Femtosecond Laser 3D Fabrication in Porous Glass for Micro- and Nanofluidic Applications
Abstract
:1. Introduction
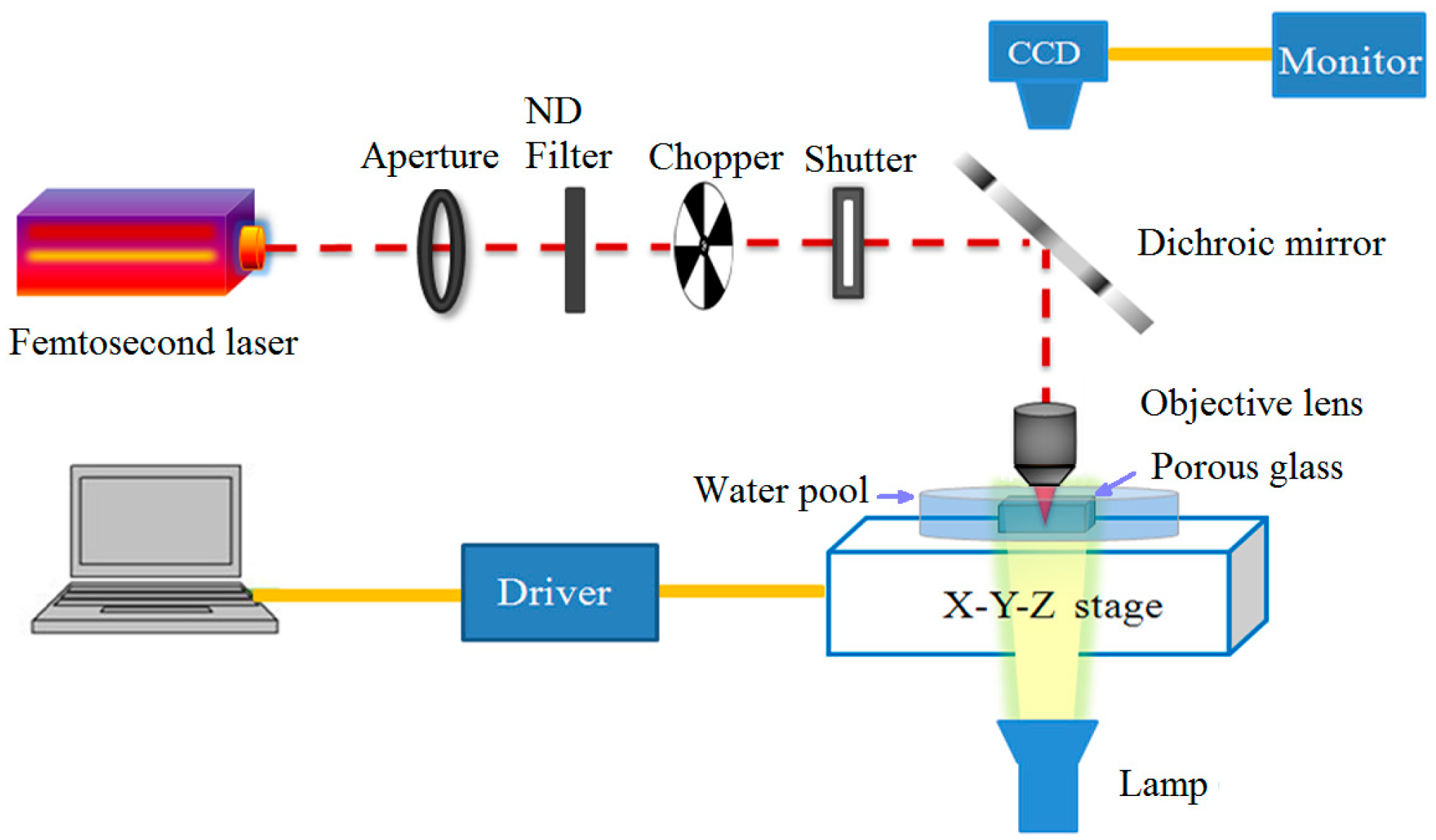
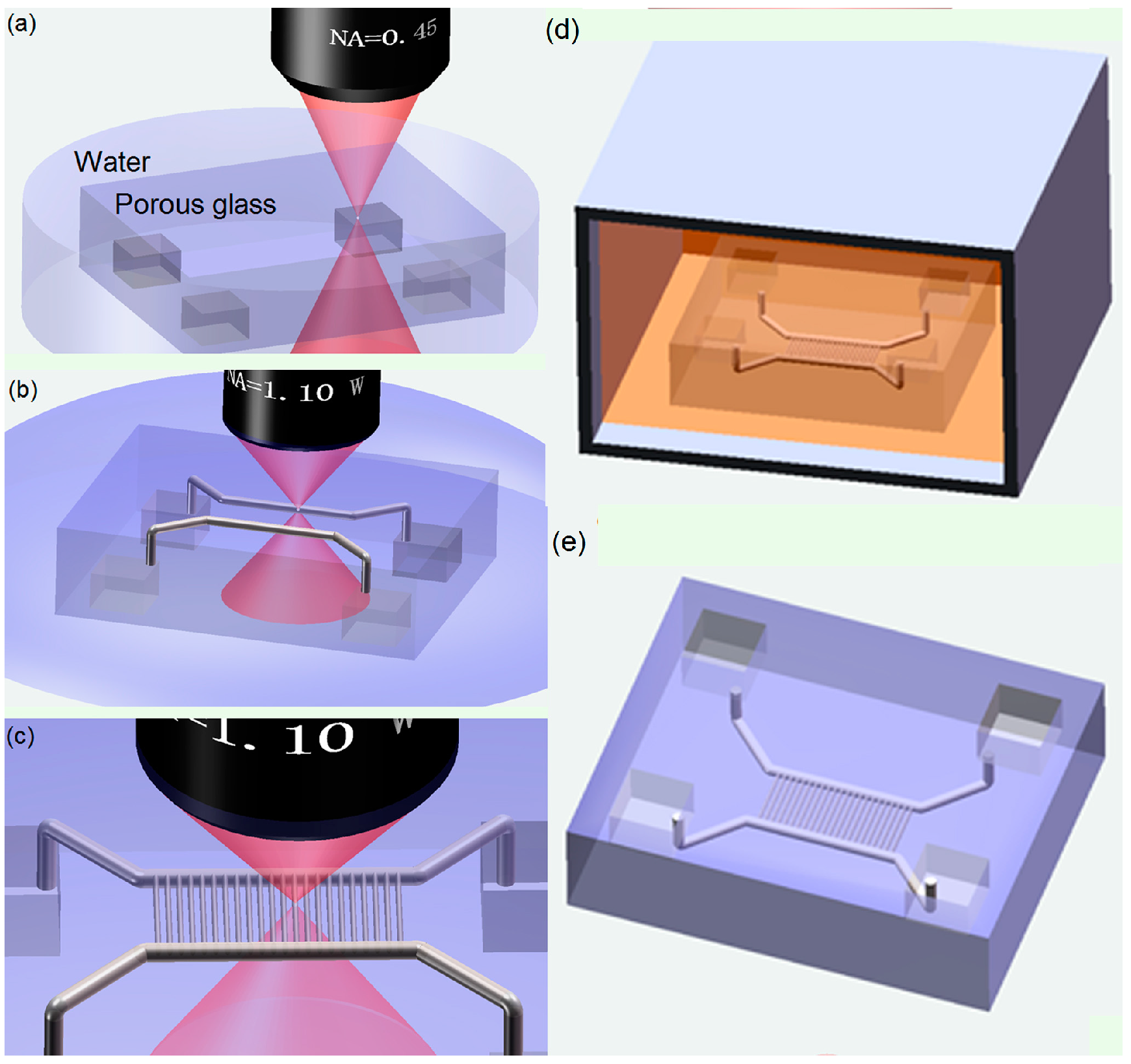
2. Experimental Setup and Fabrication Process
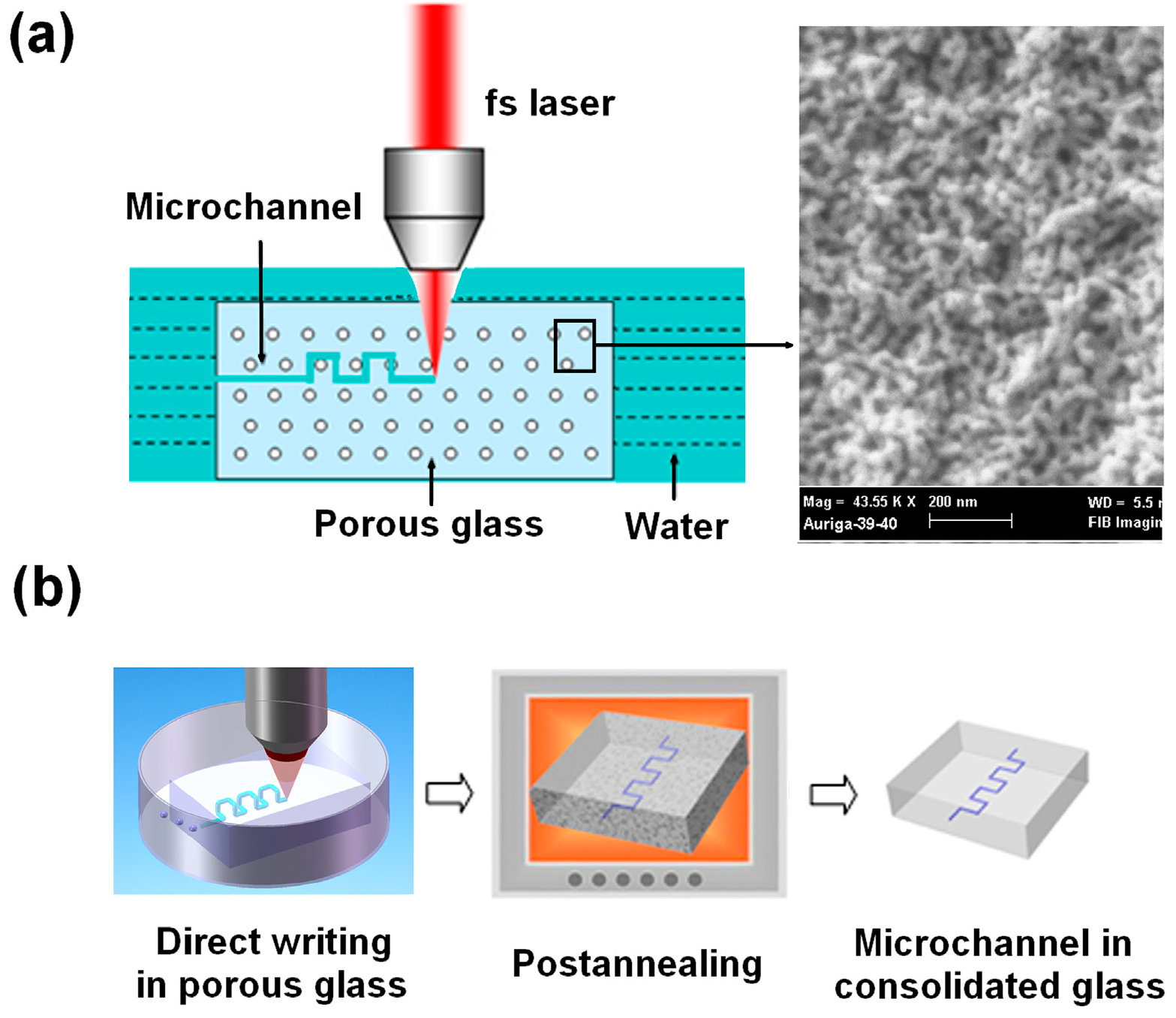
3. Laser Direct Writing of Microchannels
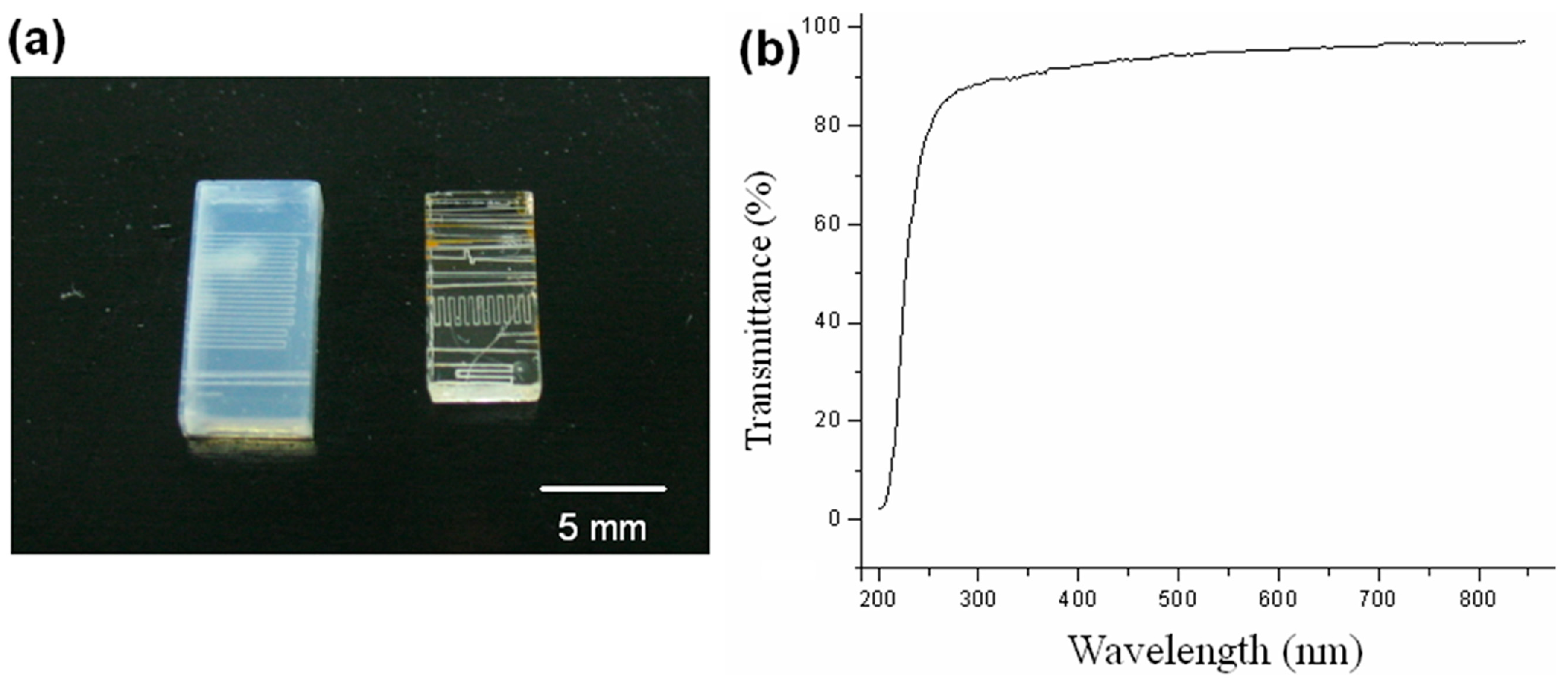
3.1. Fabrication of Multi-Centimeter-Long Microchannels
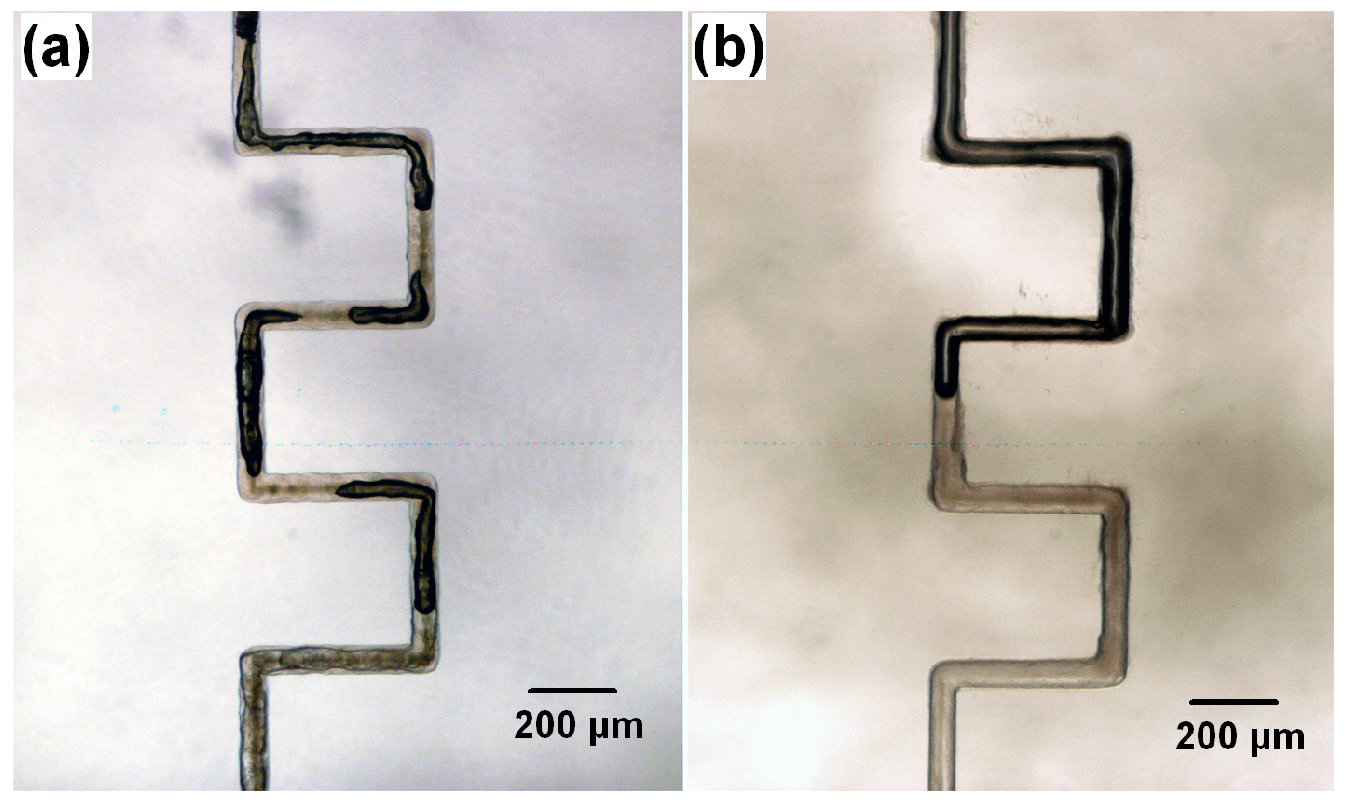
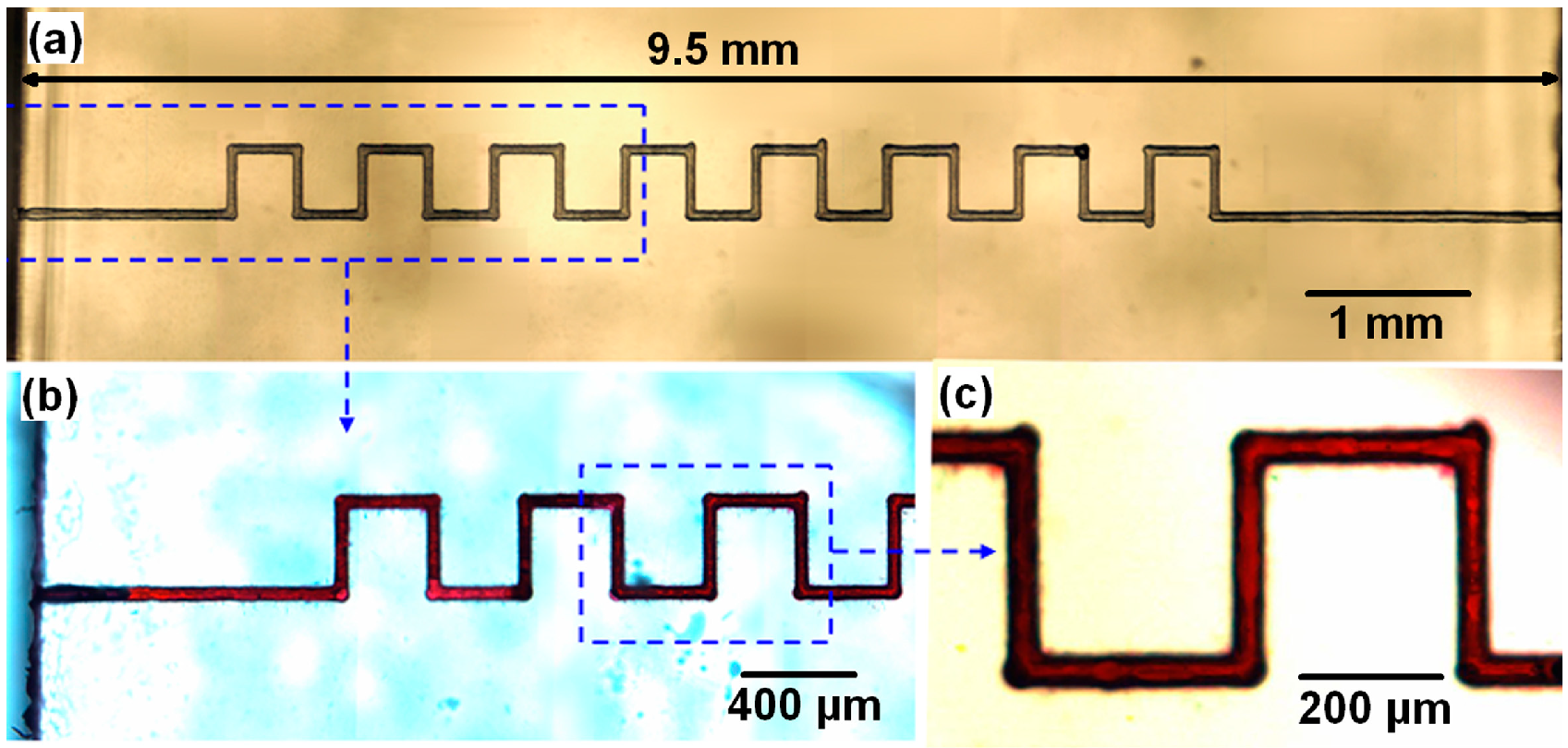
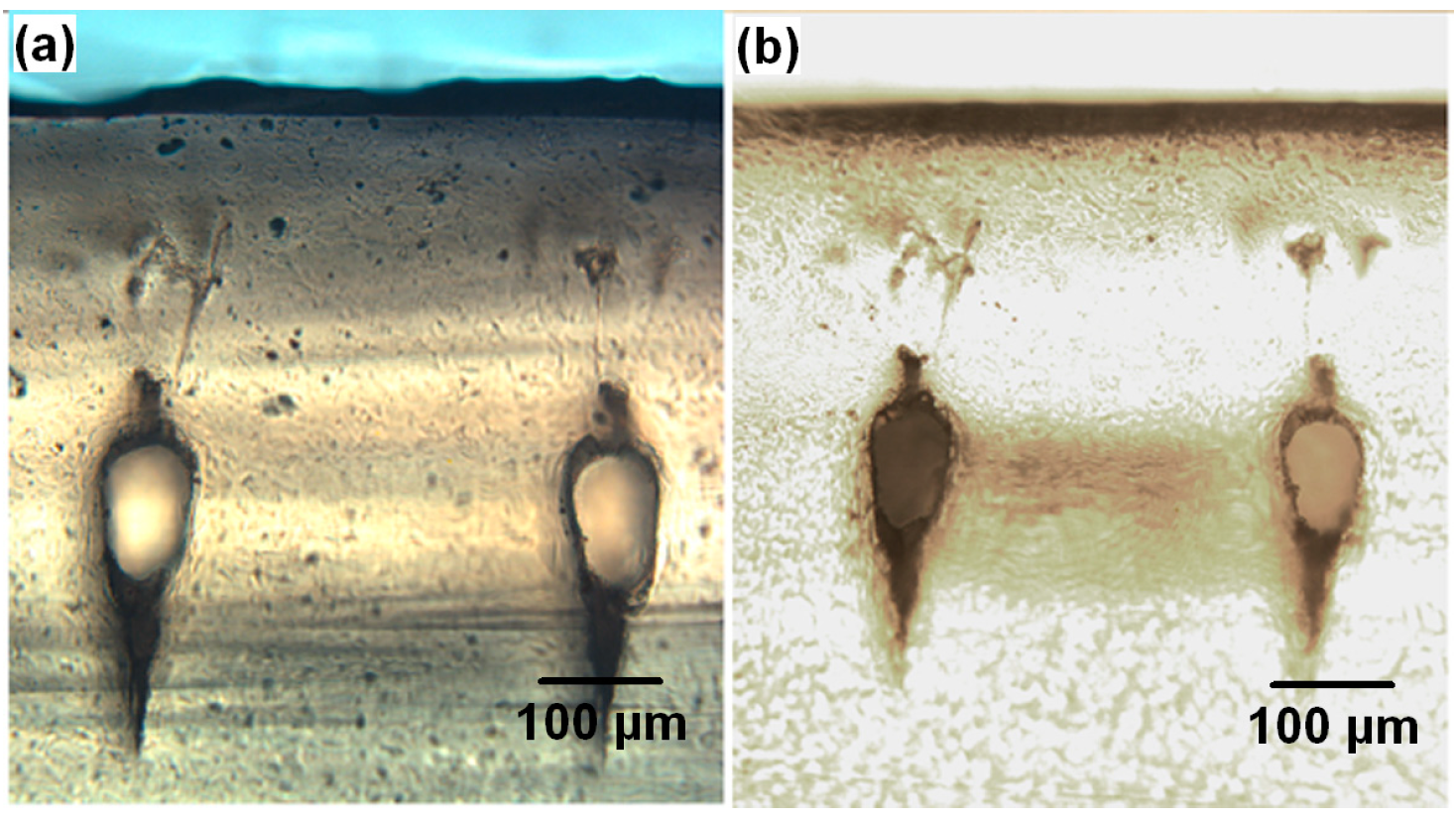
| Description | Cross section size (transverse) | Cross section size (vertical) | Channel length |
|---|---|---|---|
| Before annealing | 74 μm | 114 μm | 9.50 mm |
| After annealing | 63 μm | 97 μm | 8.00 mm |
| Shrinkage factor | 14.9% | 14.9% | 15.8% |
3.2. Mechanism of Efficient Removal of Debris

3.3. High-Aspect-Ratio 3D Microchannels
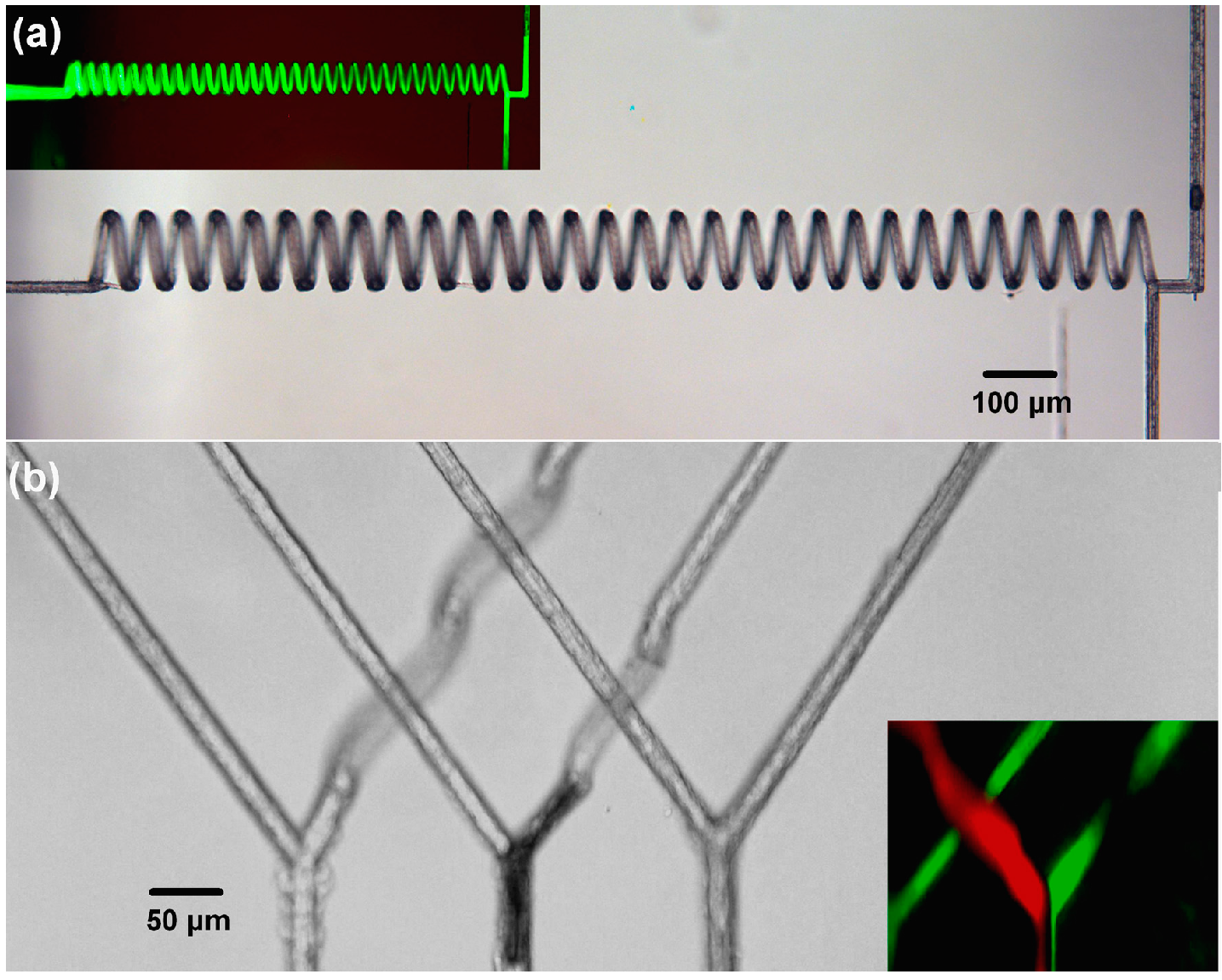
3.4. Microchannels Fabricated by Nanosecond Laser
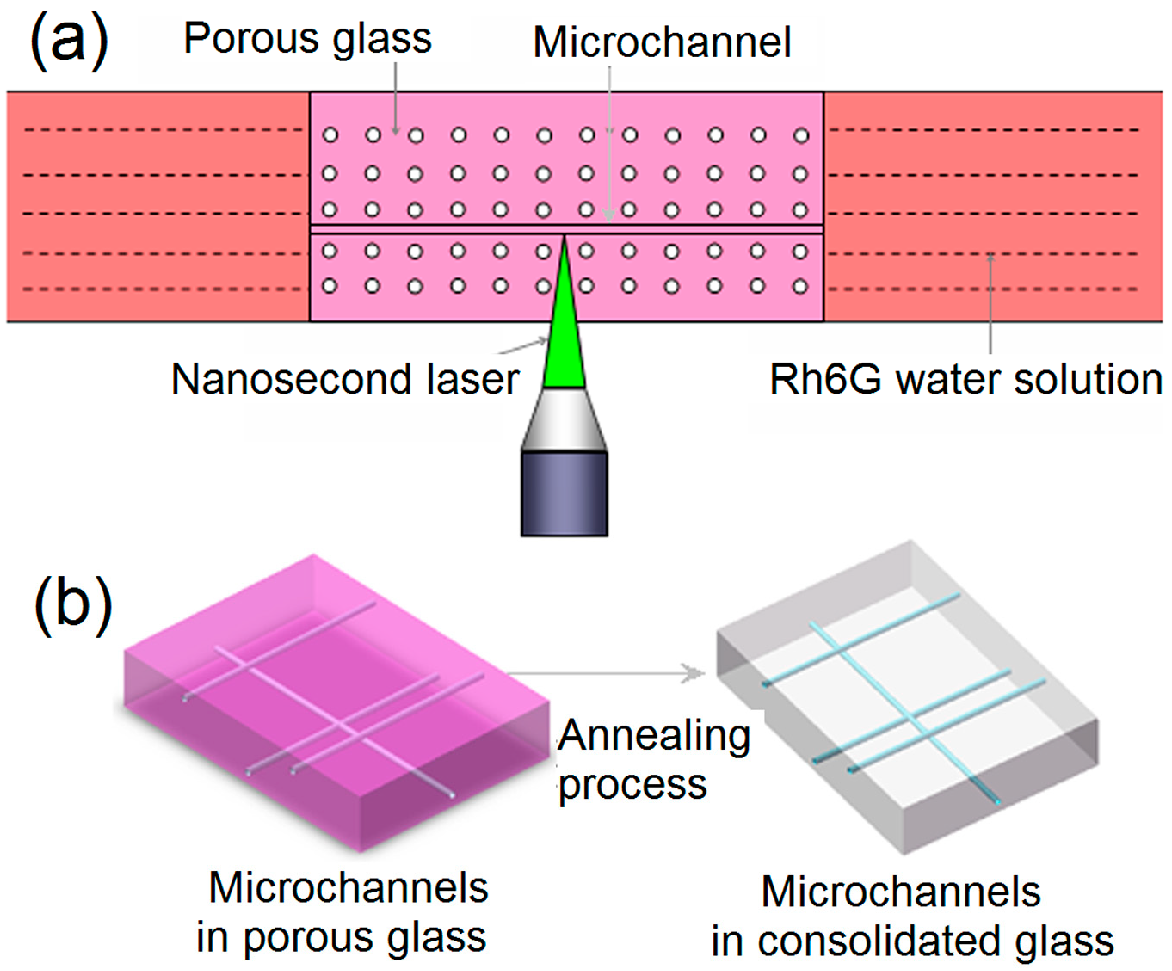
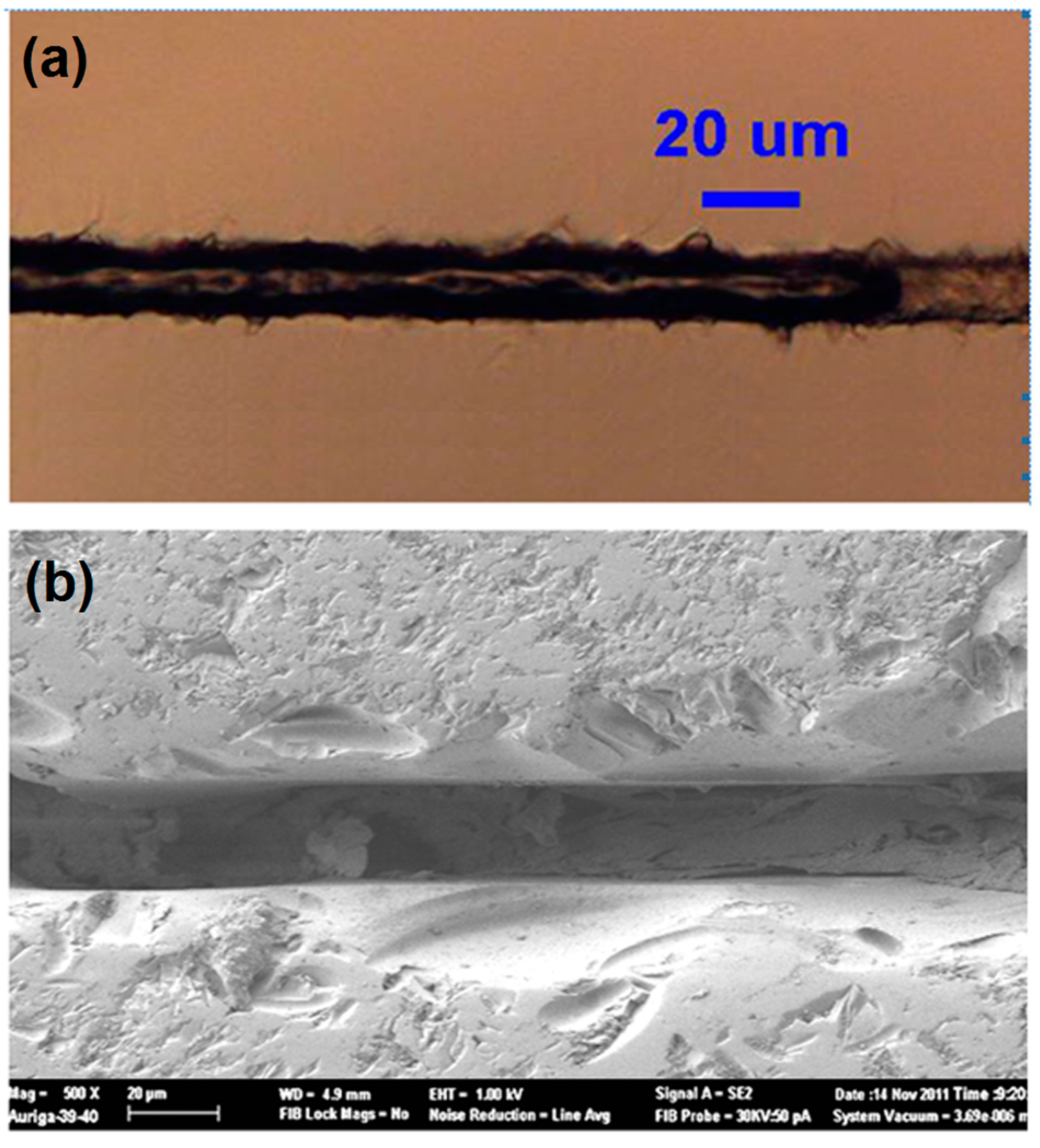
4. 3D Microfluidic Devices
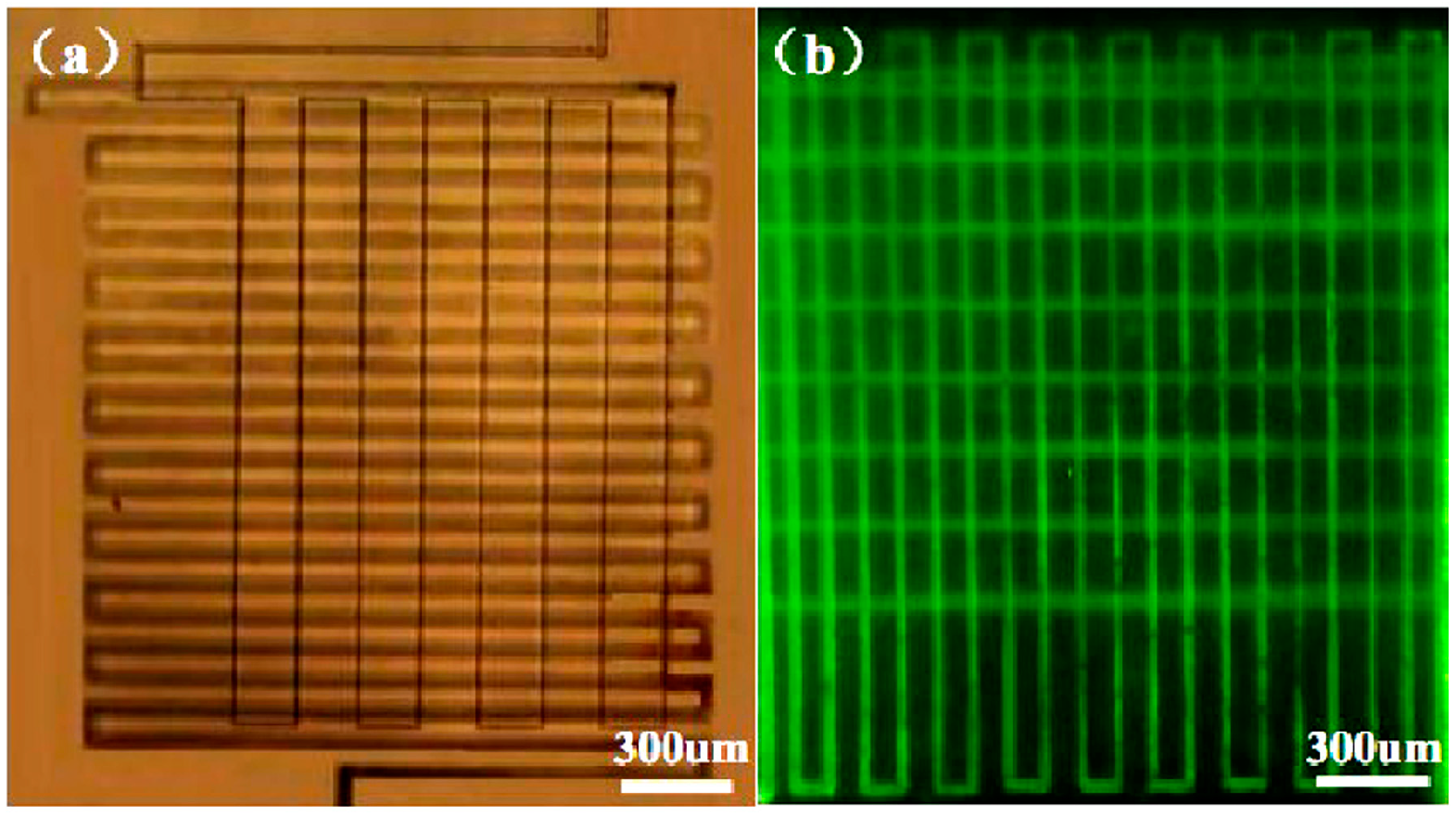
4.1. Large-Volume Hollow Chamber
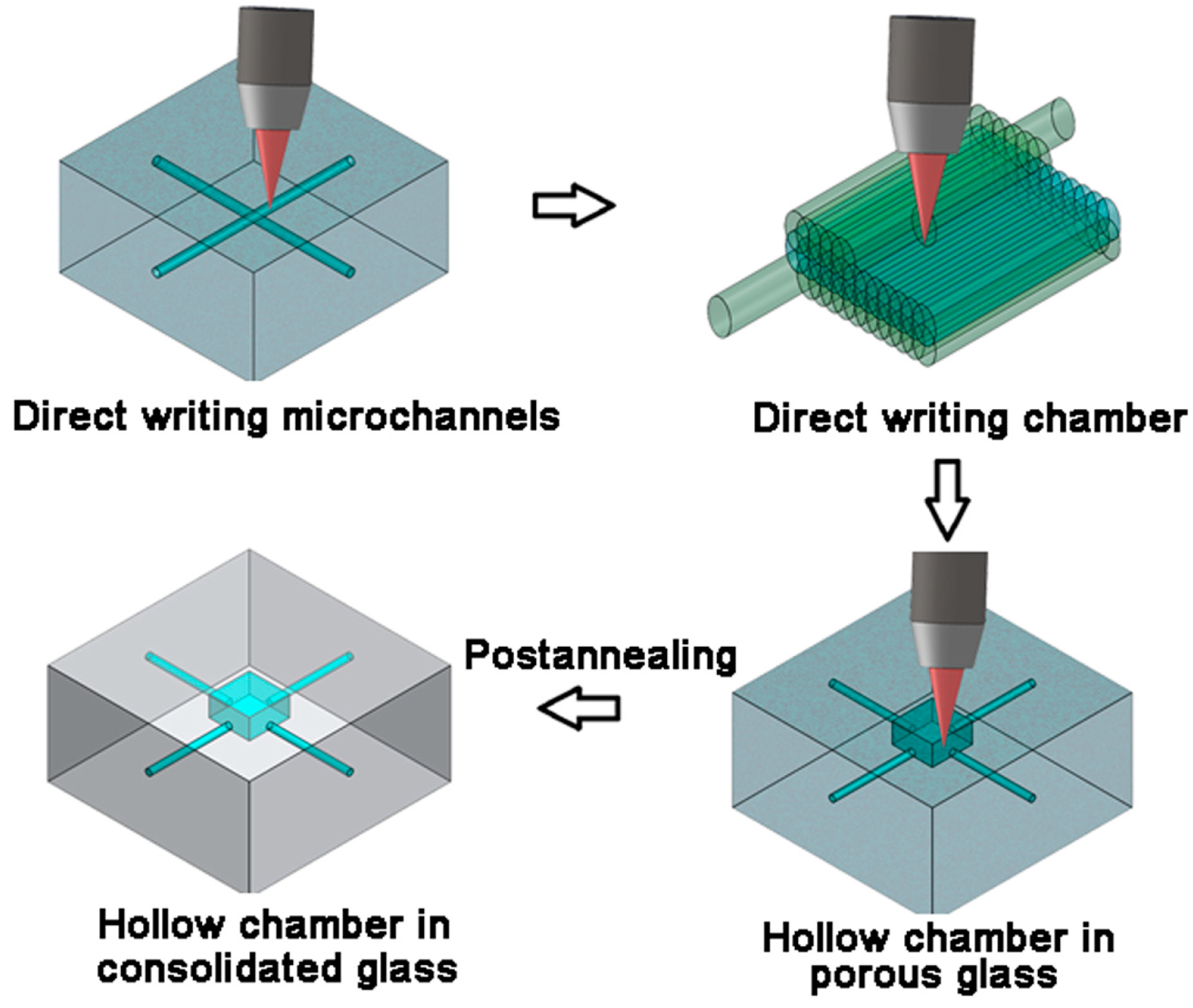

4.2. Three-Dimensional Microfluidic Mixer
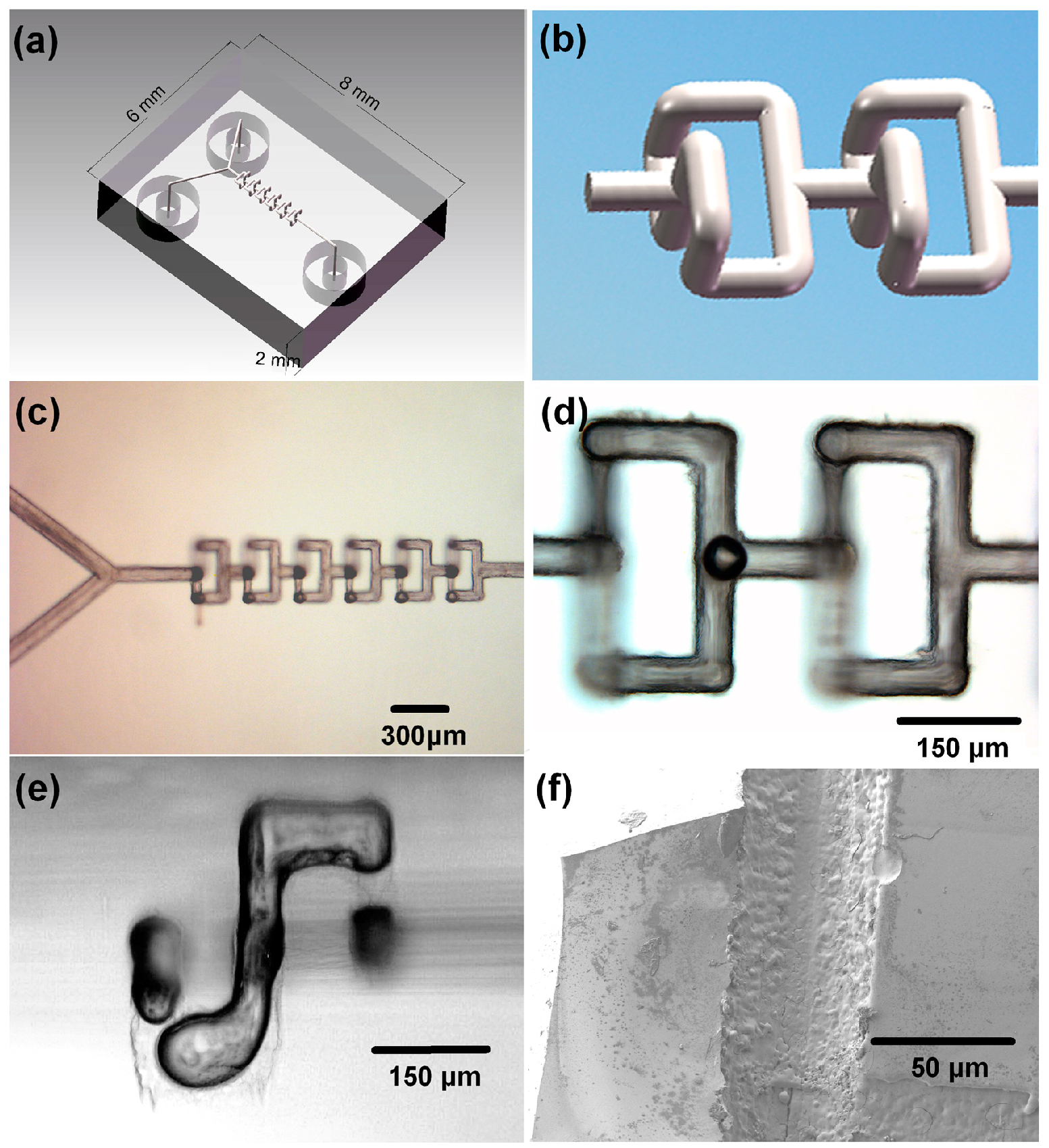

4.3. Electrophoresis Microchip

5. 3D Nanofluidic Channels and Devices
5.1. Direct Writing of Nanochannels

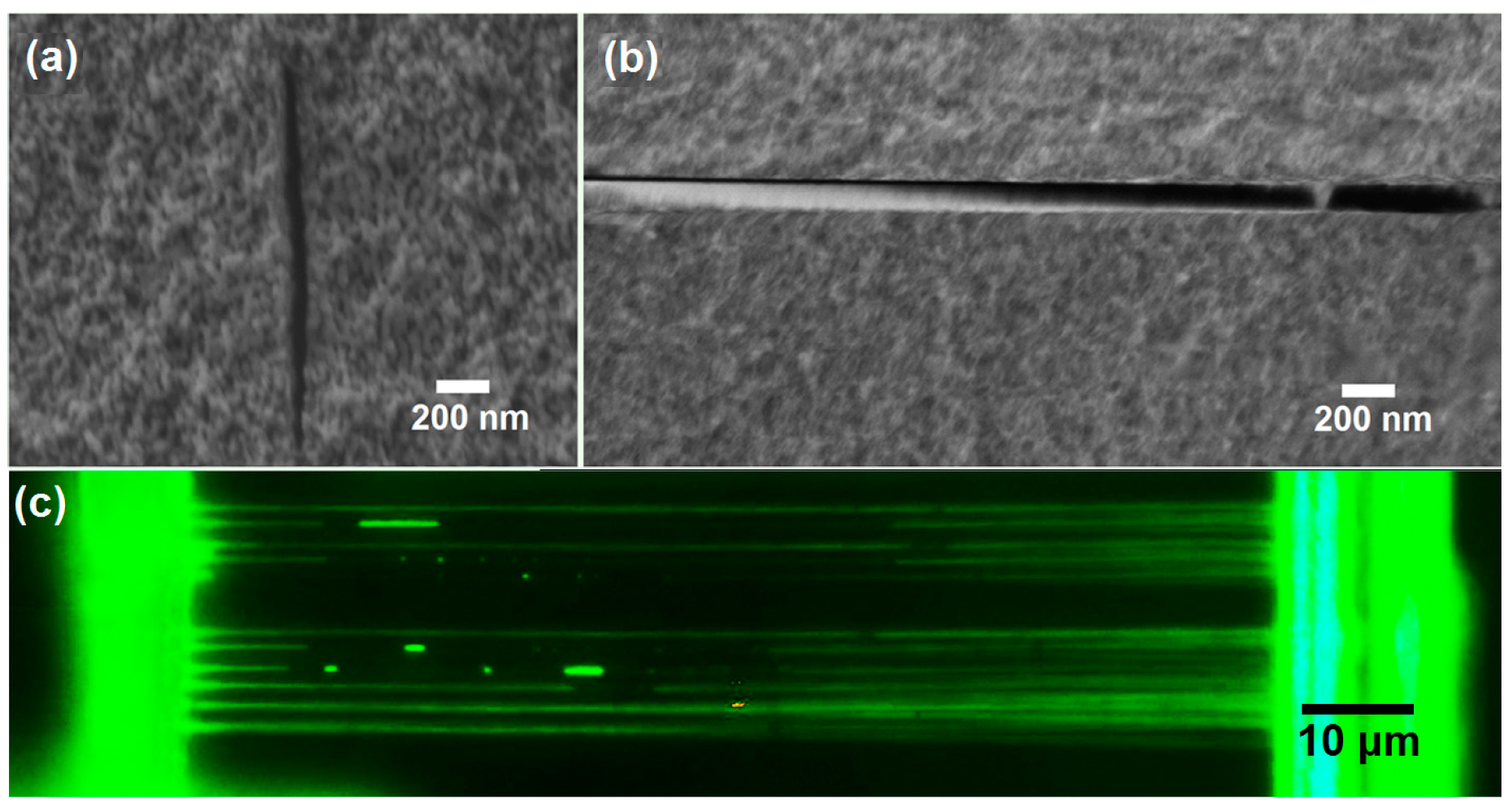
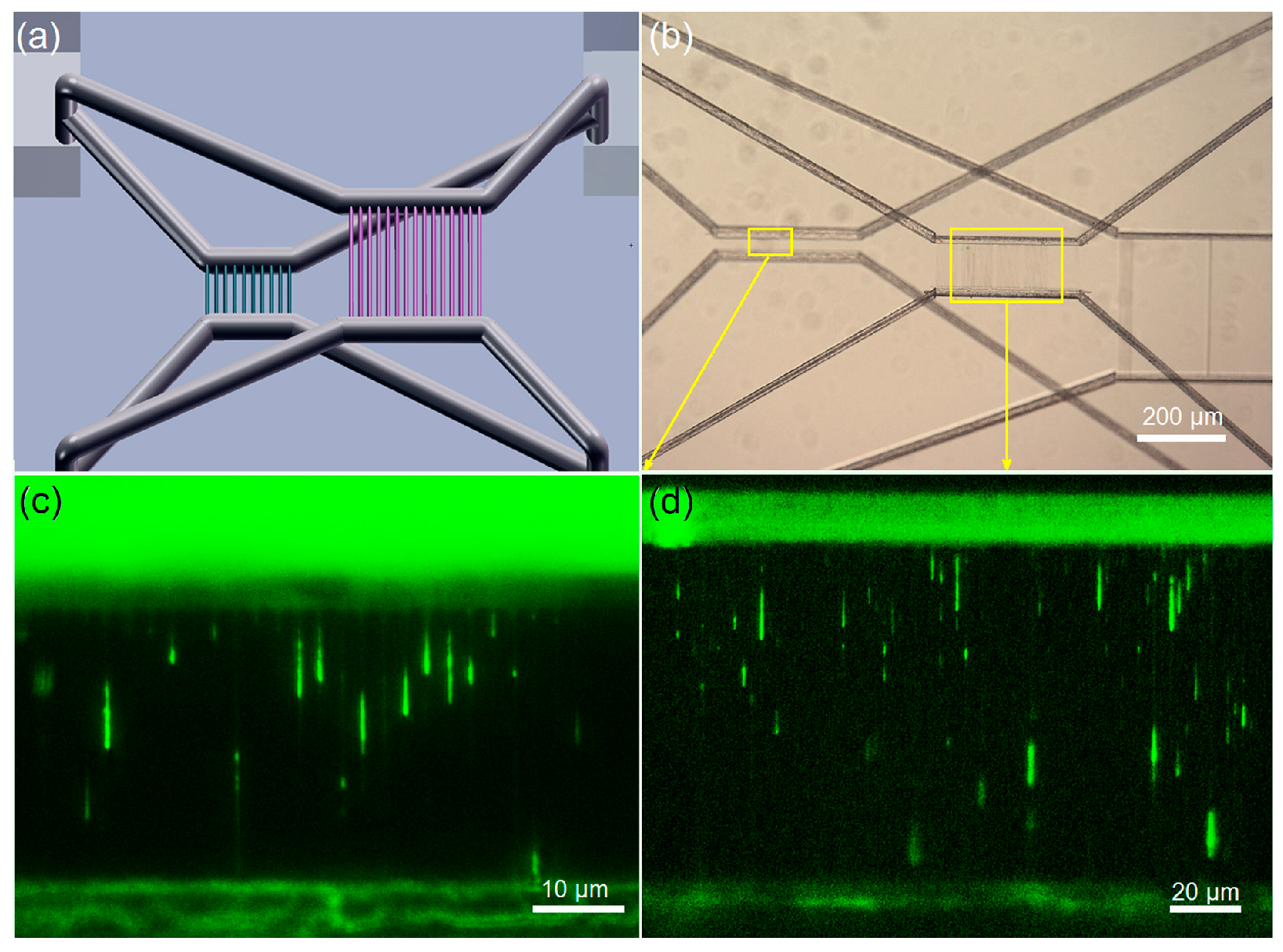
5.2. Rapid Prototyping of 3D Nanofluidic Chip

6. Mechanism Investigation
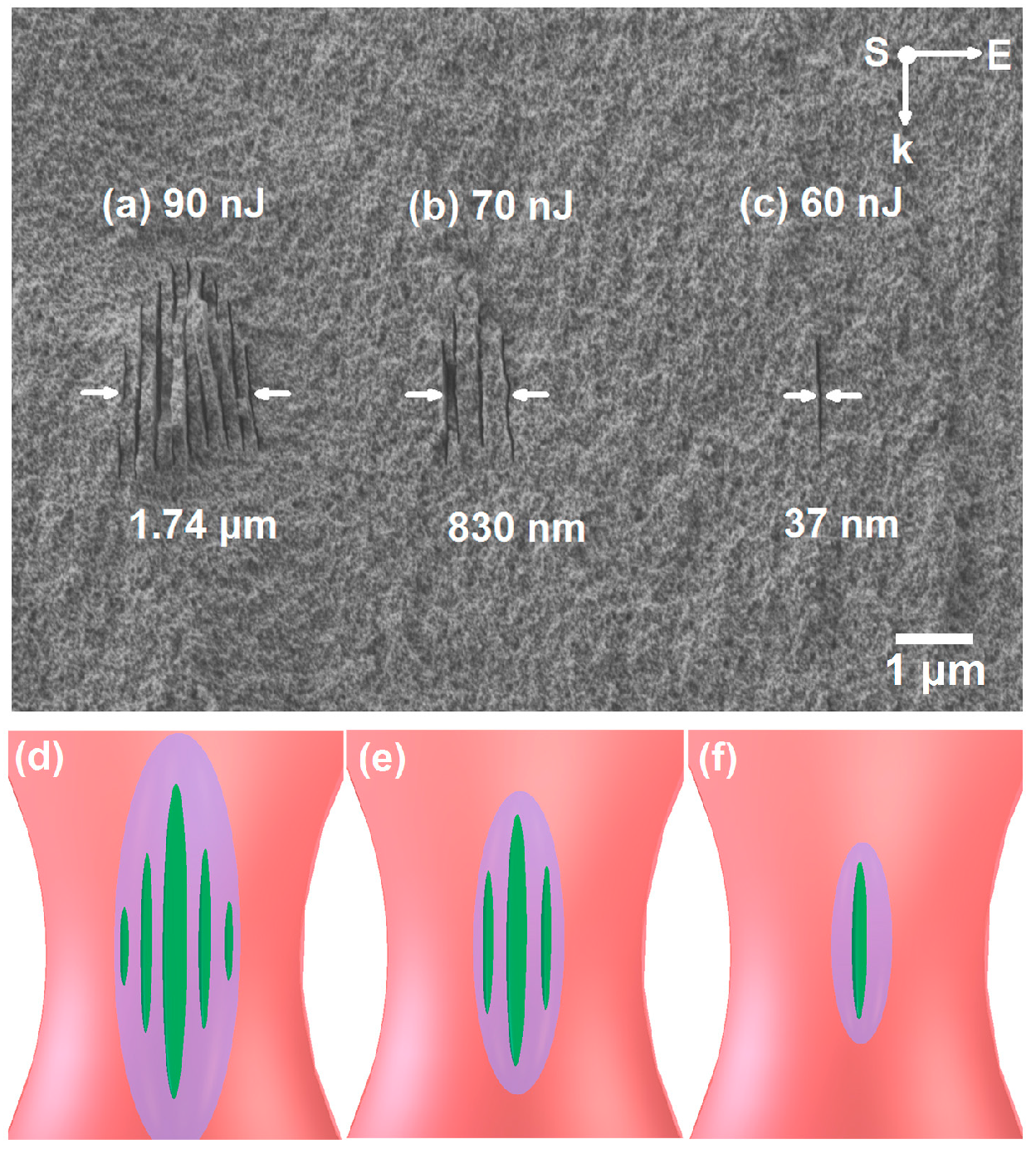
6.1. Pulse Duration Dependence
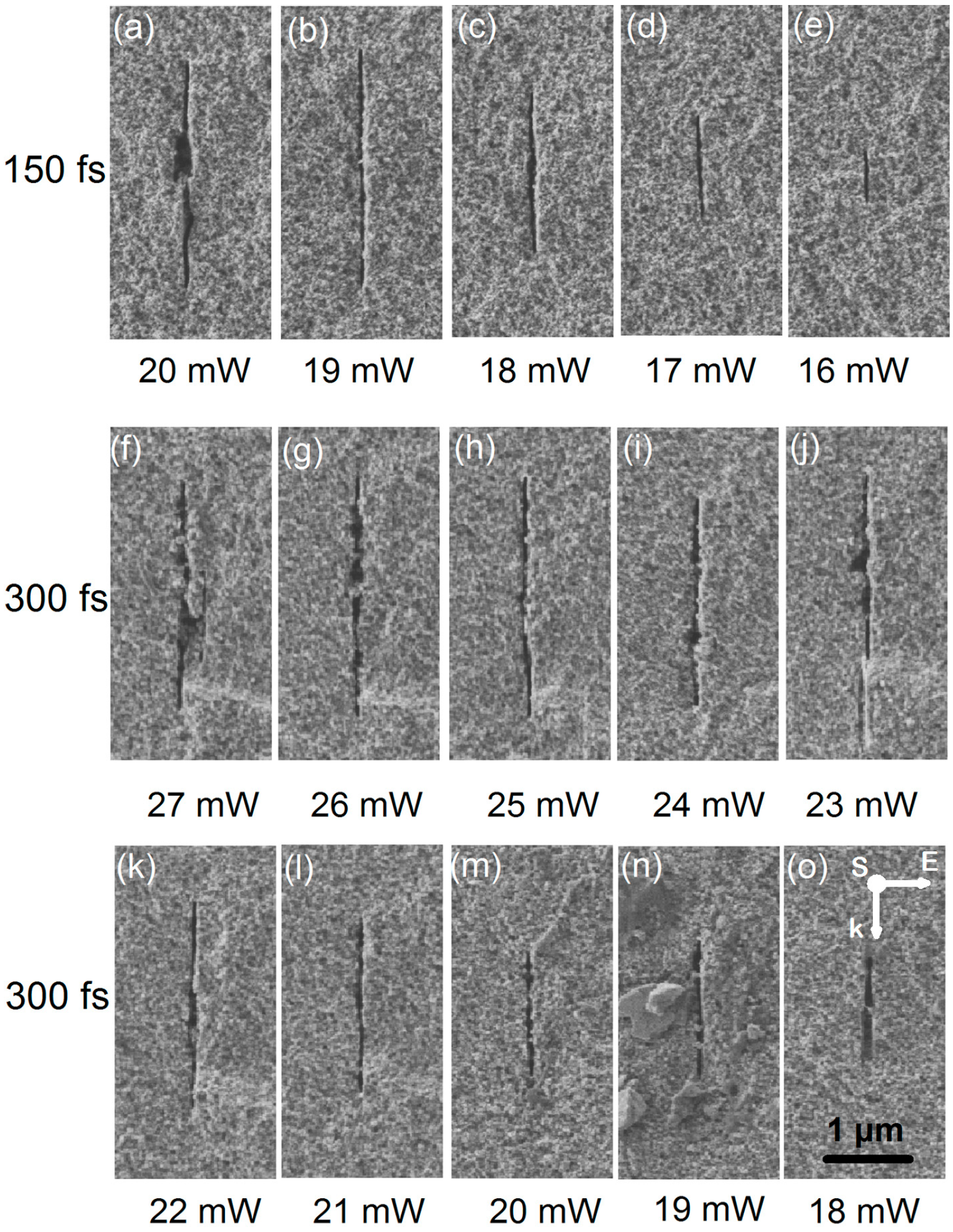

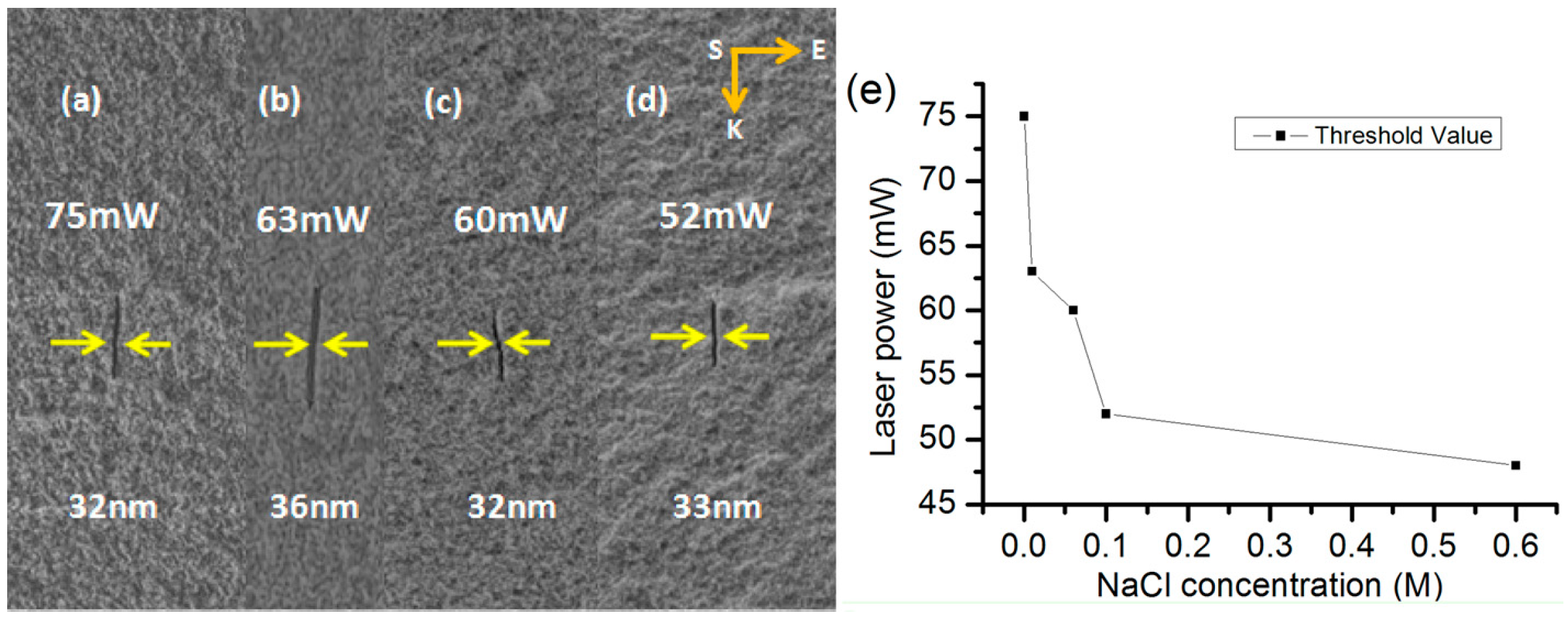
6.2. Influence of Ionization Property of Immersion Liquid

7. Conclusions and Future Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B 1990, 1, 244–248. [Google Scholar]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar]
- Bellouard, Y.; Champion, A.; Lenssen, B.; Matteucci, M.; Schaap, A.; Beresna, M.; Corbari, C.; Gecevičius, M.; Kazansky, P.; Chappuis, O.; Kral, M.; Clavel, R.; Barrot, F.; Breguet, J.-M.; Mabillard, Y.; Bottinelli, S.; Hopper, M.; Hoenninger, C.; Mottay, E.; Lopez, J. The femtoprint project. J. Laser Micro/Nanoeng. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Xiong, W.; Zhou, Y.S.; He, X.N.; Gao, Y.; Samani, M.M.; Jiang, L.; Baldacchini, T.; Lu, Y.F. Simultaneous additive and subtractive three-dimensional micro/nano-fabrication using integrated two-photon polymerization and multi-photon ablation. Light Sci. Appl. 2012, 1, e6. [Google Scholar] [CrossRef]
