Recent Trends on Micro/Nanofluidic Single Cell Electroporation
Abstract
:1. Introduction
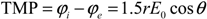
2. Bulk, Single and Localized Single Cell Membrane Electroporation
2.1. Bulk Electroporation (BEP)
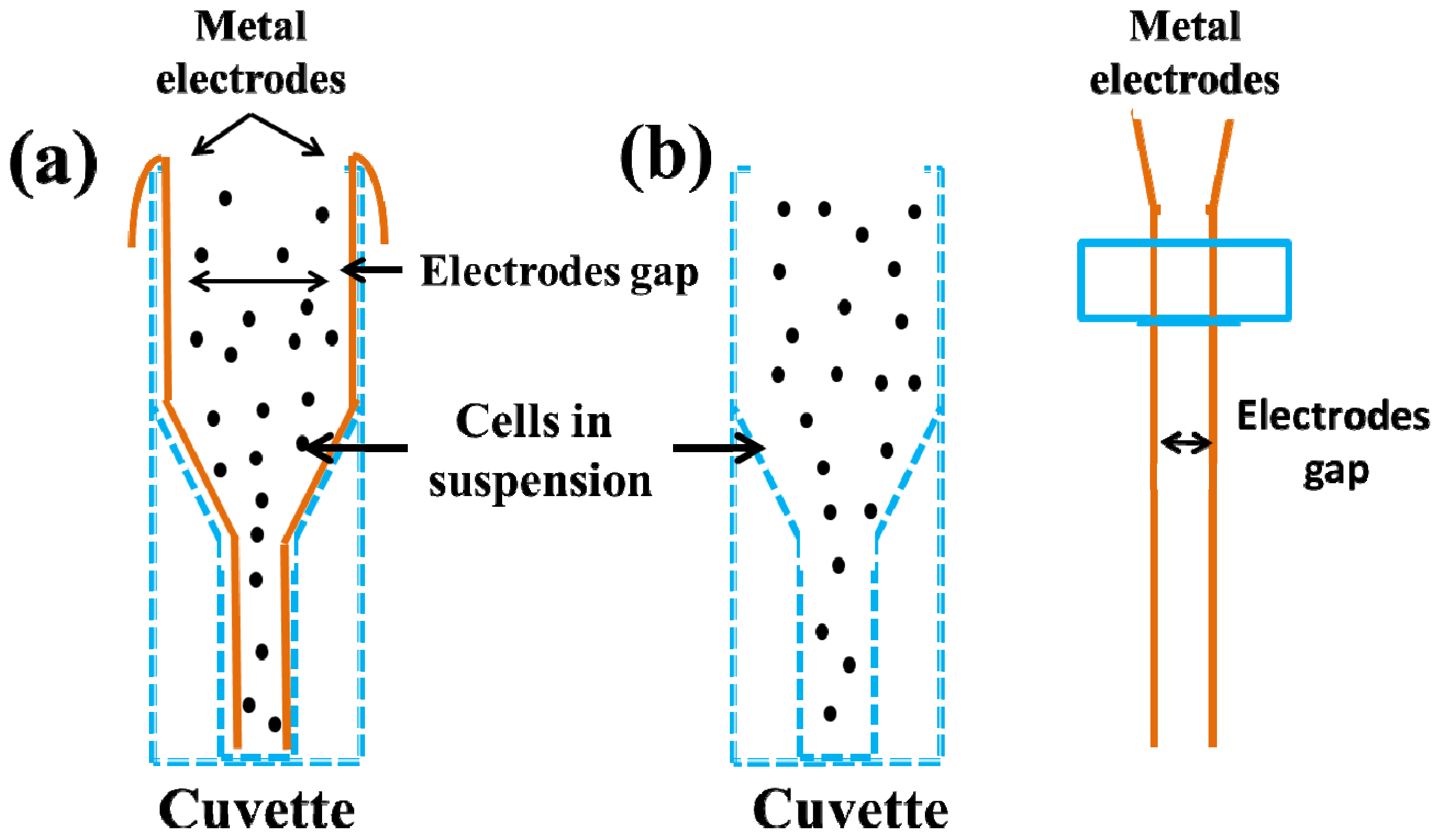
2.2. Single Cell Electroporation (SCEP)
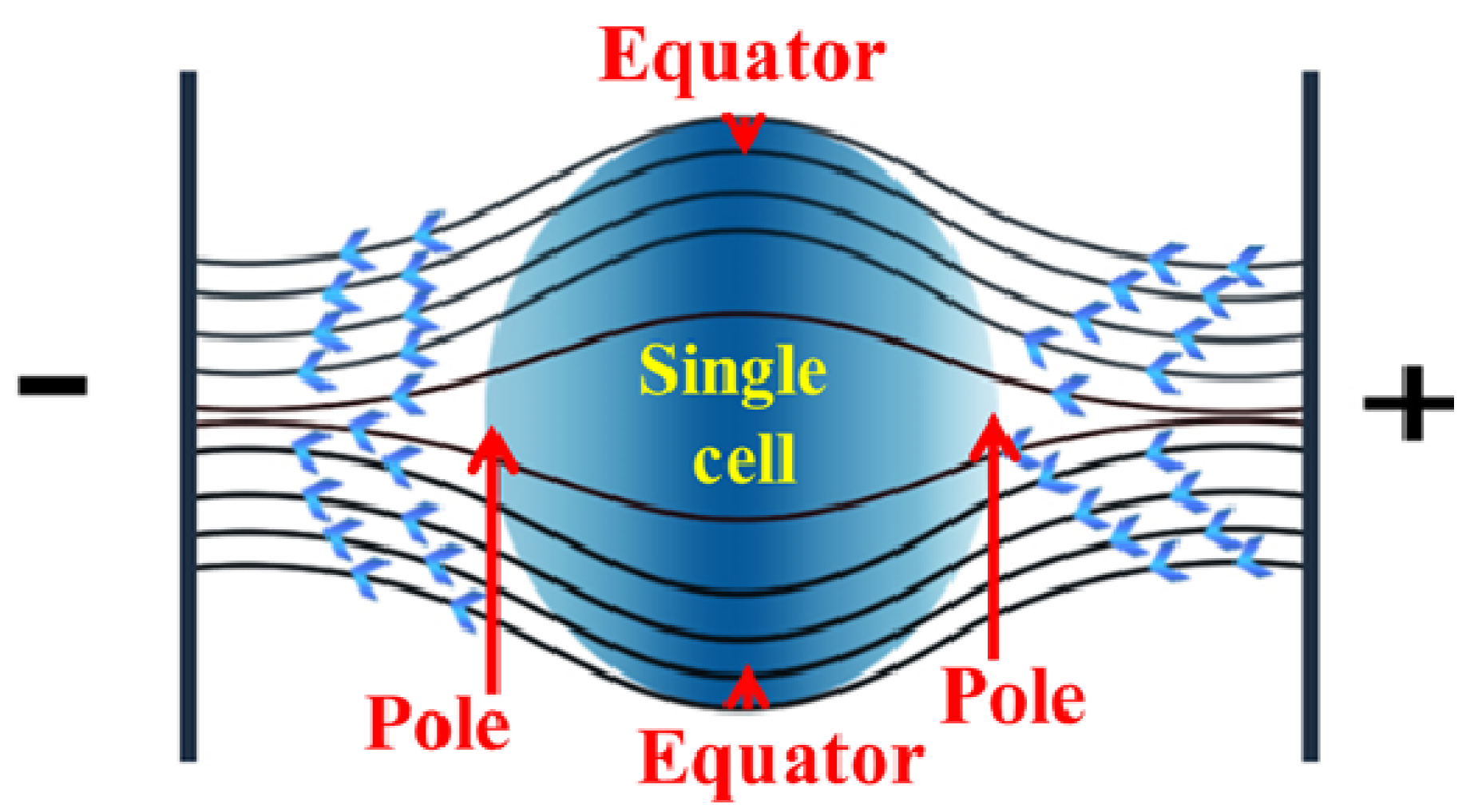
2.3. Localized Single Cell Membrane Electroporation (LSCMEP)
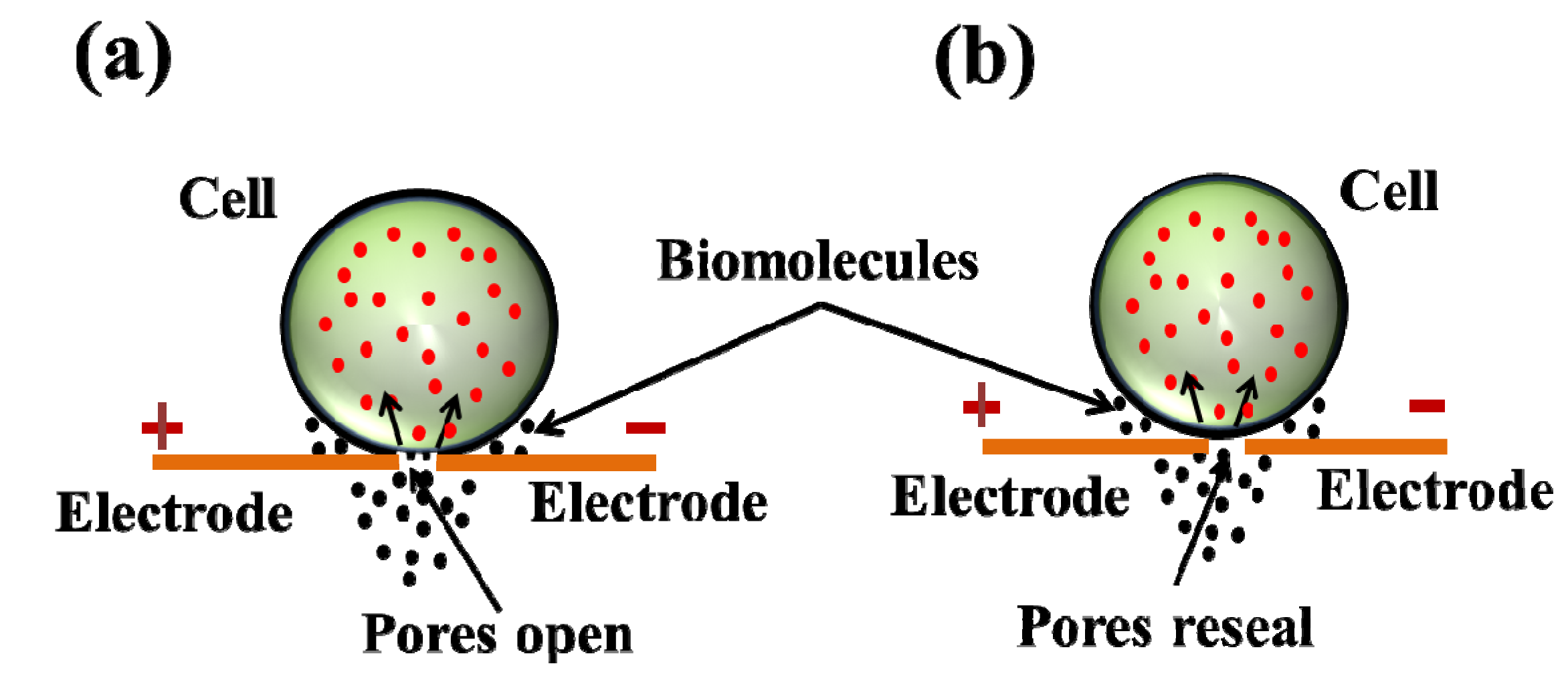
3. Micro/Nanofluidic Devices for Single Cell Electroporation
| Year and References | Potential difference | Pulse time | Cell type | Spices | Purpose |
|---|---|---|---|---|---|
| 1999 [66] | 0–60 V | 2 µs–100 ms | ND-1
ATCC#CRL-1439 | Human Rat | Transfection and lysis |
| 2001 [41] | 10 V | 5 ms | Huh-7 | Human | Transfection |
| 2003 [67] | 1125 V | Continuous DC | Jurkat | Human | Lysis |
| 2003 [38] | 20 V | ±20 s AC | S. cerevisiae | Yeast | Lysis |
| 2004 [68] | 1400 | 1 s | Erythrocytes | Human | Lysis |
| 2005 [69] | 0.1–1 V | 10 KHz AC | HeLa | Human | Transfection |
| 2006 [70] | 400 V/cm, 600–1200 V/cm | 10 µs–20 ms, 30 ms | CHO | Hamster | Transfection and lysis |
| 2006 [71] | 2000 V/cm | Continuous DC | E. coli | Bacterial | Lysis |
| 2007 [72] | 10 VPP | 1 MHz | Zucchini protoplast cells | Plant | Lysis |
| 2008 [73] | 1–3 V | 6 ms | C2C12 | Mouse | Transfection |
| 2008 [29] | 1 VPP | 0.5 Hz | Fibroblast cell | Rat | Transfection |
| 2008 [46] | <20 V | 100 KHz–1 MHz | A431 squamous cell | Human | Lysis |
| 2009 [74] | 5 Vrms | 40 Hz | FITC-BSA-laden vesicle | - | Lysis |
| 2010 [75] | <3 V | Continuous DC | - | Yeast | Transfection |
| 2012 [27] | 10 µs, 20 ms | 8 VPP | HeLa | Human | Tranfection |
| 2012 [30] | 1–60 ms | 60–260 V/2 mm | K 562, Jurkat | Human | Transfection |
| 2012 [76] | 1.3 V | Continuous DC | Algal cell | Plant | Transfection |
| 2013 [77] | 600–90 m Vpp | 2 ms | HT-29 | Human | Lysis |
3.1. Single Cell Transfection
3.1.1. Trapping Based Single Cell Transfection
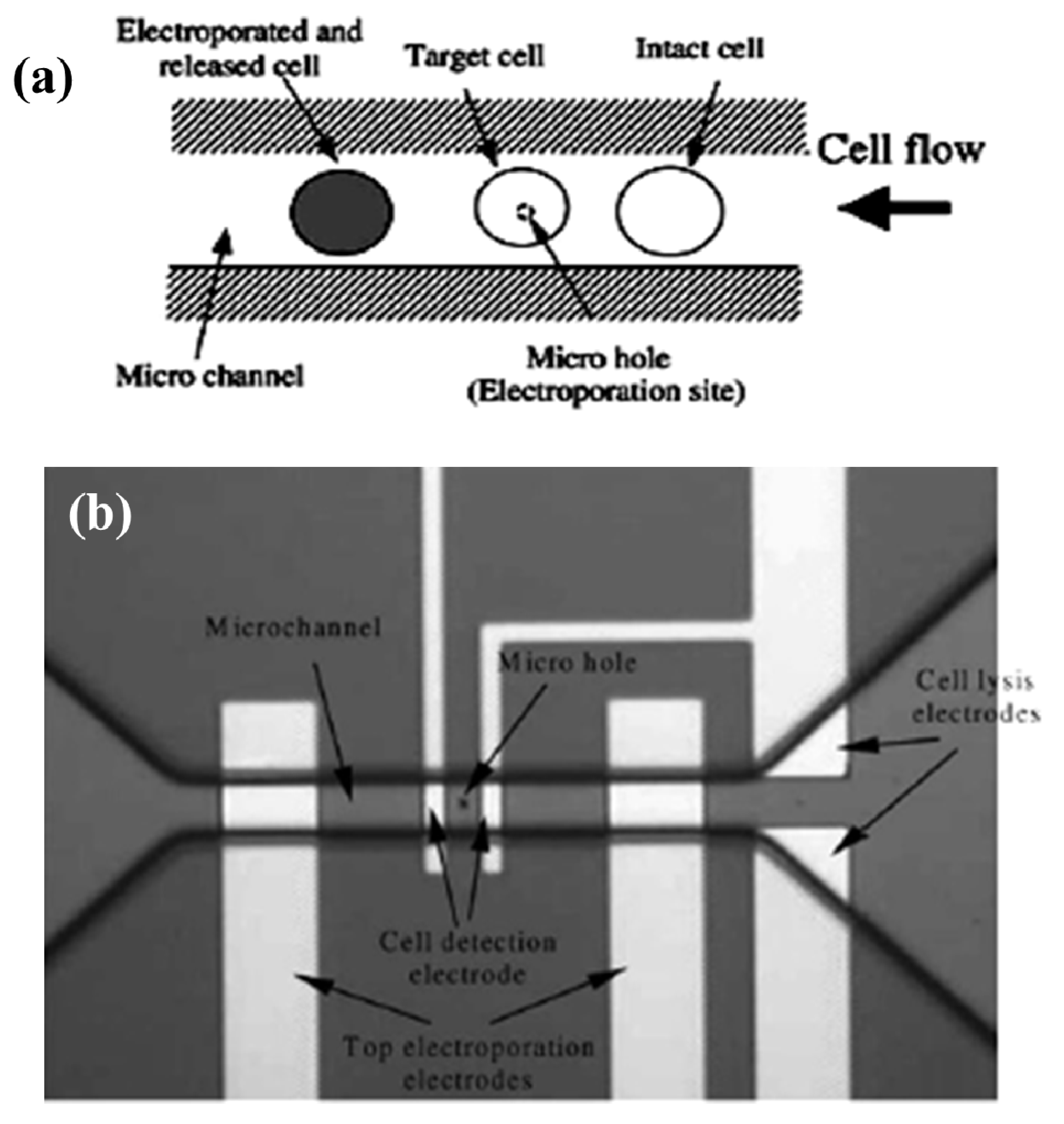
