Stress-Free Bonding Technology with Pyrex for Highly Integrated 3D Fluidic Microsystems
Abstract
:1. Introduction
2. Fundamentals of the Reflow Fusion Bond
2.1. Fluidic System
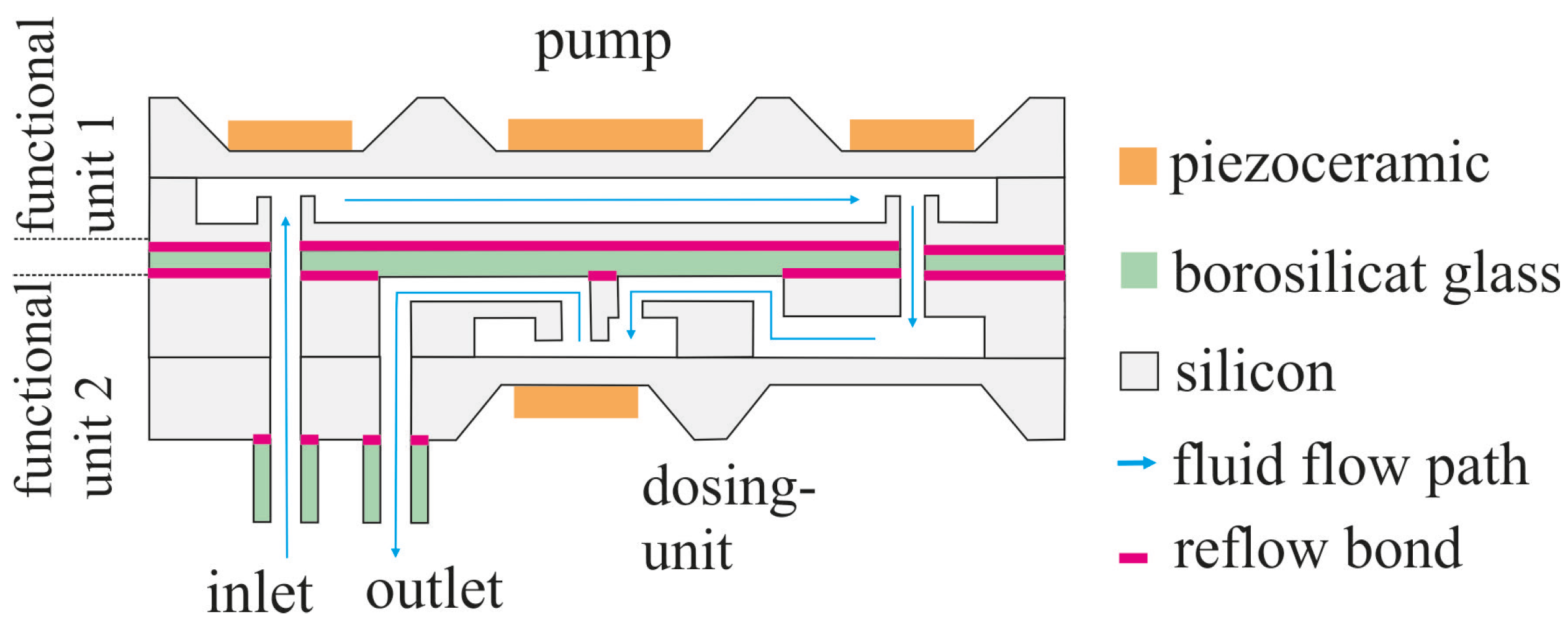
2.2. Fundamentals of the Pyrex Bonding Technology
2.2.1. Components of Borosilicate Glass
| Weight % | Compound |
|---|---|
| 81 | SiO2 |
| 13 | Bi2O3 |
| 4 | Na2O + K2O |