- Davis, K.M.; Miura, K.; Sugimoto, N.; Hirao, K. Writing waveguides in glass with a femtosecond laser. Opt. Lett. 1996, 21, 1729–1731. [Google Scholar] [CrossRef]
- Ams, M.; Dekker, P.; Marshall, G.D.; Withford, M.J. Monolithic 100 mW Yb waveguide laser fabricated using the femtosecond-laser direct-write technique. Opt. Lett. 2009, 34, 247–249. [Google Scholar] [CrossRef]
- Chen, F.; Vazquez de Aldana, J.R. Optical waveguides in crystalline dielectric materials produced by femtosecond- laser micromachining. Laser Photon. Rev. 2014, 8, 251–275. [Google Scholar] [CrossRef]
- Marcinkevicius, A.; Juodkazis, S.; Watanabe, M.; Miwa, M.; Matsuo, S.; Misawa, H.; Nishii, J. Femtosecond laser-assisted three-dimensional microfabrication in silica. Opt. Lett. 2001, 26, 277–279. [Google Scholar] [CrossRef]
- Masuda, M.; Sugioka, K.; Cheng, Y.; Aoki, N.; Kawachi, M.; Shihoyama, K.; Toyoda, K.; Helvajian, H.; Midorikawa, K. 3-D microstructuring inside photosensitive glass by femtosecond laser excitation. Appl. Phys. A 2003, 76, 857–860. [Google Scholar] [CrossRef]
- Matsuo, S.; Kiyama, S.; Shichijo, Y.; Tomita, T.; Hashimoto, S.; Hosokawa, Y.; Masuhara, H. Laser microfabrication and rotation of ship-in-a-bottle optical rotators. Appl. Phys. Lett. 2008, 93, 051107. [Google Scholar] [CrossRef]
- Masuda, M.; Sugioka, K.; Cheng, Y.; Hongo, T.; Shihoyama, K.; Takai, H.; Miyaloto, I.; Midorikawa, K. Direct fabrication of freely movable microplate inside photosensitive glass by femtosecond laser for lab-on-chip application. Appl. Phys. A 2004, 78, 1029–1032. [Google Scholar] [CrossRef]
- Cheng, Y.; Sugioka, K.; Midorikawa, K.; Masuda, M.; Toyoda, K.; Kawachi, M.; Shihoyama, K. Three-dimensional micro-optical components embedded in photosensitive glass by a femtosecond laser. Opt. Lett. 2003, 28, 1144–1146. [Google Scholar] [CrossRef]
- Cheng, Y.; Tsai, H.L.; Sugioka, K.; Midorikawa, K. Fabrication of 3D microoptical lenses in photosensitive glass using femtosecond laser micromachining. Appl. Phys. A 2006, 85, 11–14. [Google Scholar] [CrossRef]
- Wang, Z.; Sugioka, K.; Midorikawa, K. Fabrication of integrated microchip for optical sensing by femtosecond laser direct writing of Foturan glass. Appl. Phys. A 2007, 89, 951–955. [Google Scholar] [CrossRef]
- Cheng, Y.; Sugioka, K.; Midorikawa, K. Microfluidic laser embedded in glass by three-dimensional femtosecond laser microprocessing. Opt. Lett. 2004, 29, 2007–2009. [Google Scholar] [CrossRef]
- Hanada, Y.; Sugioka, K.; Kawano, H.; Ishikawa, I.S.; Miyawaki, A.; Midorikawa, K. Nano-aquarium for dynamic observation of living cells fabricated by femtosecond laser direct writing of photostructurable glass. Biomed. Microdevices 2008, 10, 403–410. [Google Scholar] [CrossRef]