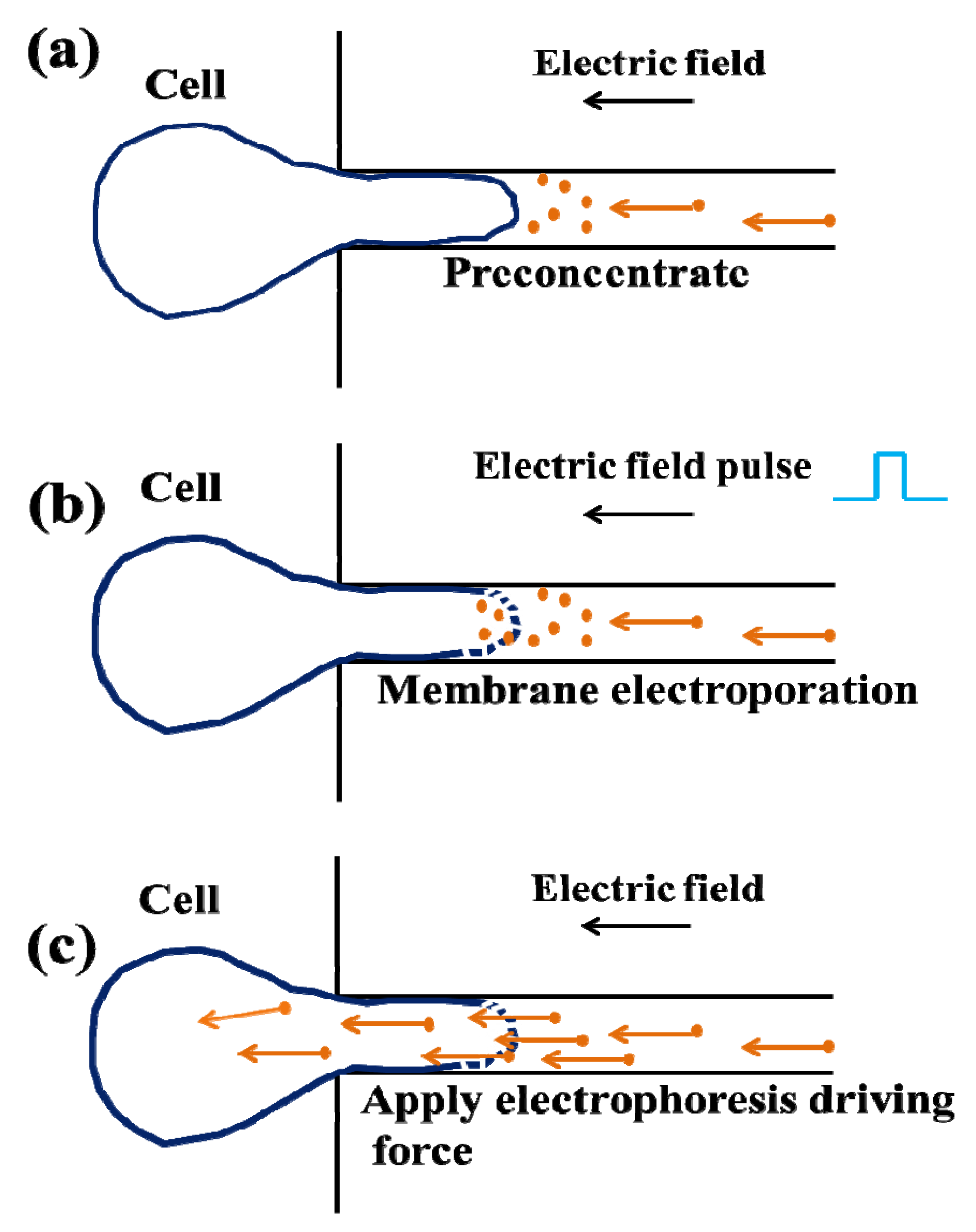
3.1.2. Droplet Microfluidics for Single Cell Transfection
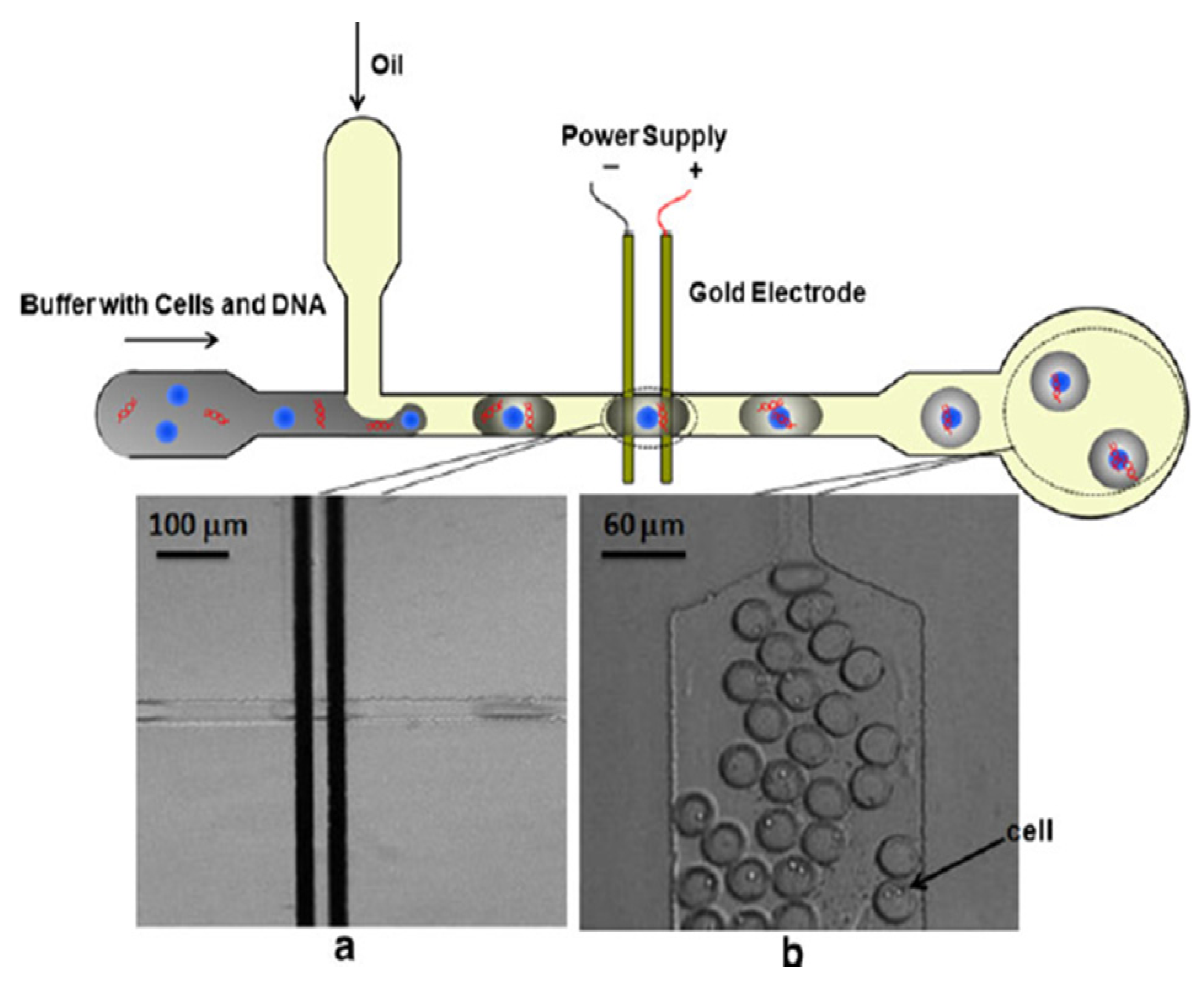

3.2. Single Cell Lysis
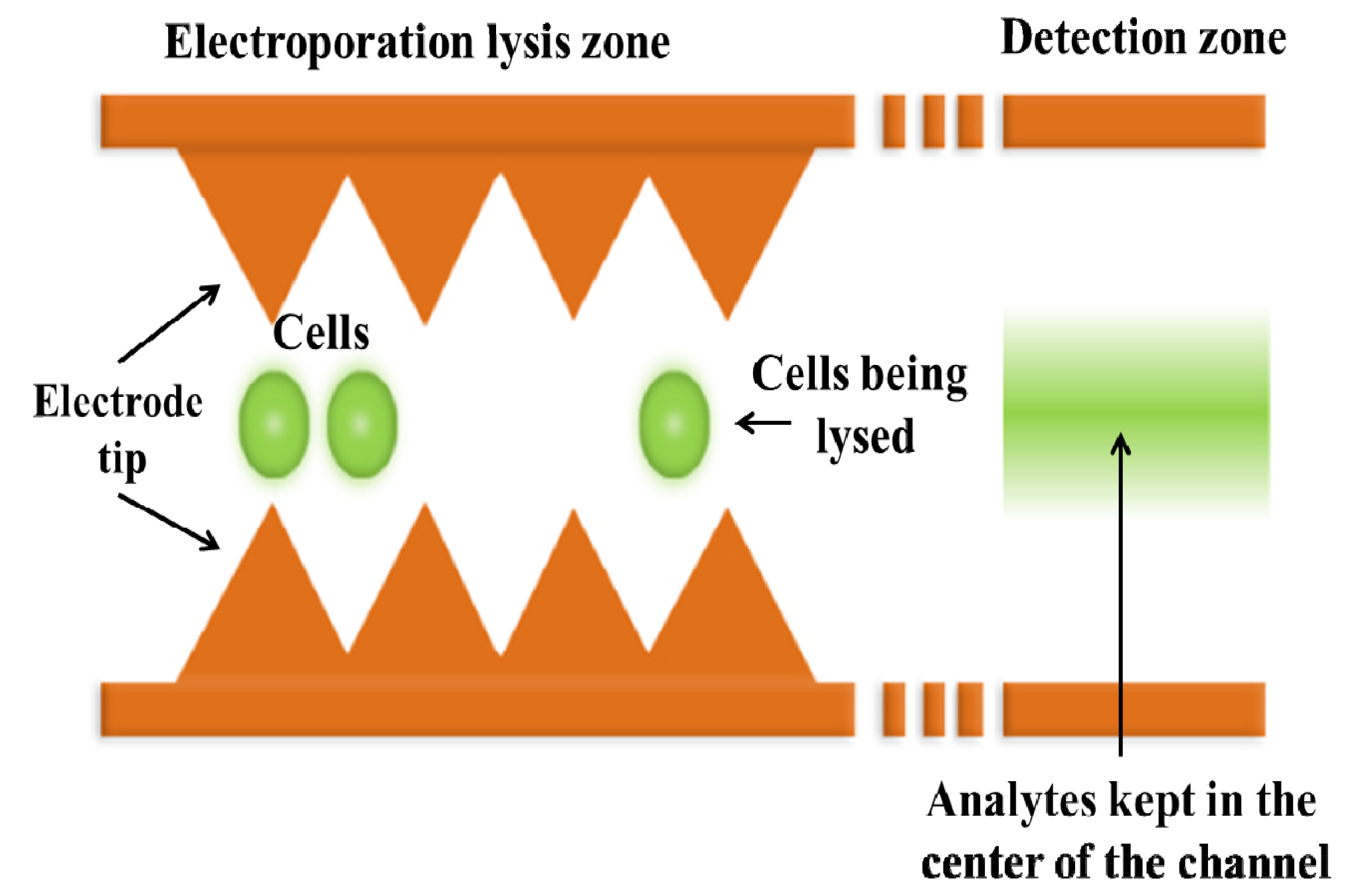
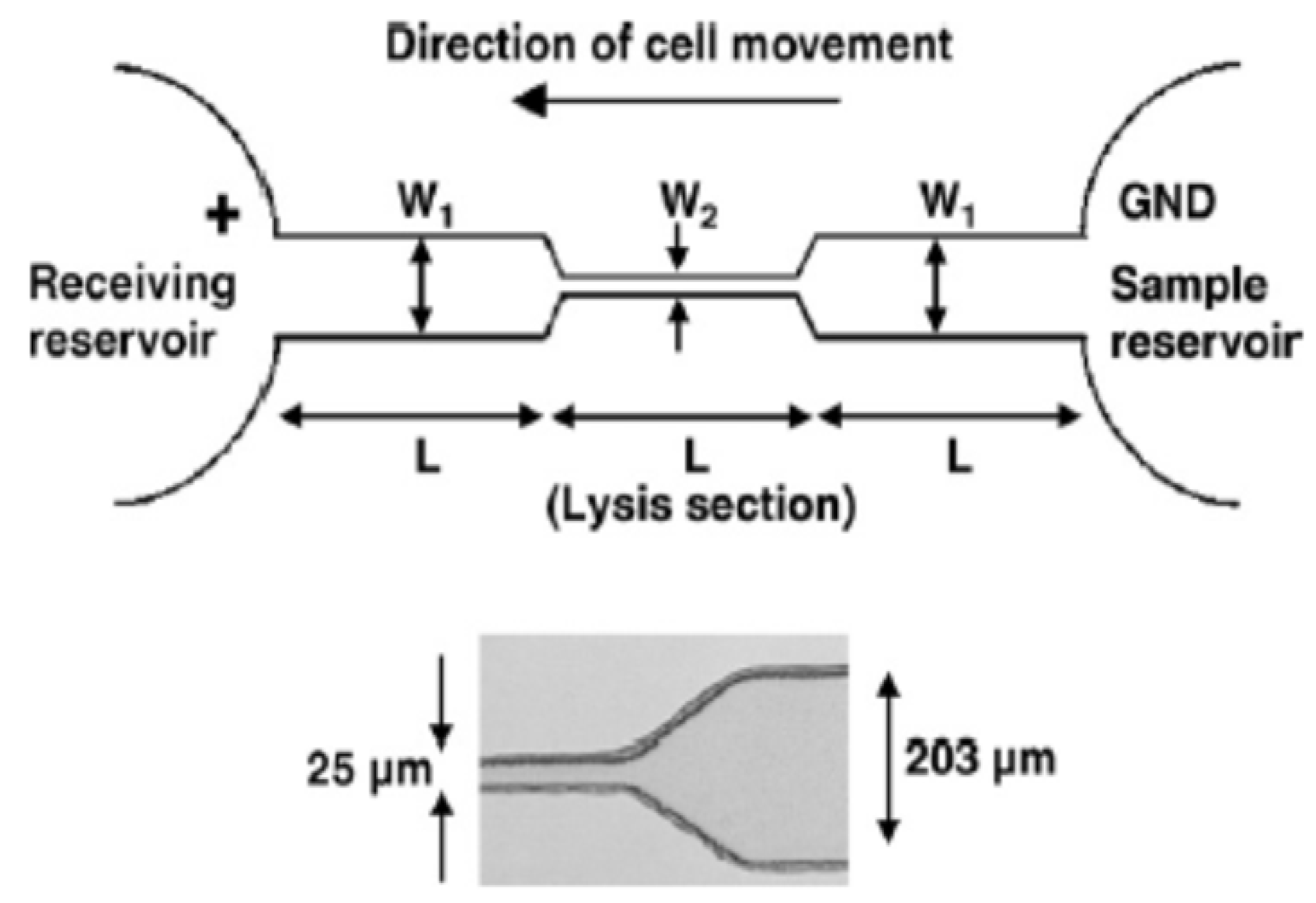

4. Localized Single Cell Membrane Electroporation (LSCMEP)
4.1. LSCMEP for Cell Transfection
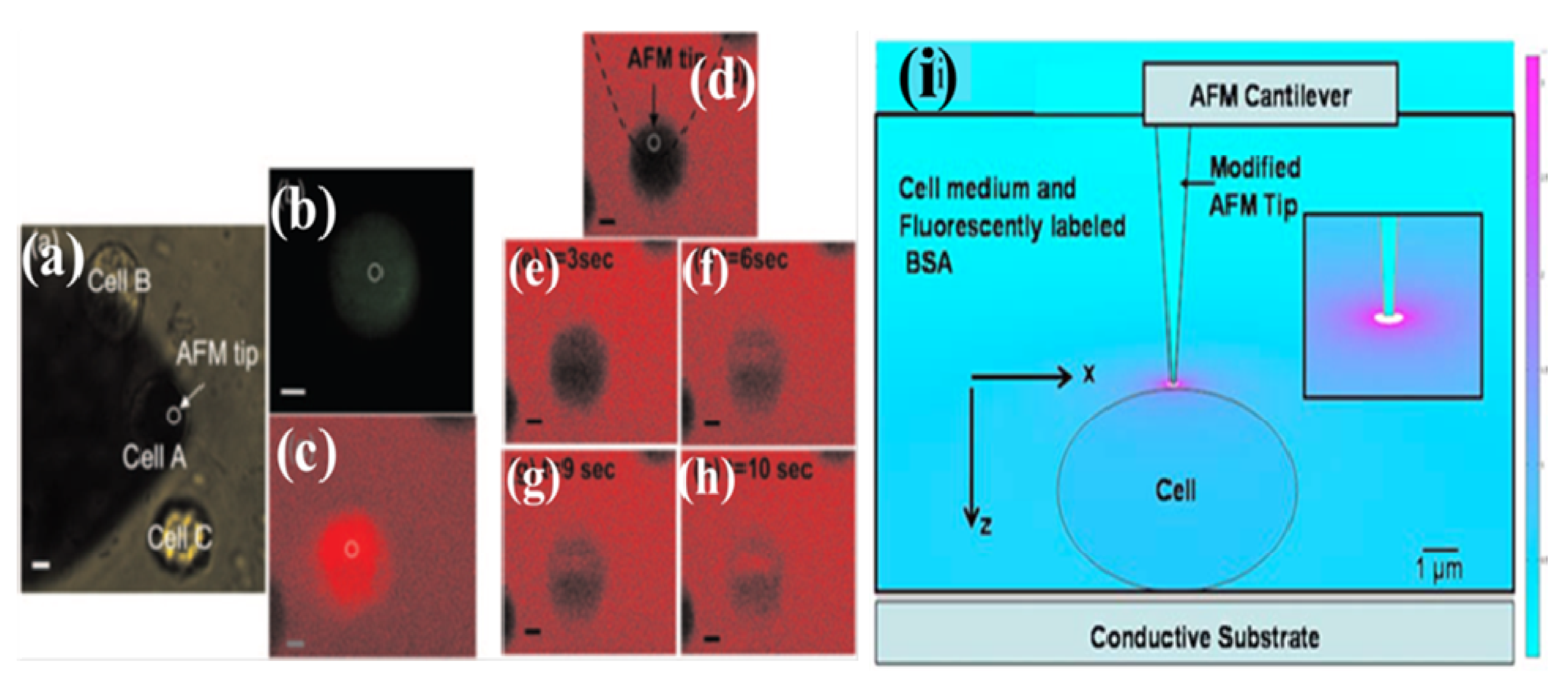
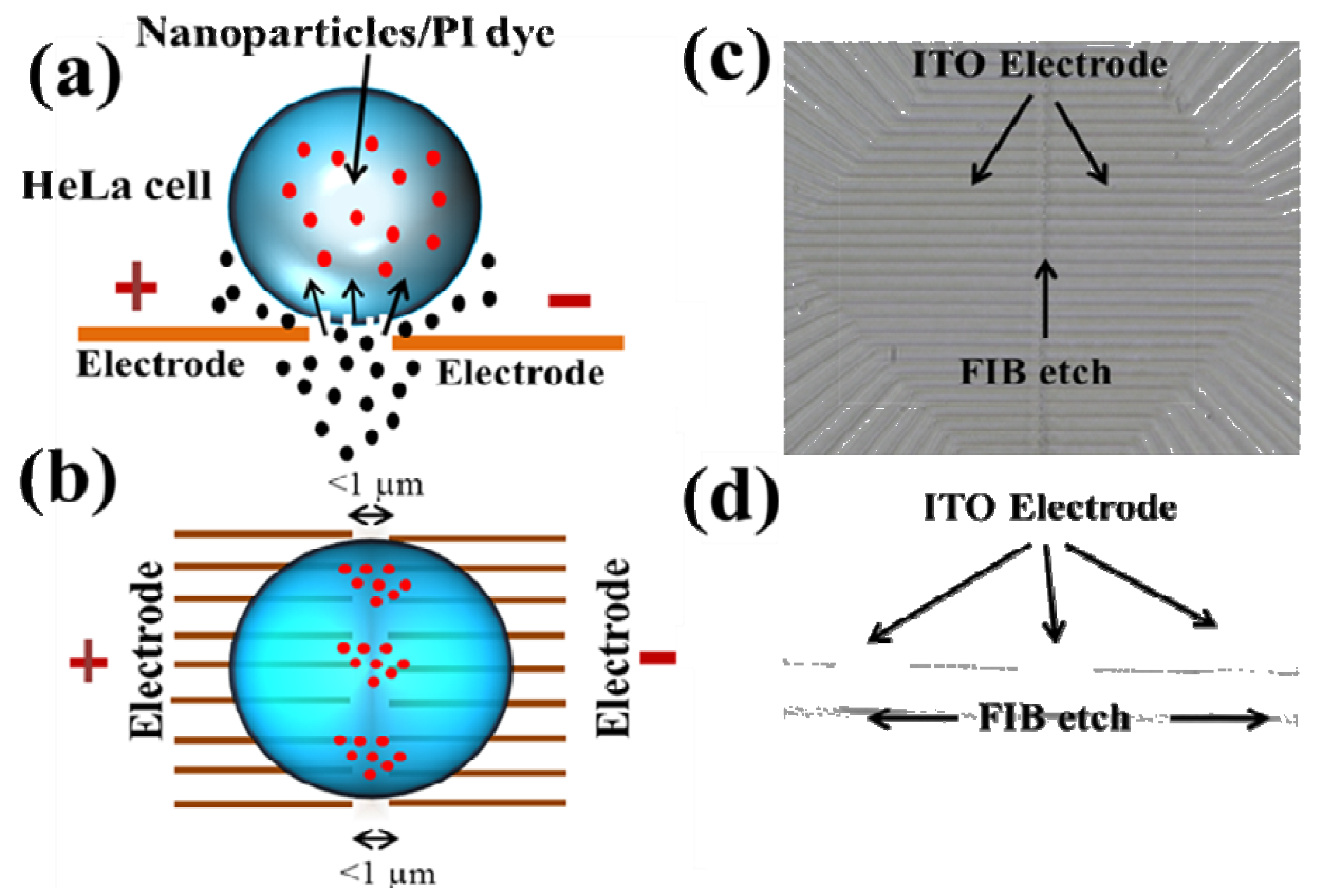
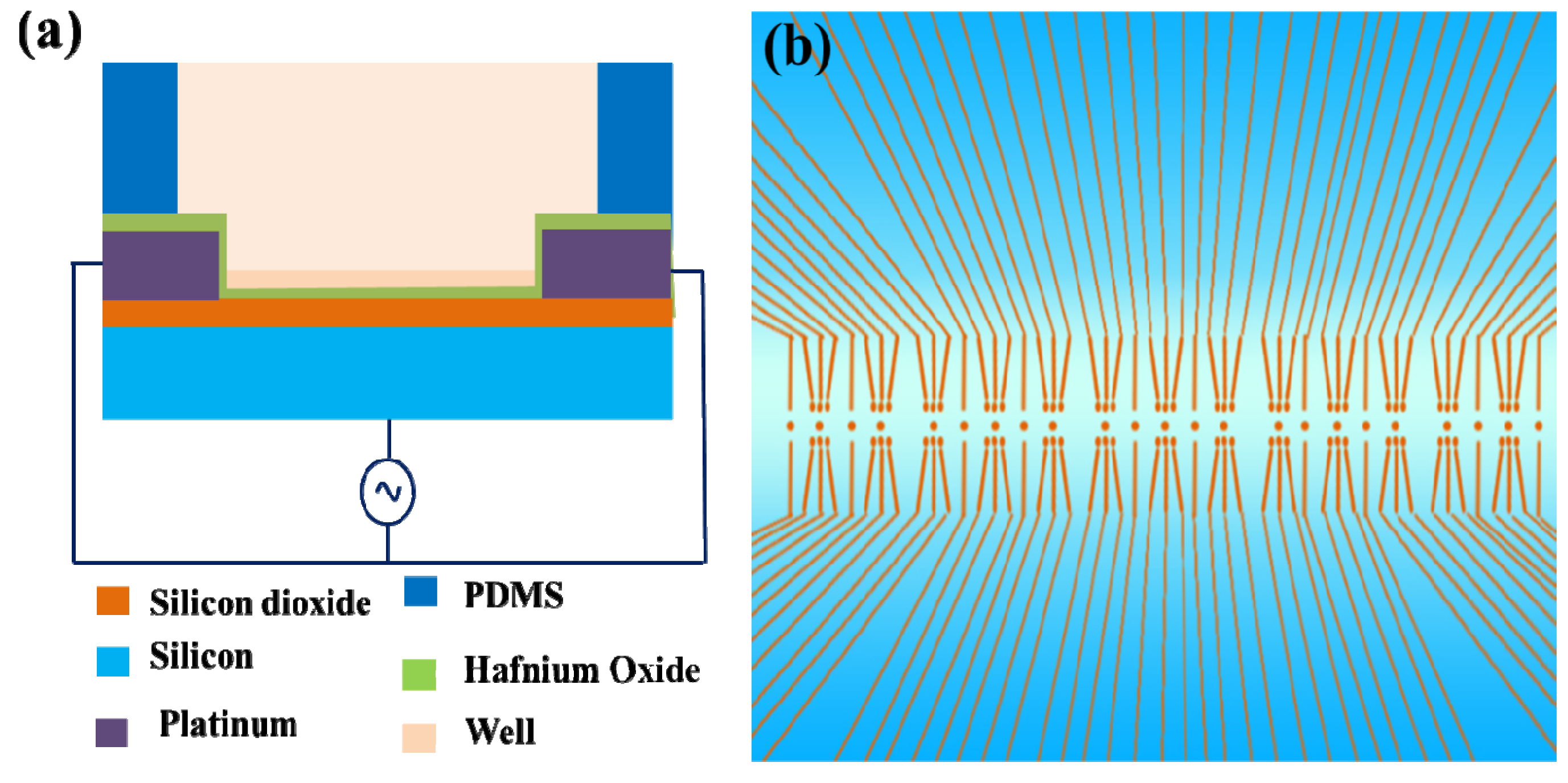
4.2. LSCMEP for Cell Lysis
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Zimmermann, U.; Pilwat, G.; Friemann, F. Dielectric breakdown of cell membrane. Biophy. J. 1974, 14, 881–899. [Google Scholar] [CrossRef]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerget. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanism of cell membrane electropermeabilization: A minireview of our present (lack of ?) knowledge. Biochim. Biophys. Acta. 2005, 1724, 270–280. [Google Scholar] [CrossRef]
- Escoffre, J.M.; Porter, T.; Wasungu, L.; Teissie, J.; Dean, D.; Rols, M.P. What is (still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Mol. Biotechnol. 2009, 41, 286–295. [Google Scholar] [CrossRef]
- Neu, W.K.; Neu, J.C. Theory of Electroporation. In Cardiac Bioelectric Therapy; Efimov, I.R., Kroll, M.W., Tchou, P.J., Eds.; Springer: New York, NY, USA, 2009; pp. 133–134. [Google Scholar]
- Ho, S.Y.; Mittal, G.S.; Cross, J.D. Effect of high electric field pulses on the activity of selected enzymes. J. Food Eng. 1997, 31, 69–84. [Google Scholar] [CrossRef]
- Prasanna, G.L.; Panda, T. Electroporation: Basic principles, practical consideration and applications in molecular biology. Bioprocess Eng. 1997, 16, 261–264. [Google Scholar] [CrossRef]
- Serpersu, E.H.; Tsong, T.Y.; Kinosita, K. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim. Biophys. Acta 1985, 812, 779–785. [Google Scholar] [CrossRef]
- Tsong, T.Y.; Kinosita, K. Use of voltage pulses for the pore opening and drug loading and the subsequent resealing of red blood cells. Bibliotheca Haematologica 1985, 51, 108–114. [Google Scholar]