| 2 | Al2O3 |
2.2.2. Physical and Chemical Principles of the Bond
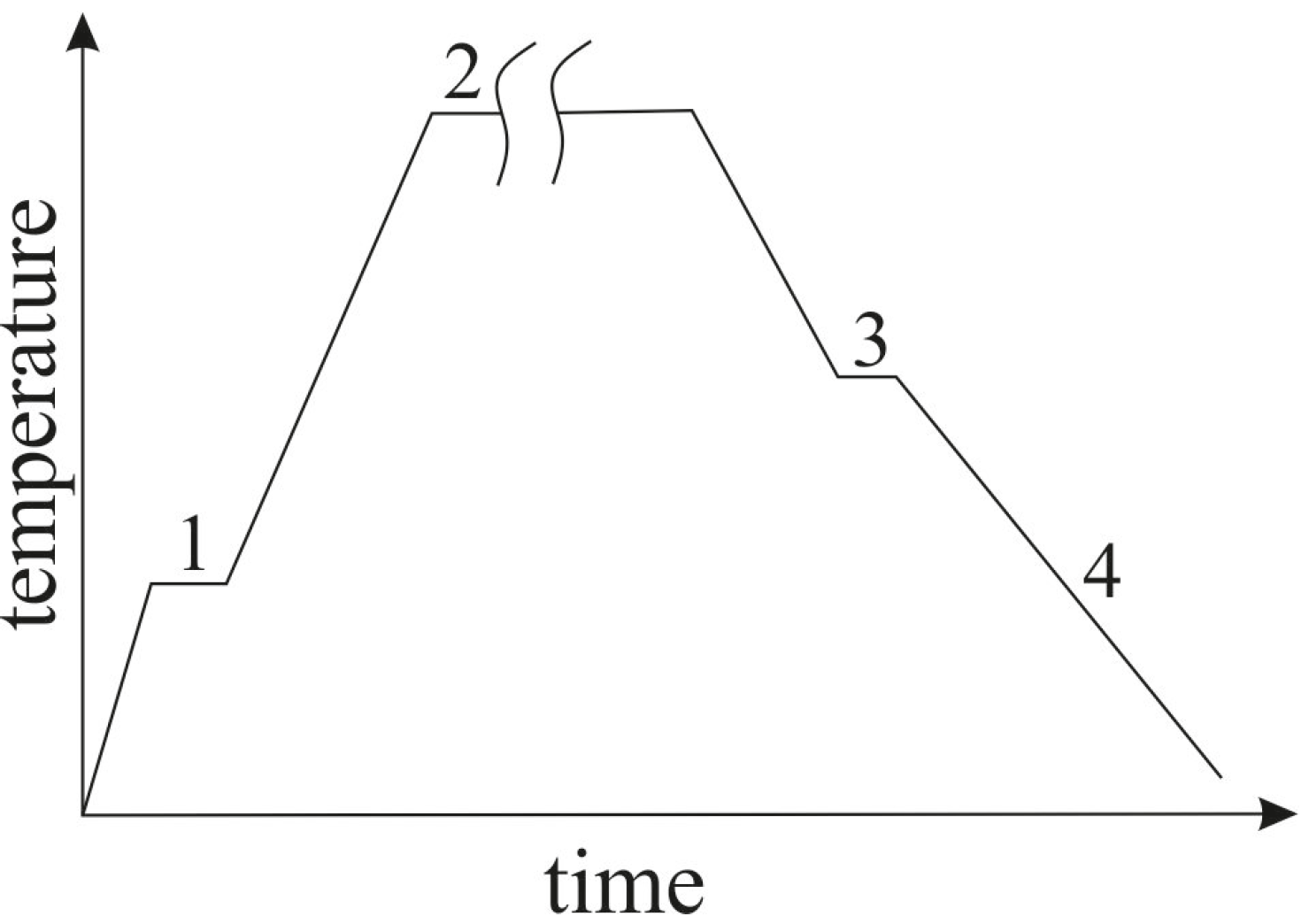
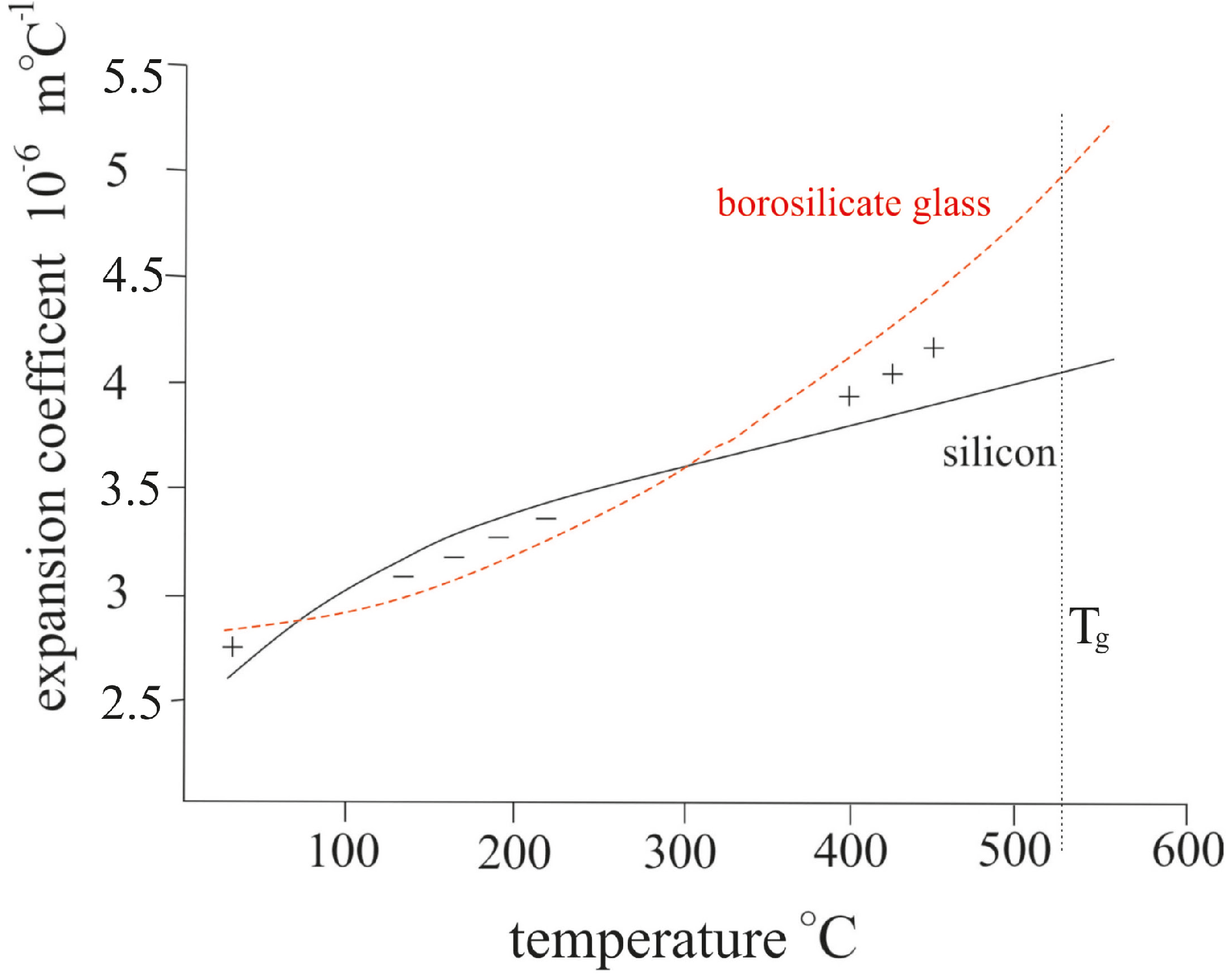
2.3. Simulation of the Bonded Assembly
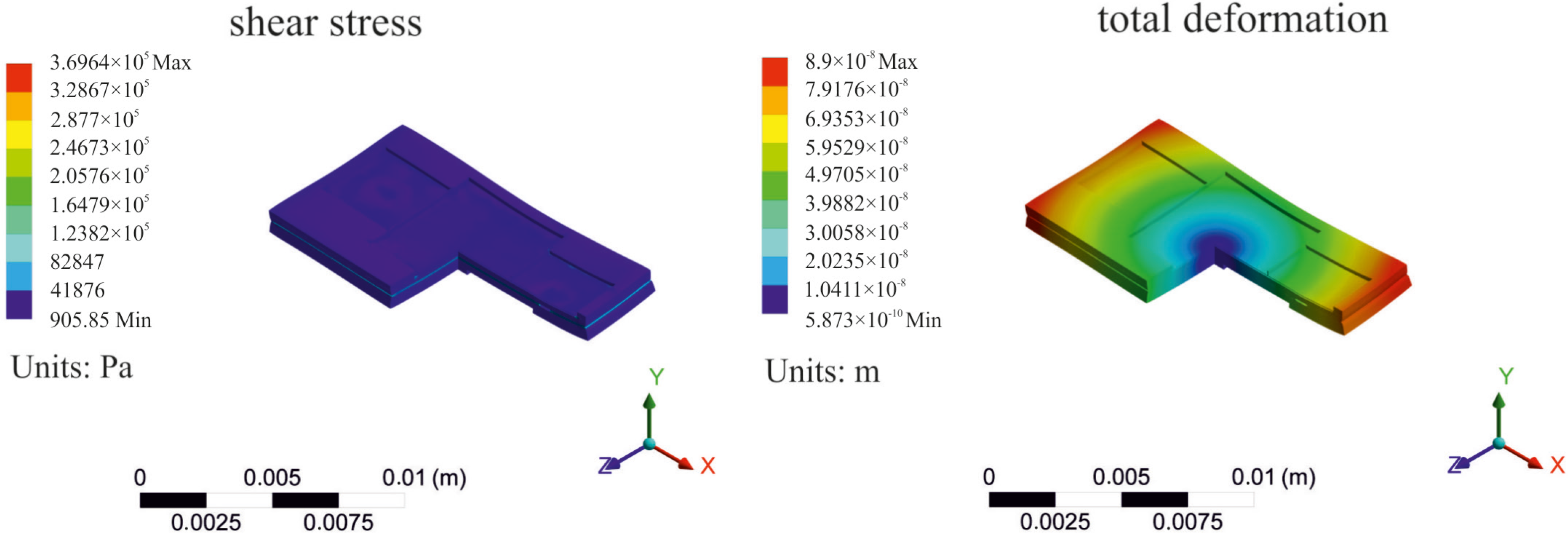
3. Preliminary Tests
3.1. Fabrication and Experiments
Preparation of the Bonded Components
3.2. Preliminary Experiments: Tube on Silicon
3.2.1. Design of Experiments
| Factor | Unit | Condition 1 | Condition 2 |
|---|---|---|---|
| Cleaning step before bond | N/A | Piranha etch | Piranha etch and HF |
| Heating rate (Step 1 to 2) | °C/min | 7 | 15 |
| Bonding temperature | °C | 730 | 770 |
| Cool-down wait-time (Step 3) | h | 6 | 12 |
3.2.2. Test Setup
- Speed of the axes is 100 µm/s;
- Shear height is 100 µm;
- Load cell is DS 100 Kg.