- Hanada, Y.; Sugioka, K.; Ishikawa, I.S.; Kawano, H.; Miyawaki, A.; Midorikawa, K. 3D microfluidic chips with integrated functional microelements fabricated by a femtosecond laser for studying the gliding mechanism of cyanobacteria. Lab Chip 2011, 11, 2109–2115. [Google Scholar] [CrossRef]
- Crespi, A.; Gu, Y.; Ngamsom, B.; Hoekstra, H.J.W.M.; Dongre, C.; Pollnau, M.; Ramponi, R.; van den Vlekkert, H.H.; Watts, P.; Cerullo, G.; Osellame, R. Three-dimensional Mach-Zehnder interferometer in a microfluidic chip for spatially-resolved label-free detection. Lab. Chip 2010, 10, 1167–1173. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, D.J.; Jeon, H.; Hiromatsu, K.; Grigoropoulos, C.P. Single cell detection using a glass-based optofluidic device fabricated by femtosecond laser pulses. Lab. Chip 2009, 9, 311–318. [Google Scholar] [CrossRef]
- Bragheri, F.; Ferrara, L.; Bellini, N.; Vishnubhatla, K.C.; Minzionil, P.; Ramponi, R.; Osellame, R.; Cristiani, I. Optofluidic chip for single cell trapping and stretching fabricated by a femtosecond laser. J. Biophoton. 2010, 3, 234–243. [Google Scholar] [CrossRef]
- Schaap, A.; Bellouard, Y.; Rohrlack, T. Optofluidic lab-on-a-chip for rapid algae population screening. Biomed. Opt. Express 2011, 2, 658–664. [Google Scholar] [CrossRef]
- Xu, J.; Liao, Y.; Zeng, H.D.; Sun, H.Y.; Song, J.; Wang, X.S.; Cheng, Y.; Xu, Z.Z.; Sugioka, K.; Midorikawa, K. Selective metallization on insulator surfaces with femtosecond laser pulses. Opt. Express 2007, 15, 12743–12748. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, J.; Sun, H.Y.; Song, J.; Wang, X.S.; Cheng, Y. Fabrication of microelectrodes deeply embedded in LiNbO3 using a femtosecond laser. Appl. Surf. Sci. 2008, 254, 7018–7021. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, J.; Cheng, Y.; Zhou, Z.H.; He, F.; Sun, H.Y.; Song, J.; Wang, X.S.; Xu, Z.Z.; Sugioka, K.; Midorikawa, K. Electro-optic integration of embedded electrodes and waveguides in LiNbO3 using a femtosecond laser. Opt. Lett. 2008, 33, 2281–2283. [Google Scholar] [CrossRef]
- Xu, J.; Wu, D.; Hanada, Y.; Chen, C.; Wu, S.Z.; Cheng, Y.; Sugioka, K.; Midorikawa, K. Electrofluidics fabricated by space-selective metallization in glass microfluidic structures using femtosecond laser direct writing. Lab Chip 2013, 13, 4608–4616. [Google Scholar] [CrossRef]
- Liao, Y.; Qiao, L.; Wang, Z.; Wang, M.; Liu, L.; Sugioka, K.; Cheng, Y. Fabrication of a liquid crystal light modulator by use of femtosecond-laser-induced nanoripples. Opt. Mater. Express 2013, 3, 1698–1704. [Google Scholar] [CrossRef]