- Schoenbach, K.H.; Beebe, S.J.; Buescher, E.S. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics 2001, 22, 440–448. [Google Scholar] [CrossRef]
- Chen, N.; Schoenbach, K.H.; Kolb, J.F.; Swanson, R.J.; Garner, A.L.; Yang, J.; Joshi, R.P.; Beebe, S.J. Leukemic cell intracellular responses to nanosecond electric fields. Biochem. Biophys. Res. Commun. 2004, 317, 421–427. [Google Scholar] [CrossRef]
- DeBruin, K.A.; Krassowska, W. Modelling electroporation in a single cell. I. Effects of field strength and rest potential. Boiphys. J. 1999, 77, 1213–1224. [Google Scholar] [CrossRef]
- DeBruin, K.A.; Krassowska, W. Modelling electroporation in a single cell. II. Effects of ionic concentrations. Biophys. J. 1999, 77, 1225–1233. [Google Scholar] [CrossRef]
- Movahed, S.; Li, D. Microfluidics cell electroporation. Microfluid Nanofluid 2011, 10, 703–734. [Google Scholar] [CrossRef]
- Chang, D.C.; Chassy, B.M.; Saunders, J.A. Guide to Electroporation and Electrofusion; Academic: San Diego, CA, USA, 1992. [Google Scholar]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Neumann, E.; Sowers, A.E.; Jordan, C.A. Electroporation and Electrofusion in Cell Biology; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- Teissie, J.; Rols, M.P. An experimental evalution of the critical potential difference inducing cell membrane electropermeabilization. Biophys. J. 1993, 65, 409–413. [Google Scholar] [CrossRef]
- Zimmermann, U. Electric field-mediated fusion and related electric phenomena. Biochim. Biophys. Acta 1982, 694, 222–227. [Google Scholar]
- Stampfli, R. Reversible electric breakdown of the excitable membrane of a Ranvier node. Ann. Acad. Bras. Cien. 1958, 30, 57–63. [Google Scholar]
- Rubinsky, B. Irreversible electroporation in medicine. Technol. Cancer Res. Treat. 2007, 6, 255–259. [Google Scholar]
- Nollet, J.A. Researches Sur Les Causes Particulieres Des Phenomenes Electriques; (in French). Chez H.L. Guerin & L.F. Delatour: Paris, France, 1754. [Google Scholar]