3.2.3. Results for Tube Bonding
| Experiment | Conditions | Mean shear force | Std. dev. | Mean shear strength |
|---|---|---|---|---|
| Bonding Temperature\Bonding Time\Cleaning | (N) | (N) | (N/mm2) | |
| 1 | 730 °C\6 h\Piranha | 140.6 | 70.7 | 6.6 |
| 2 | 770 °C\12 h\Piranha + HF | 203.3 | 27.6 | 9.6 |
| 3 | 730 °C\12 h\Piranha | 163.9 | 32.7 | 7.7 |
| 4 | 770 °C\6 h \Piranha + HF | 282.9 | 31.6 | 13.3 |
| 5 | 770 °C\6 h\Piranha | 287.0 | 54.9 | 13.5 |
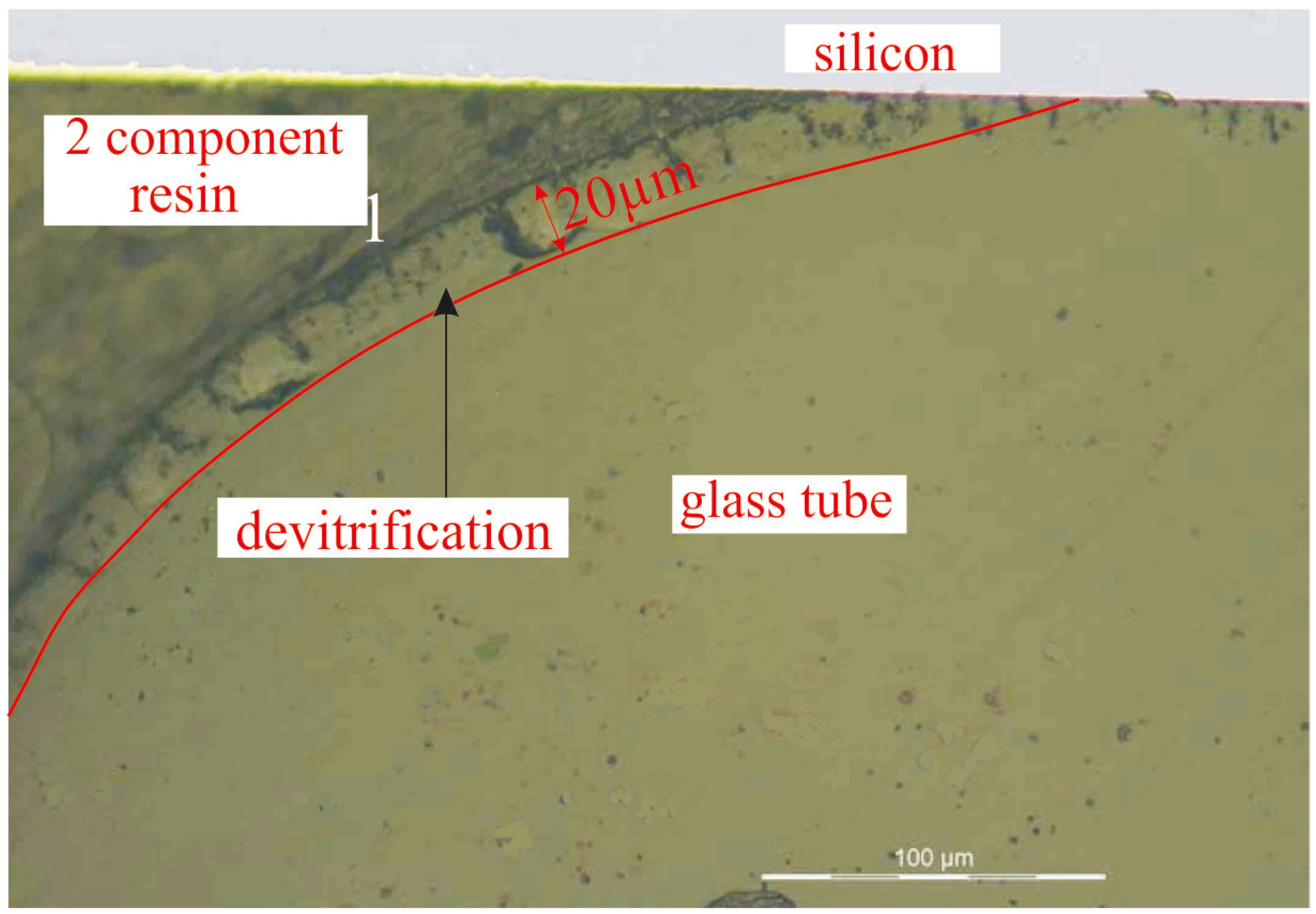
3.3. Preliminary Experiments: Silicon Chips Bonded to a Pyrex Chip
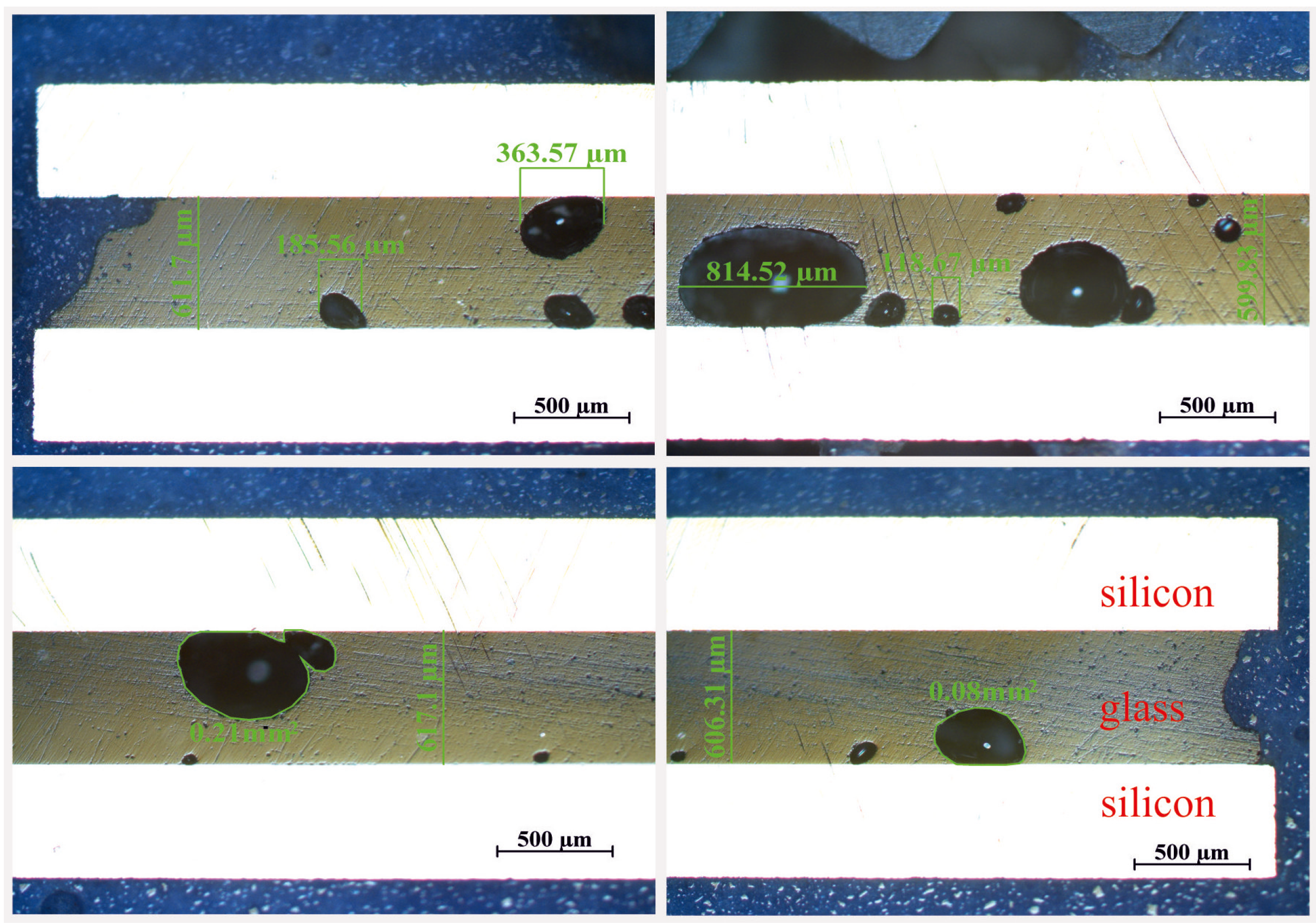