- Bellouard, Y.; Said, A.; Bado, P. Integrating optics and micro-mechanics in a single substrate: A step toward monolithic integration in fused silica. Opt. Express 2005, 13, 6635–6644. [Google Scholar] [CrossRef]
- Lenssen, B.; Bellouard, Y. Optically transparent glass micro-actuator fabricated by femtosecond laser exposure and chemical etching. Appl. Phys. Lett. 2012, 101, 103503. [Google Scholar]
- Hnatovsky, C.; Taylor, R.S.; Simova, E.; Rajeev, P.P.; Rayner, D.M.; Bhardwaj, V.R.; Corkum, P.B. Fabrication of microchannels in glass using focused femtosecond laser radiation and selective chemical etching. Appl. Phys. A 2006, 84, 47–61. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y.; Midorikawa, K. Three-dimensional micromachining of glass using femtosecond laser for lab-on-a-chip device manufacture. Appl. Phys. A 2005, 81, 1–10. [Google Scholar] [CrossRef]
- Sugioka, K.; Hanada, Y.; Midorikawa, K. Three-dimensional femtosecond laser micromachining of photosensitive glass for biomicrochips. Laser Photonics Rev. 2010, 4, 386–400. [Google Scholar] [CrossRef]
- Li, Y.; Itoh, K.; Watanabe, W.; Yamada, K.; Kuroda, D.; Nishii, J.; Jiang, Y.Y. Three-dimensional hole drilling of silica glass from the rear surface with femtosecond laser pulses. Opt. Lett. 2001, 26, 1912–1914. [Google Scholar] [CrossRef]
- Maruo, S.; Fourkas, J.T. Recent progress in multiphoton microfabrication. Laser Photon. Rev. 2008, 2, 100–111. [Google Scholar] [CrossRef]
- Ke, K.; Hasselbrink, E.F.; Hunt, A.J. Rapidly prototyped three-dimensional nanofluidic channel networks in glass substrates. Anal. Chem. 2005, 77, 5083–5088. [Google Scholar] [CrossRef]
- Liao, Y.; Ju, Y.; Zhang, L.; He, F.; Zhang, Q.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Three-dimensional microfluidic channel with arbitrary length and configuration fabricated inside glass by femtosecond laser direct writing. Opt. Lett. 2010, 35, 3225–3227. [Google Scholar] [CrossRef]
- Liu, C.N.; Liao, Y.; He, F.; Lin, D.; Song, J.X.; Lin, J.T.; Cheng, Y.; Sugioka, K.; Midorikawa, K. Compact 3D microfluidic channel structures embedded in glass fabricated by femtosecond laser direct writing. J. Laser Micro/Nanoeng. 2013, 8, 170–174. [Google Scholar] [CrossRef]
- Liao, Y.; Shen, Y.L.; Qiao, L.L.; Chen, D.P.; Cheng, Y.; Sugioka, K.; Midorikawa, K. Femtosecond laser nanostructuring in porous glass with sub-50nm feature sizes. Opt. Lett. 2013, 38, 187–189. [Google Scholar] [CrossRef]
- Ju, Y.; Liao, Y.; Zhang, L.; Zhang, Q.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Fabrication of large-volume microfluidic chamber embedded in glass using three-dimensional femtosecond laser micromachining. Microfluid. Nanofluid. 2011, 11, 111–117. [Google Scholar] [CrossRef]