- Fox, M.B.; Esveld, D.C.; Valero, A.; Luttge, R.; Mastwijk, H.C.; Bartels, P.V.; ven den Berg, A.; Boom, R.M. Electroporation of cells in microfluidic devices: A review. Anal. Bional Chem. 2006, 385, 474–485. [Google Scholar] [CrossRef]
- Lee, W.G.; Demirci, U.; Khademhosseini, A. Microscale electroporation: Challenges and perspectives for clinical applications. Integr. Biol. 2009, 1, 242–251. [Google Scholar] [CrossRef]
- Wang, S.; Lee, L.J. Micro-/Nanofluidics based cell electroporation. 2013, 7. [Google Scholar] [CrossRef]
- Wang, M.; Orwar, O.; Olofsson, J.; Weber, S.G. Single cell electroporation: Review. Anal. Bional. Chem. 2010, 397, 3225–3248. [Google Scholar] [CrossRef]
- Chen, S.-C.; Santra, T.S.; Chang, C.-J.; Chen, T.-J.; Wang, P.-C.; Tseng, F.-G. Delivery of molecules into cells using localized single cell electroporation on ITO microelectrode based transparent chip. Biomed. Microdevices 2012, 14, 811–817. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, J.H.; Kim, K.P.; Chung, T.K. Continuous low voltage dc electroporation on a microfluidic chip with polyelectrolytic salt bridges. Anal. Chem. 2007, 79, 7761–7766. [Google Scholar] [CrossRef]
- Nawarathna, D.; Unal, K.; Wickramasinghe, H.K. Localized electroporation and molecular delivery into single living cells by atomic force microscopy. Appl. Phys. Lett. 2008, 93, 153111. [Google Scholar] [CrossRef]
- Boukany, P.E.; Morss, A.; Liao, W.-C.; Henslee, B.; Jung, H.C.; Zhang, X.; Yu, B.; Wang, X.; Wu, Y.; Li, L.; et al. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat. Nanotecnol. 2011, 6, 747–754. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation of cell and tissues. IEEE Trans. Plasma Sci. 2000, 28, 24–33. [Google Scholar] [CrossRef]
- Kinosita, K.; Hibino, M.; Itoh, H.; Shigemori, M.; Hirano, K.; Kirino, Y.; Hayakawa, T. Events of Membrane Electroporation Visualized on a Time Scale from Microsecond to Nanoseconds. In Guide to Electroporationand Electrofusion; Chang, D.C., Chassy, B.M., Saunders, J.A., Sowers, A.E., Eds.; Academic Press: Orlando, FL, USA, 1992; pp. 29–46. [Google Scholar]
