed. 2011, 6, 591–603. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Chu, H.-T.; Liou, Y.-M.; Huang, K.-S. Light-Addressable Electrodeposition of Magnetically-Guided Cells Encapsulated in Alginate Hydrogels for Three-Dimensional Cell Patterning. Micromachines 2014, 5, 1173-1187. https://doi.org/10.3390/mi5041173
Huang S-H, Chu H-T, Liou Y-M, Huang K-S. Light-Addressable Electrodeposition of Magnetically-Guided Cells Encapsulated in Alginate Hydrogels for Three-Dimensional Cell Patterning. Micromachines. 2014; 5(4):1173-1187. https://doi.org/10.3390/mi5041173
Chicago/Turabian StyleHuang, Shih-Hao, Hsiao-Tzu Chu, Yan-Min Liou, and Kuo-Sheng Huang. 2014. "Light-Addressable Electrodeposition of Magnetically-Guided Cells Encapsulated in Alginate Hydrogels for Three-Dimensional Cell Patterning" Micromachines 5, no. 4: 1173-1187. https://doi.org/10.3390/mi5041173