- Liao, Y.; Song, J.; Li, E.; Luo, Y.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Rapid prototyping of three-dimensional microfluidic mixers in glass by femtosecond laser direct writing. Lab Chip 2012, 12, 746–749. [Google Scholar] [CrossRef]
- Ju, Y.; Liu, C.; Liao, Y.; Liu, Y.; Zhang, L.; Shen, Y.; Chen, D.; Cheng, Y. Direct fabrication of a microfluidic chip for electrophoresis analysis by water-assisted femtosecond laser writing in porous glass. Chin. Opt. Lett. 2013, 11, 072201. [Google Scholar] [CrossRef]
- Liao, Y.; Cheng, Y.; Liu, C.N.; Song, J.X.; He, F.; Shen, Y.L.; Chen, D.P.; Xu, Z.Z.; Fan, Z.C.; Wei, X.B.; Sugioka, K.; Midorikawa, K. Direct laser writing of sub-50nm nanofluidic channels buried in glass for threedimensional micro-nanofluidic integration. Lab Chip 2013, 13, 1626–1631. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, B.; Qiao, L.L.; Liu, L.Q.; Sugioka, K.; Cheng, Y. Threshold effect in femtosecond laser induced nanograting formation in glass: influence of the pulse duration. Appl. Phys. A 2014, 114, 223–230. [Google Scholar] [CrossRef]
- Umran, F.A.; Liao, Y.; Elias, M.M.; Sugioka, K.; Stoian, R.; Cheng, G.H.; Cheng, Y. Formation of nanogratings in a transparent material with tunable ionization property by femtosecond laser irradiation. Opt. Express 2013, 21, 15259–15267. [Google Scholar] [CrossRef]
- Elmer, T.H. Porous and Reconstructed Glasses. In Engineered Materials Handbook; ASM International: Metals Park, OH, USA, 1992; Volume 4, pp. 427–432. [Google Scholar]
- Song, J.; Wang, X.; Hu, X.; Dai, Y.; Qiu, J.; Cheng, Y.; Xu, Z. Formation mechanism of self-organized voids in dielectrics induced by tightly focused femtosecond laser pulses. Appl. Phys. Lett. 2008, 92, 092904. [Google Scholar] [CrossRef]
- Hwang, D.J.; Choi, T.Y.; Grigoropoulos, C.P. Liquid-assisted femtosecond laser drilling of straight and three-dimensional microchannels in glass. Appl. Phys. A 2004, 79, 605–612. [Google Scholar] [CrossRef]
- Herman, P.R.; Oettl, A.; Chen, K.P.; Marjoribanks, R.S. Laser micromachining of transparent fused silica with 1-ps pulses and pulse trains. Proc. SPIE 1999, 3616, 148–155. [Google Scholar]
- Anderson, J.R.; Chiu, D.T.; Jackman, R.J.; Cherniavskaya, O.; McDonald, J.C.; Wu, H.; Whitesides, S.H.; Whitesides, G.M. Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal. Chem. 2000, 72, 3158–3164. [Google Scholar] [CrossRef]
- Zhu, J.J.; Xuan, X.C. Particle electrophoresis and dielectrophoresis in curved microchannels. J. Colloid Interface Sci. 2009, 340, 285–290. [Google Scholar] [CrossRef]