- Neumann, E.; Rosenheck, R. Permeability induced by electric impulsions in vesicular membranes. J. Membr. Biol. 1972, 10, 279–290. [Google Scholar] [CrossRef]
- Gabriel, B.; Teissie, J. Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane. Biophys. J. 1997, 73, 2630–2637. [Google Scholar] [CrossRef]
- Lundqvist, J.A.; Sahlin, F.; Aberg, M.A.; Strimberg, A.; Eriksson, P.S.; Orwar, O. Altering the biochemical state of individual cultured cells and organelles with ultramicroelectrodes. Proc. Natl. Acad. Sci. USA 1998, 95, 10356–10360. [Google Scholar] [CrossRef]
- Valero, A.; Merino, F.; Wolbers, F.; Luttge, R.; Vermes, I.; Andersson, H.; van den Berg, A. Apoptotic cell death dynamics of HL 60 cells studied using a microfluidic cell tarp device. Lab Chip 2005, 5, 49–55. [Google Scholar] [CrossRef]
- Suehiro, J.; Yatsunami, R.; Hamada, R.; Hara, M. Quantitative estimation of biological cell concentration suspended in aqueous medium by using dielectrophoretic impedance measurement method. J. Phys. D Appl. Phys. 1999, 32, 2814–2820. [Google Scholar] [CrossRef]
- Suehiro, J.; Shutou, M.; Hatano, T.; Hra, M. High sensitive detection of biological cells using dielectrophoretic impedance measurement method combined with electropermeabilization. Sens. Actuators B 2003, 96, 144–151. [Google Scholar] [CrossRef]
- Suehiro, J.; Hatano, T.; Shutou, M.; Hra, M. Improvement of electric pulse shape of electropermeabilization-assisted dielectrophoretic impedance measurement for high sensitive bacteria detection. Sens. Actuators B 2005, 109, 209–215. [Google Scholar] [CrossRef]
- Loomis-Husselbee, J.W.; Cullen, P.J.; Irvine, R.F.; Dawson, A.P. Electroporation can cause artifacts due to solubilization of cations from the electrode plates. Biochem. J. 1991, 277, 883–885. [Google Scholar]