3.4. Discussion of the Preliminary Test
4. Experimental Result of the System
4.1. Preparation of the Fluidic System
4.2. Integration of Micropump and Dosing Unit

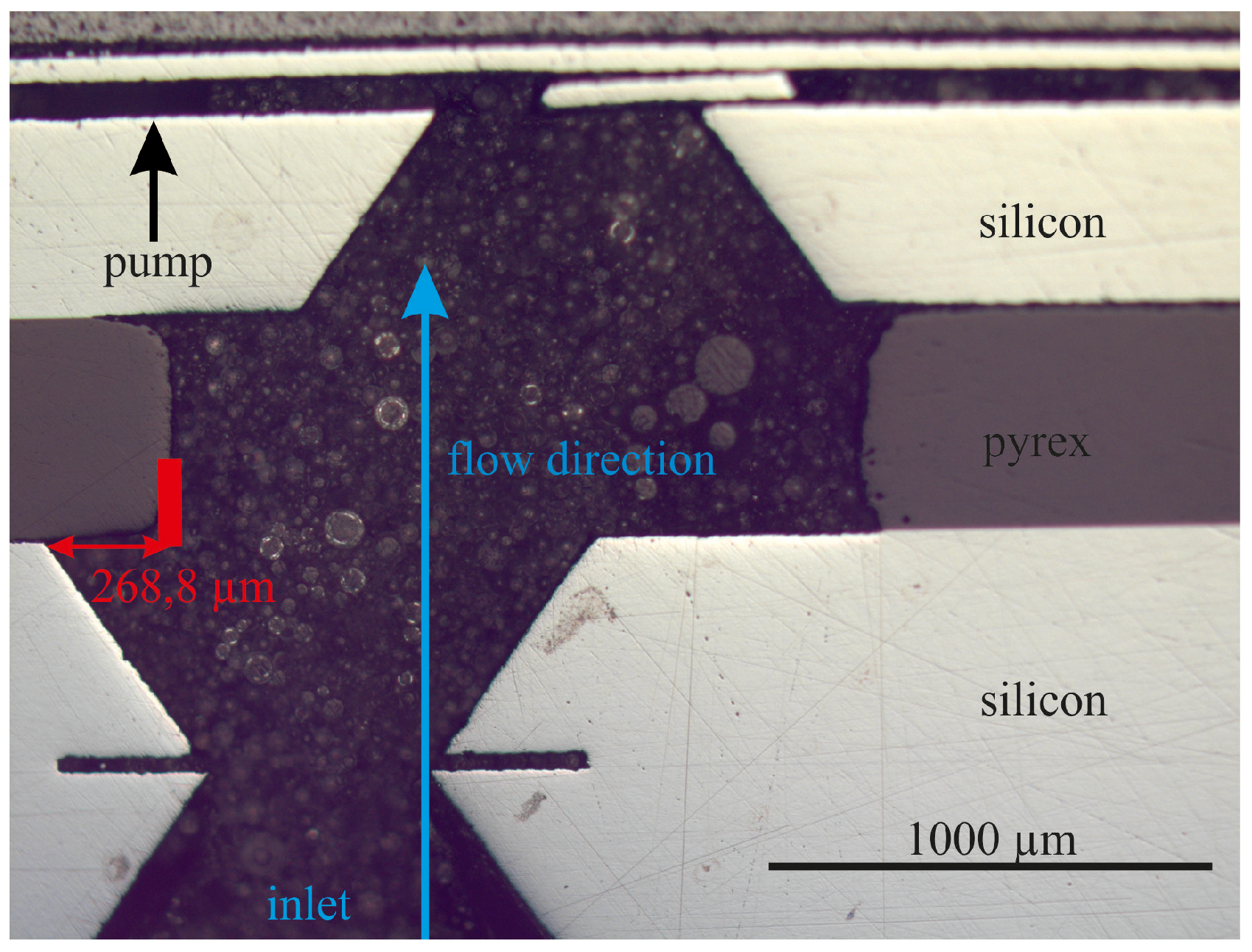
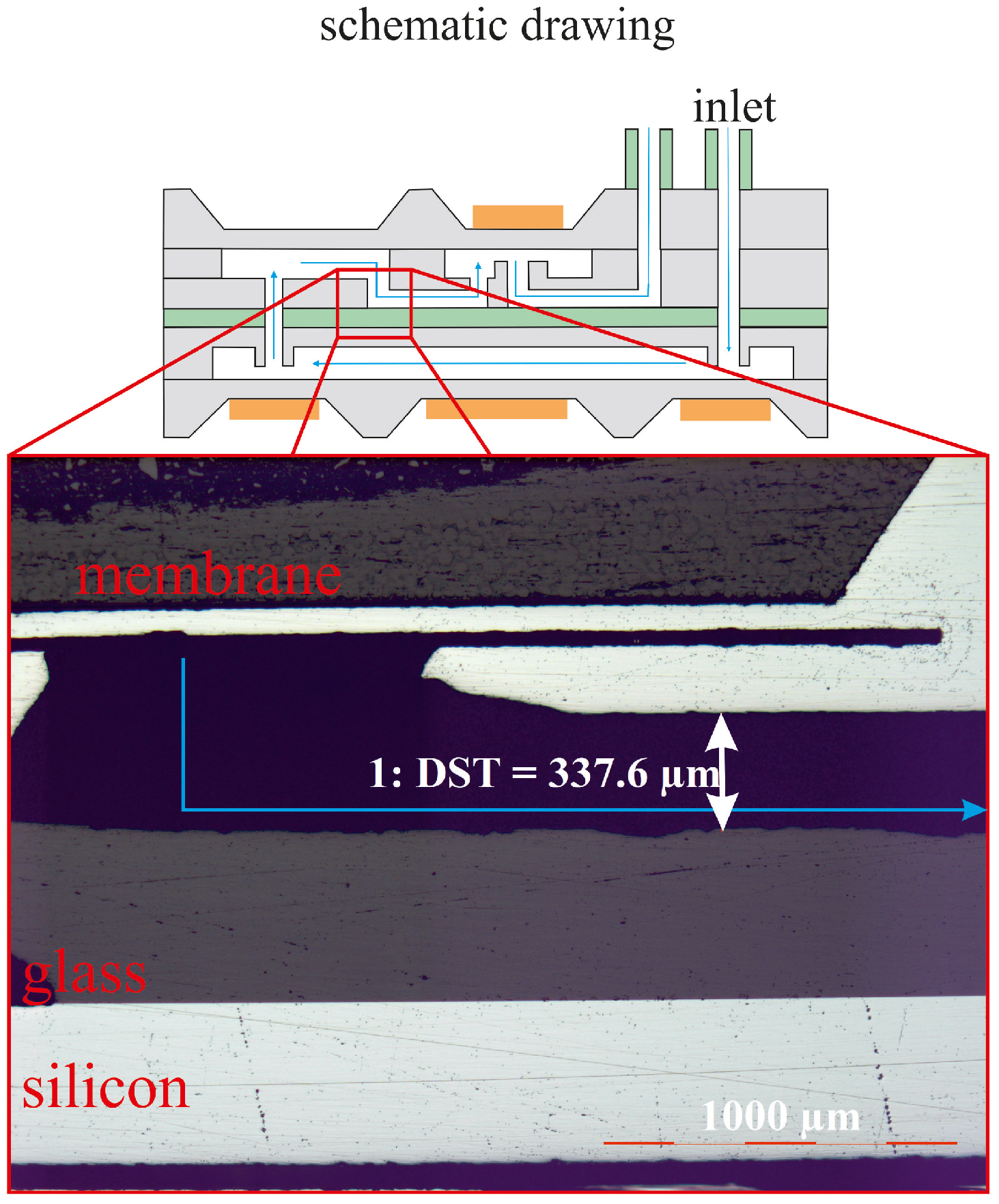
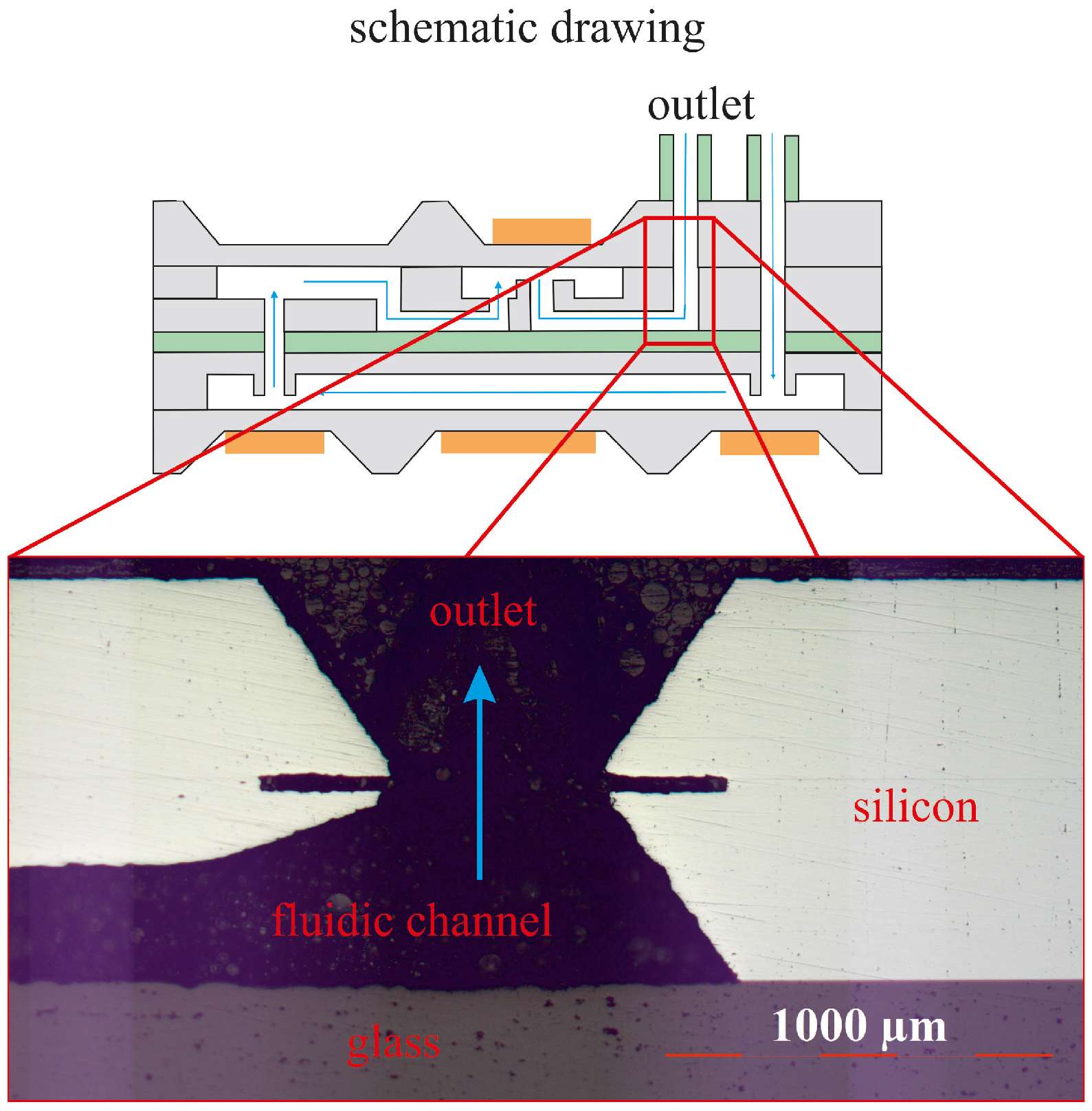