- Garcia, A.L.; Ista, L.K.; Petsev, D.N.; O’Brien, M.J.; Bisong, P.; Mammoli, A.A.; Brueck, S.R. J.; Lopez, G.P. Electrokinetic molecular separation in nanoscale fluidic channels. Lab Chip 2005, 11, 1271–1276. [Google Scholar] [CrossRef]
- Liu, C.N.; Liao, Y.; He, F.; Shen, Y.L.; Chen, D.P.; Cheng, Y.; Xu, Z.Z.; Sugioka, K.; Midorikawa, K. Fabrication of three-dimensional microfluidic channels inside glass using nanosecond laser direct writing. Opt. Express 2012, 20, 4291–4296. [Google Scholar] [CrossRef]
- Wang, J.; Niino, H.; Yabe, A. One-step microfabrication of fused silica by laser ablation of an organic solution. Appl. Phys. A 1999, 68, 111–113. [Google Scholar] [CrossRef]
- Niino, H.; Yasui, Y.; Ding, X.; Narazaki, A.; Sato, T.; Kawaguchi, Y.; Yabe, A. Surface micro-fabrication of silica glass by excimer laser irradiation of organic solvent. J. Photochem. Photo. A Chem. 2003, 158, 179–182. [Google Scholar] [CrossRef]
- Vass, C.; Osvay, K.; Hopp, B. Fabrication of 150 nm period grating in fused silica by two-beam interferometric laser induced backside wet etching method. Opt. Express 2006, 14, 8354–8359. [Google Scholar] [CrossRef]
- Abbott, A. Cell culture: Biology’s new dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wu, Z.G. Micromixers—A review. J. Micromech. Microeng. 2005, 15, R1. [Google Scholar] [CrossRef]
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olsen, M.G.; Santiago, J.G.; Adrian, R.J.; Aref, H.; Beebe, D.J. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromech. Syst. 2000, 9, 190–197. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Therriault, D.; White, S.R.; Lewis, J.A. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2003, 2, 265–271. [Google Scholar] [CrossRef]
- Chen, H.; Meiners, J.C. Topologic mixing on a microfluidic chip. Appl. Phys. Lett. 2004, 84, 2193–2195. [Google Scholar] [CrossRef]
- Schönfeld, F.; Hessel, V.; Hofmann, C. An optimised split-and-recombine micro-mixer with uniform “chaotic” mixing. Lab Chip 2004, 4, 65–69. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, J.K.; Park, J.; Chung, S.; Chung, C.; Chang, J.K. Rapid three-dimensional passive rotation micromixer using the breakup process. J. Micromech. Microeng. 2004, 14, 6–14. [Google Scholar] [CrossRef]
- Xia, H.M.; Wan, S.Y. M.; Shu, C.; Chew, Y.T. Chaotic micromixers using two-layer crossing channels to exhibit fast mixing at low Reynolds numbers. Lab Chip 2005, 5, 748–755. [Google Scholar] [CrossRef]
- Yasui, T.; Omoto, Y.; Osato, K.; Kaji, N.; Suzuki, N.; Naito, T.; Watanabe, M.; Okamoto, Y.; Tokeshi, M.; Shamoto, E.; Baba, Y. Microfluidic baker’s transformation device for three-dimensional rapid mixing. Lab Chip 2011, 11, 3356–3360. [Google Scholar] [CrossRef]