- Lin, Y.C.; Jen, C.M.; Huang, M.Y.; Wu, C.Y.; Lin, X.Y. Electroporation microchips for continuous gene transfection. Sens. Actuators B 2001, 79, 137–143. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, M.; Fan, C.S.; Wu, L.W. A microchip for electroporation of primary endothelial cells. Sens. Actuators A 2003, 108, 12–19. [Google Scholar] [CrossRef]
- Knorr, D.; Angersbach, A.; Eshtiaghi, M.N.; Heinz, V.; Lee, D. Processing concepts based on high intensity electric field pulses. Trends Food Sci. Technol. 2001, 12, 129–135. [Google Scholar] [CrossRef]
- Pol, I.E. Pulsed-electric field treatment enhances the bactericidal action of nisin against Bacillus cereus. App. Enviro. Microbial. 2000, 66, 428–430. [Google Scholar] [CrossRef]
- Fox, M.B.; Esveld, E.; Luttge, R.; Boom, R. A new pulsed electric field microreactor: Comparison between the laboratory and microscale. Lab Chip 2005, 5, 943–948. [Google Scholar] [CrossRef]
- Sedgwick, H.; Caron, F.; Monaghan, P.B.; Kolch, W.; Cooper, J.M. Lab-on-a-chip technologies for proteomic analysis from isolated cells. J. R. Soc. Interface 2008, 5, S123–S130. [Google Scholar] [CrossRef]
- De la Rosa, C.; Prakash, R.; Tilley, P.A.; Fox, J.D.; Kaler, K.V. Integrated microfluidic systems for sample preparation and detection of respiratory pathogen bordetella pertussis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 2007, 6303–6306. [Google Scholar]
- Wang, J.; Bao, N.; Paris, L.L.; Wang, H.Y.; Geahlen, R.L.; Lu, C. Detection of kinase translocation using microfluidic electroporative flow cytometry. Anal. Chem. 2008, 80, 1087–1093. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lu, C. Microfluidic electroporation for delivery of small molecules and genes into cells using a common DC power supply. Biotechnol. Bioeng. 2008, 8, 62–67. [Google Scholar]
- Olofsson, J.; Levin, M.; Stromberg, A.; Weber, S.G.; Ryttsen, F.; Orwar, O. Scanning electroporation of selected areas of adherent cell cultures. Anal. Chem. 2007, 79, 4410–4418. [Google Scholar] [CrossRef]
- Zudans, I.; Agarwal, A.; Orwar, O.; Weber, S.G. Numerical calculations of single-cell electroporation with an electrolyte-filled capillary. Biophys. J. 2007, 92, 3696–3705. [Google Scholar] [CrossRef]