4.3. Fluidic Measurement
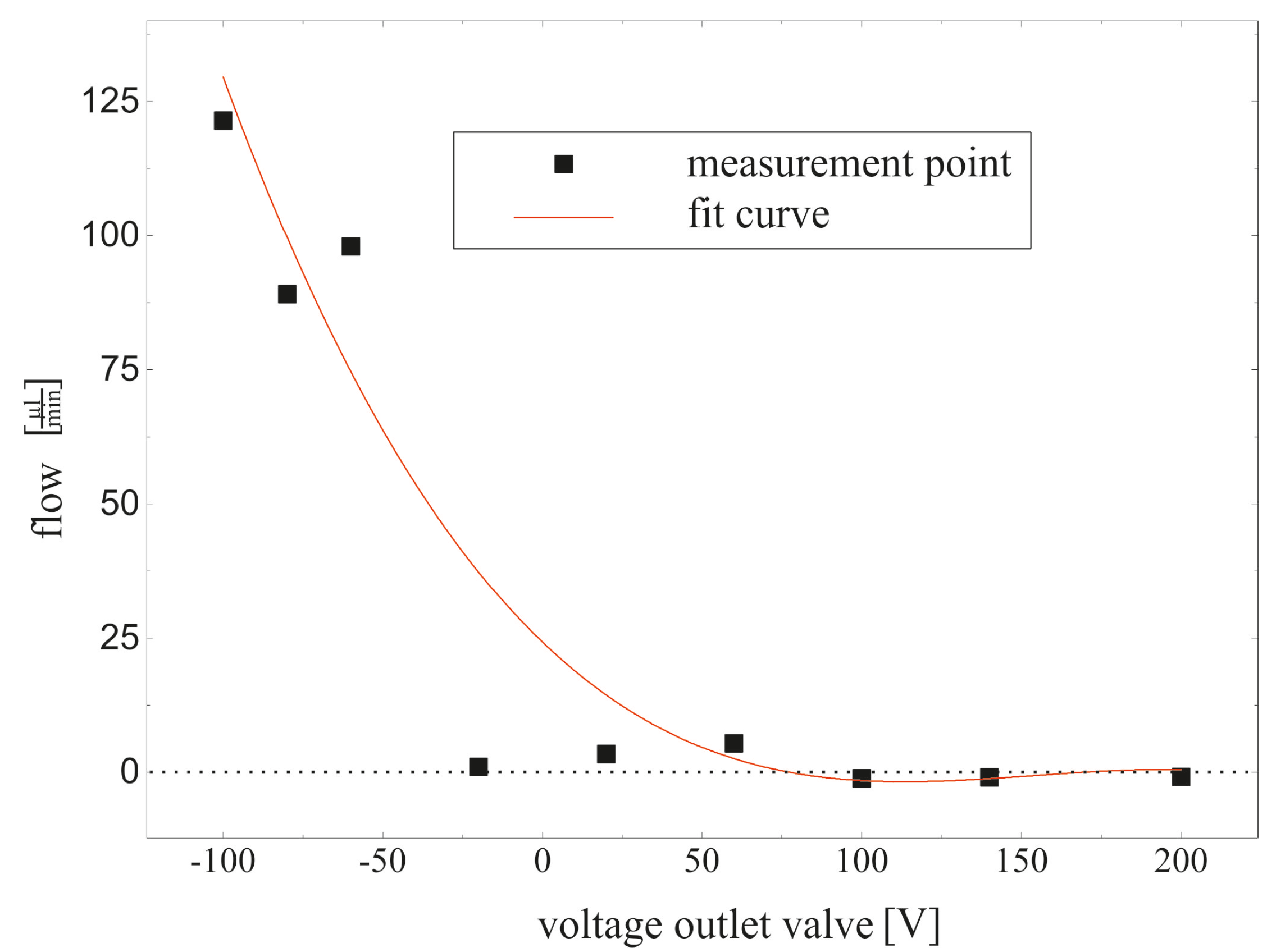

5. Conclusions
Author Contributions
Conflicts of Interest
References
- Woias, P. Micropumps: Summarizing the first two decades. In Proceedings of Microfluidics and BioMEMS, San Francisco, CA, USA, 28 September 2001; pp. 39–52.