- Wiggins, S.; Ottino, J.M. Foundations of chaotic mixing. Philos. Trans. R. Soc. London Ser. A 2004, 362, 937–970. [Google Scholar] [CrossRef]
- Carrière, P. On a three-dimensional implementation of the baker’s transformation. Phys. Fluids 2007, 19, 118110. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Femtosecond laser processing for optofluidic fabrication. Lab Chip 2012, 12, 3576–3589. [Google Scholar] [CrossRef]
- Dongre, C.; van Weerd, J.; Bellini, N.; Osellame, R.; Cerullo, G.; van Weeghel, R.; Hoekstra, H.J.W.M.; Pollnau, M. Dual-point dual-wavelength fluorescence monitoring of DNA separation in a lab on a chip. Biomed. Opt. Express 2010, 1, 729–735. [Google Scholar] [CrossRef]
- Abgrall, P.; Nguyen, N.T. Nanofluidic devices and their applications. Anal. Chem. 2008, 80, 2326–2341. [Google Scholar] [CrossRef]
- Kovarik, M.L.; Jacobson, S.C. Nanofluidics in Lab-on-a-Chip Devices. Anal. Chem. 2009, 81, 7133–7140. [Google Scholar] [CrossRef]
- Kuo, T.C.; Cannon, D.M.; Shannon, M.A.; Bohn, P.W.; Sweedler, J.V. Hybrid threedimensional nanofluidic/microfluidic devices using molecular gates. Sens. Actuators A 2003, 102, 223–233. [Google Scholar] [CrossRef]
- Jeon, S.; Malyarchuk, V.; White, J.O.; Rogers, J.A. Optically fabricated three dimensional nanofluidic mixers for microfluidic devices. Nano Lett. 2005, 5, 1351–1356. [Google Scholar] [CrossRef]
- Lee, S.; An, R.; Hunt, A.J. Liquid glass electrodes for nanofluidics. Nat. Nanotechnol. 2010, 5, 412–416. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Kazansky, P.; Qiu, J.; Hirao, K. Self-organized nanogratings in glass irradiated by ultrashort light pulses. Phys. Rev. Lett. 2003, 91, 247405. [Google Scholar] [CrossRef]
- Bhardwaj, V.R.; Simova, E.; Rajeev, P.P.; Hnatovsky, C.; Taylor, R.S.; Rayner, D.M.; Corkum, P.B. Optically produced arrays of planar nanostructures inside fused silica. Phys. Rev. Lett. 2006, 96, 057404. [Google Scholar] [CrossRef]
- Hnatovsky, C.; Taylor, R.S.; Simova, E.; Bhardwaj, V.R.; Rayner, D.M.; Corkum, P.B. Polarization-selective etching in femtosecond laser-assisted microfluidic channel fabrication in fused silica. Opt. Lett. 2005, 30, 1867–1869. [Google Scholar] [CrossRef]
- Korte, F.; Serbin, J.; Koch, J.; Egbert, A.; Fallnich, C.; Ostendorf, A.; Chichkov, B.N. Towards nanostructuring with femtosecond laser pulses. Appl. Phys. A 2003, 77, 229–235. [Google Scholar]
- Joglekar, A.P.; Liu, H.; Meyhofer, E.; Mourou, G.; Hunt, A.J. Optics at critical intensity: Applications to nanomorphing. Proc. Natl. Acad. Sci. USA 2004, 101, 5856–5861. [Google Scholar] [CrossRef]