- Agarwal, A.; Zudans, I.; Weber, E.A.; Olofsson, J.; Orwar, O.; Weber, S.G. Effect of cell size and shape on single-cell electroporation. Anal. Chem. 2007, 79, 3589–3596. [Google Scholar] [CrossRef]
- Olofsson, J.; Nolkrantz, K.; Ryttsen, F.; Lambie, B.A.; Weber, S.G.; Orwar, O. Single cell electroporation. Curr. Opin. Biotechnol. 2003, 14, 29–34. [Google Scholar]
- Ryttsen, F.; Farre, C.; Brennan, C.; Weber, S.G.; Nolkrantz, K.; Jardemark, K.; Chiu, D.T.; Orwar, O. Characterization of single cell electroporation by using patch-clamp and fluorescence microscopy. Biophys. J. 2000, 79, 1993–2001. [Google Scholar] [CrossRef]
- Nolkrantz, K.; Farre, C.; Brederlau, A.; Karlsson, R.I.; Brennan, C.; Erikssson, P.S.; Weber, S.G.; Sandberg, M.; Orwar, O. Electroporation of single cells and tissues with an electrolyte filled capillary. Anal. Chem. 2001, 73, 4469–4477. [Google Scholar] [CrossRef]
- Fei, Z.; Wang, S.; Xie, Y.; Henslee, B.E.; Koh, C.G.; Lee, L.J. Gene transfection of mammalian cells using membrane sandwich electroporation. Anal. Chem. 2007, 79, 5719–5722. [Google Scholar] [CrossRef]
- Fei, Z.; Hu, X.; Choi, H.-W.; Wang, S.; Farson, D.; Lee, L.J. Micronozzle array enhanced sandwich electroporation of embryonic stem cells. Anal. Chem. 2010, 82, 353–358. [Google Scholar] [CrossRef]
- Vassanelli, S.; Bandiera, L.; Borgo, M.; Cellere, G.; Santoni, L.; Bersani, C.; Salamon, M.; Zaccolo, M.; Lorenzelli, L.; Girardi, S.; et al. Space and time-resolved gene expression experiments on cultured mammalian cells by a single-cell electroporation microarray. New Biotechnol. 2008, 25, 55–67. [Google Scholar]
- Vally, J.K.; Hsu, H.-Y.; Neale, S.; Ohta, A.T.; Jamshidi, A.; Wu, M.C. Assessment of Single Cell Viability Following Light Induced Electroporation through Use of On-Chip Microfluidics. In Proceedings of the IEEE 22nd International Conference on Micro Electro Mechanical Systems, Sorrento, Italy, 25–29 January 2009; pp. 411–414.