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar]
- Haque, R.-U.M.; Wise, K.D. A Glass-in-Silicon Reflow Process for Three-Dimensional Microsystems. J. Microelectromech. Syst. 2013, 22, 1470–1477. [Google Scholar]
- Schrag, H.-J.; Ruthmann, O.; Doll, A.; Woias, P.; Hopt, U.T. German Artificial Sphincter System-GASS II: Erste in vivo Evaluation eines neuen hochintegrativen Neosphinkters zur Therapie der hochgradigen Stuhlinkontinenz / Short time in vivo evaluation of a novel and highly integrated sphincter prosthesis for therapy of major fecale incontinence. Biomedizinische Technik/Biomed. Eng. 2005, 50, 371–374. [Google Scholar]
- Unnikrishnan, S.; Jansen, H.; Berenschot, E.; Mogulkoc, B.; Elwenspoek, M. MEMS within a Swagelok®: A new platform for microfluidic devices. Lab Chip 2009, 9, 1966–1969. [Google Scholar]
- Albaugh, K.B.; Cade, P.E.; Rasmussen, D.H. Mechanisms of anodic bonding of silicon to pyrex glass. In Proceedings of the IEEE Solid-State Sensor and Actuator Workshop, Hilton Head Island, SC, USA, 6–9 June 1988; pp. 109–110.
- Despont, M.; Gross, H.; Arrouy, F.; Stebler, C.; Staufer, U. Fabrication of a silicon-Pyrex-silicon stack by a.c. anodic bonding. Sens. Actuators A Phys. 1996, 55, 219–224. [Google Scholar]
- Doll, A.; Goldschmidtboeing, F.; Woias, P. Low temperature plasma-assisted wafer bonding and bond interface stress characterization. In Proceedings of the IEEE Solid-State Sensor and Actuator Workshop, Hilton Head Island, SC, USA, 6–9 June 1988; pp. 665–668.
- Schott AG. Duran: Röhren, Stäbe und Kapillaren aus Borosilikatglas. Available online: http://www.schott.com/rohrglas (accessed on 18 September 2014).
- Harendt, C.; Appel, W.; Graf, H.-G.; Hofflinger, B.; Penteker, E. Wafer fusion bonding and its application to silicon-on-insulator fabrication. J. Micromech. Microeng. 1991, 1, 145. [Google Scholar]
- Fazal, I.; Elwenspoek, M.C. Fusion-bonded fluidic interconnects. J. Micromech. Microeng. 2008, 18, 055011. [Google Scholar]
- Moğulkoç, B.; Jansen, H.V.; Berenschot, J.W.; Ter Brake, H.J.M.; Knowles, K.M.; Elwenspoek, M.C. Characterization of MEMS-on-tube assembly: Reflow bonding of borosilicate glass (Duran®) tubes to silicon substrates. J. Micromech. Microeng. 2009, 19, 085027. [Google Scholar]
- Moğulkoç, B.; Knowles, K.M.; Jansen, H.V.; Ter Brake, H.J.M.; Elwenspoek, M.C. Surface devitrification and the growth of Cristobalite in Borofloatr® (Borosilicate 8330) glass. J. Am. Ceram. Soc. 2010, 93, 2713–2719. [Google Scholar]
- Inzinga, R.A.; Lin, T.-W.; Yadav, M.; Johnson, H.T.; Horn, G.P. Characterization and control of residual stress and curvature in anodically bonded devices and substrates with etched features. Exp. Mech. 2012, 52, 637–648. [Google Scholar]
- Dage Deutschland GmbH. Datenblatt: Dage Series 4000 Multifunktionstester. Available online: http://www.nordson.com/DE-De/Divisions/Dage/Products/Bondtesters/Pages/4000Optima.aspx (accessed on 18 September 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thoma, F.; Goldschmidtböing, F.; Cobry, K.; Woias, P. Stress-Free Bonding Technology with Pyrex for Highly Integrated 3D Fluidic Microsystems. Micromachines 2014, 5, 783-796. https://doi.org/10.3390/mi5030783
Thoma F, Goldschmidtböing F, Cobry K, Woias P. Stress-Free Bonding Technology with Pyrex for Highly Integrated 3D Fluidic Microsystems. Micromachines. 2014; 5(3):783-796. https://doi.org/10.3390/mi5030783
Chicago/Turabian StyleThoma, Florian, Frank Goldschmidtböing, Keith Cobry, and Peter Woias. 2014. "Stress-Free Bonding Technology with Pyrex for Highly Integrated 3D Fluidic Microsystems" Micromachines 5, no. 3: 783-796. https://doi.org/10.3390/mi5030783