- Fischer, J.; Wegener, M. Three-dimensional optical laser lithography beyond the diffraction limit. Laser Photonics Rev. 2013, 7, 22–44. [Google Scholar] [CrossRef]
- Juodkazis, S.; Nishimura, K.; Tanaka, S.; Misawa, H.; Gamaly, E.G.; Luther-Davies, B.; Hallo, L.; Nicolai, P.; Tikhonchuk, V.T. Laser-induced microexplosion confined in the bulk of a sapphire crystal: evidence of multimegabar pressures. Phys. Rev. Lett. 2006, 96, 166101. [Google Scholar] [CrossRef]
- Tegenfeldt, J.O.; Prinz, C.; Cao, H.; Huang, R.L.; Austin, R.H.; Chou, S.Y.; Cox, E.C.; Sturm, J.C. Micro- and nanofluidics for DNA analysis. Anal. Bioanal. Chem. 2004, 378, 1678–1692. [Google Scholar] [CrossRef]
- Taylor, R.; Hnatovsky, C.; Simova, E. Applications of femtosecond laser induced self-organized planar nanocracks inside fused silica glass. Laser Photon. Rev. 2008, 2, 26–46. [Google Scholar] [CrossRef]
- Cheng, G.; Mishchik, K.; Mauclair, C.; Audouard, E.; Stoian, R. Ultrafast laser photoinscription of polarization sensitive devices in bulk silica glass. Opt. Express 2009, 17, 9515–9525. [Google Scholar] [CrossRef]
- Richter, S.; Heinrich, M.; Doring, S.; Tünnermann, A.; Nolte, S.; Peschel, U. Nanogratings in fused silica: Formation, control, and applications. J. Laser Appl. 2012, 24, 042008. [Google Scholar] [CrossRef]
- Richter, S.; Heinrich, M.; Doring, S.; Tuennermann, A.; Nolte, S. Formation of femtosecond laser-induced nanogratings at high repetition rates. Appl. Phys. A 2011, 104, 503–507. [Google Scholar] [CrossRef]
- Mauclair, C.; Zamfirescu, M.; Colombier, J.P.; Cheng, G.; Mishchik, K.; Audouard, E.; Stoian, R. Control of ultrafast laser-induced bulk nanogratings in fused silica via pulse time envelopes. Opt. Express 2012, 20, 12997–13005. [Google Scholar] [CrossRef]
- Kazansky, P.G.; Shimotsuma, Y. Self-assembled sub-wavelength structures and form birefringence created by femtosecond laser writing in glass: properties and applications. J. Ceram. Soc. Jpn. 2008, 116, 1052–1062. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Cheng, Y. Femtosecond Laser 3D Fabrication in Porous Glass for Micro- and Nanofluidic Applications. Micromachines 2014, 5, 1106-1134. https://doi.org/10.3390/mi5041106
Liao Y, Cheng Y. Femtosecond Laser 3D Fabrication in Porous Glass for Micro- and Nanofluidic Applications. Micromachines. 2014; 5(4):1106-1134. https://doi.org/10.3390/mi5041106
Chicago/Turabian StyleLiao, Yang, and Ya Cheng. 2014. "Femtosecond Laser 3D Fabrication in Porous Glass for Micro- and Nanofluidic Applications" Micromachines 5, no. 4: 1106-1134. https://doi.org/10.3390/mi5041106