- Valley, J.K.; Neale, S.; Hsu, H.-Y.; Ohta, A.T.; Jamshidi, A.; Wu, M.C. Parallel single-cell light-induced electroporeation and dielectrophoretic manipulation. Lab Chip 2009, 9, 1714–1720. [Google Scholar] [CrossRef]
- Brennan, D.; Justice, J.; Corbett, B.; McCarthy, T.; Galvin, P. Emerging optofluidic technologies for point-of-care genetic analysis systems: A review. Anal. Bioanal. Chem. 2009, 395, 621–636. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lee, G.-B. An optically induced cell lysis device using dielectrophoresis. Appl. Phys. Lett. 2009, 94, 033901. [Google Scholar] [CrossRef]
- Sott, K.; Eriksson, E.; Petelenz, E.; Goksor, M. Optical system for single cell analysis. Expert. Opin. Drug Discov. 2008, 3, 1323–1344. [Google Scholar] [CrossRef]
- Yang, S.-M.; Yu, T.-M.; Huang, H.-P.; Ku, M.-Y.; Hsu, L.; Liu, C.H. Dynamic manipulation and patterning of microparticles and cells by using TiOPc-based optoelectronic dielectrophoresis. Opt. Lett. 2010, 35, 1959–1961. [Google Scholar] [CrossRef]
- Yang, S.-M.; Yu, T.-M.; Huang, H.-P.; Ku, M.-Y.; Tseng, S.-Y.; Tsai, C.-L.; Chen, H.P.; Hsu, L.; Liu, C.-H. Light-driven manipulation of pico-bubbles on a TiOPc-based optoelectronic chip. Appl. Phys. Lett. 2011, 98, 153512. [Google Scholar] [CrossRef]
- Huang, Y.; Rubinsky, B. Micro-electroporation: Improving the efficiency and understanding of electrical permeabilization of cells. Biomed. Microdevice. 1999, 2, 145–150. [Google Scholar] [CrossRef]
- McClain, M.A.; Culbertson, C.T.; Jacobson, S.C.; Allabritton, N.L.; Sims, C.E.; Ramsey, J.M. Microfluidic devices for the high-throughput chemical analysis of cells. Anal. Chem. 2003, 75, 5646–5655. [Google Scholar] [CrossRef]
- Gao, J.; Yin, X.F.; Fang, Z.L. Intergation of single cell injection, cell lysis separation and detection of intracellular constituents on a microfluidic chip. Lab Chip 2004, 4, 47–52. [Google Scholar] [CrossRef]
- Shin, Y.S.; Cho, K.; Kim, J.K.; Lim, S.H.; Park, C.H.; Lee, K.B.; Park, Y.; Chung, C.; Han, D.-C.; Chang, J.K. Electrotransfection of mammalian cells using microchannel-type electroporation chip. Anal. Chem. 2004, 76, 7045–7052. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lu, C. Electroporation of mammalian cells in a microfluidic channel with geometric variation. Anal. Chem. 2006, 78, 5158–5164. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Bhunia, A.K.; Lu, C. A microfluidic flow-through device for high throughput electrical lysis of bacterial cells based on continuous DC voltage. Biosens. Bioelectron. 2006, 22, 582–588. [Google Scholar] [CrossRef]
- Ikeda, N.; Tanaka, N.; Yanagida, Y.; Hatsuzawa, T. On-chip single-cell lysis for extracting intracellular material. Jpn. J. Appl. Phys. 2007, 46, 6410–6414. [Google Scholar] [CrossRef]
- Valero, A.; Post, J.N.; van Nieuwkasteele, J.W.; Ter Braak, P.M.; Kruijer, W.; van den Berg, A. Gene transfer and protein dynamics in stem cells using single cell electroporation in a microfluidic device. Lab Chip 2008, 8, 62–67. [Google Scholar] [CrossRef]
- Lim, J.K.; Zhou, H.; Tilton, R.D. Liposome rupture and contents release over coplanar microelectrodes arrays. J. Colloid Interface Sci. 2009, 332, 113–121. [Google Scholar] [CrossRef]
- Zhu, T.; Luo, C.; Huang, J.; Xiong, C.; Quyang, Q.; Fang, J. Electroporation based on hydrodynamic focusing of microfluidics with low DC voltage. Biomed. Microdevices 2010, 12, 35–40. [Google Scholar] [CrossRef]
- Qu, B.; Eu, Y.-J.; Jeong, W.-J.; Kim, D.-P. Droplet electroporation in microfluidics for efficient cell transformation with or without cell wall removal. Lab Chip 2012, 12, 4483–4488. [Google Scholar] [CrossRef]
- Jokilaakso, N.; Salm, E.; Chen, A.; Millet, L.; Guevara, C.D.; Dorvel, B.; Reddy, B., Jr.; Karlstrom, A.E.; Chen, Y.; Ji, H.; et al. Ultra-localized single cell electroporation using silicon nanowires. Lab Chip 2013, 13, 336–339. [Google Scholar] [CrossRef]
- Huang, Y.; Rubinsky, B. Microfabricated electroporation chip for single cell membrane permeabilization. Sens. Actuators A 2001, 89, 242–245. [Google Scholar] [CrossRef]
- Huang, Y.; Rubinsky, B. Flow through microelectroporation chip for high efficiency single cell genetic manipulation. Sens. Actuators A 2003, 104, 205–212. [Google Scholar] [CrossRef]
- Khine, M.; Lau, A.; Ionescu-Zanetti, C.; Seo, J.; Lee, L.P. A single cell electroporation chip. Lab Chip 2005, 5, 38–43. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, H.; Ohoka, M.; Okonogi, A.; Kabata, H.; Kanno, I.; Washizu, M.; Kotera, H. High Throughput Cell Electroporation Array Fabricated by Single Mask Inclined uv Lithography Exposure and Oxygen Plasma Etching. In Proceedings of the IEEE 14th International Conference on Solid-State Sensors, Actuators, and Microsystems, Lyon, France, 10–14 June 2007; pp. 687–690.
- Ionescu-Zanetti, C.; Blatz, A.; Khine, M. Electrophoresis-assisted single-cell electroporation for efficient intracellular delivery. Biomed. Microdevic. 2008, 10, 113–116. [Google Scholar] [CrossRef]
- Gac, S.L.; van den Berg, A. Single cell electroporation using microfluidic devices. Methods Mol. Biol. 2012, 853, 65–82. [Google Scholar] [CrossRef]
- Koster, S.; Angile, F.E.; Duan, H.; Agresti, J.J.; Wintner, A.; Schmitz, C.; Rowat, A.C.; Merten, C.A.; Pisignano, D.; Griffiths, A.D.; et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 2008, 8, 1110–1115. [Google Scholar] [CrossRef]
- He, M.; Edgar, J.S.; Jeffries, G.D.M.; Lorenz, R.M.; Shelby, J.P.; Chiu, D.T. Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets. Anal. Chem. 2005, 77, 1539–1544. [Google Scholar] [CrossRef]
- Luo, C.; Yang, X.; Fu, Q.; Sun, M.; Quyang, Q.; Chen, Y.; Ji, H. Picoliter-volume aqueous droplets in oil: Electrochemical detection and yeast cell electroporation. Electrophoresis 2006, 27, 1977–1983. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, J.; Bao, N.; Lu, C. Electroporation of cells in microfluidic droplets. Anal. Chem. 2009, 81, 2027–2031. [Google Scholar] [CrossRef]
- Lu, H.; Schmidt, M.A.; Jensen, K.F. A microfluidic electroporation device for cell lysis. Lab Chip 2005, 5, 23–29. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lu, C. High-throughput and real-time study of single cell electroporation using microfluidics: Effects of medium osmolarity. Biotechnol. Bioeng. 2006, 95, 1116–1125. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, P.C.H. Microfluidic selection and retention of a single cardiac myocyte, on-chip dye loading, cell concentration by chemical stimulation, and quantitative fluorescent analysis of intracellular calcium. Anal. Chem. 2005, 77, 4315–4322. [Google Scholar] [CrossRef]
- Di Carlo, D.; Aghdam, N.; Lee, L.P. Single cell enzyme concentrations, kinetics, and inhibition analysis using high-density hydrodynamic cell isolation arrays. Anal. Chem. 2006, 78, 4925–4930. [Google Scholar] [CrossRef]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. Microfluidic device for single-cell analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef]
- Hargis, A.D.; Alarie, J.P.; Ramsey, J.M. Characterization of cell lysis events on a microfluidic device for high-throughput single cell analysis. Electrophoresis 2011, 32, 3172–3179. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Tibbits, G.F.; Li, P.C. Real-time monitoring of intracellular calcium dynamic mobilization of a single cardiomyocyte in a microfluidic chip pertaining. Electrophoresis 2007, 28, 4723–4733. [Google Scholar] [CrossRef]
- Mellors, J.S.; Jorabachi, K.; Smith, L.M.; Ramsey, J.M. Integrated microfluidic device for automated single cell analysis using electrophoretic separation and electrospray ionization mass spectrometry. Anal. Chem. 2010, 82, 967–973. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Santra, T.S.; Tseng, F.G. Recent Trends on Micro/Nanofluidic Single Cell Electroporation. Micromachines 2013, 4, 333-356. https://doi.org/10.3390/mi4030333
Santra TS, Tseng FG. Recent Trends on Micro/Nanofluidic Single Cell Electroporation. Micromachines. 2013; 4(3):333-356. https://doi.org/10.3390/mi4030333
Chicago/Turabian StyleSantra, Tuhin Subhra, and Fang Gang Tseng. 2013. "Recent Trends on Micro/Nanofluidic Single Cell Electroporation" Micromachines 4, no. 3: 333-356. https://doi.org/10.3390/mi4030333







