Detection of Cyanotoxins in Algae Dietary Supplements
Abstract
:1. Introduction
2. Results and Discussion
2.1. Multi-Toxins Analysis and Validation
2.1.1. Evaluation of Extraction Methods
2.1.2. Methods Validation
2.2. Toxins Quantification in CB Dietary Supplements
2.3. Transformation Products ANA-a
2.4. Risk Assessment
3. Conclusions
4. Materials and Methods
4.1. Chemicals, Reagents, and Stock Solutions
4.2. Algal Dietary Supplement Samples
4.3. Sample Treatment Steps
4.3.1. MMPB via LDTD-APCI-HRMS
4.3.2. ANA-a via LDTD-HRMS and MCs-ANA-a via UHPLC-HESI-HRMS
4.3.3. BMAA, CYN, and STX via UHPLC-HESI-HRMS
4.4. Analytical Detection
4.4.1. LDTD Conditions
4.4.2. UHPLC Conditions
4.4.3. HRMS Conditions
4.5. Method Validation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baures, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.; Bakker, G.J. Toxins of cyanobacteria. Mol. Nutr. Food. Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- O’neil, J.; Davis, T.W.; Burford, M.A.; Gobler, C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Oliver, R.L.; Hamilton, D.P.; Brookes, J.D.; Ganf, G.G. Physiology, blooms and prediction of planktonic cyanobacteria. In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherland, 2012; pp. 155–194. [Google Scholar]
- Persson, P.B.; Zakrisson, A. Dietary supplements: Health from the ocean? Acta Physiol. 2015, 215. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.S.; Yang, Y.; Park, Y.; Lee, J. Health benefits of blue-green algae: Prevention of cardiovascular disease and nonalcoholic fatty liver disease. J. Med. Food 2013, 16, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.; Khosravi-Darani, K.; Mozafari, M. Nutritional and medical applications of Spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Drapeau, C.; Anderson, D.M. Harvesting of Aphanizomenon flos-aquae ralfs ex born. & flah. Var. Flos-aquae (cyanobacteria) from Klamath lake for human dietary use. J. Appl. Phycol. 2000, 12, 585–595. [Google Scholar]
- Saker, M.L.; Welker, M.; Vasconcelos, V.M. Multiplex pcr for the detection of toxigenic cyanobacteria in dietary supplements produced for human consumption. Appl. Microbiol. Biotechnol. 2007, 73, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Lavorini, P.; Funari, E.; Scardala, S.; Testai, E. Contamination by Microcystis and microcystins of blue–green algae food supplements (bgas) on the italian market and possible risk for the exposed population. Food Chem. Toxicol. 2012, 50, 4493–4499. [Google Scholar] [CrossRef] [PubMed]
- Rellán, S.; Osswald, J.; Saker, M.; Gago-Martinez, A.; Vasconcelos, V. First detection of anatoxin-a in human and animal dietary supplements containing cyanobacteria. Food Chem. Toxicol. 2009, 47, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in three alkaline rift valley lakes of Kenya-lakes Bogoria, Nakuru and Elmenteita. J. Plankton Res. 2004, 26, 925–935. [Google Scholar] [CrossRef]
- Petroczi, A.; Taylor, G.; Naughton, D. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem. Toxicol. 2011, 49, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharm. 2005, 203, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Draisci, R.; Ferretti, E.; Palleschi, L.; Marchiafava, C. Identification of anatoxins in blue-green algae food supplements using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2001, 18, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Menard, C. Determination of microcystins in blue-green algae, fish and water using liquid chromatography with ultraviolet detection after sample clean-up employing immunoaffinity chromatography. J. Chromatogr. A 2001, 922, 111–117. [Google Scholar] [CrossRef]
- Gilroy, D.J.; Kauffman, K.W.; Hall, R.A.; Huang, X.; Chu, F.S. Assessing potential health risks from microcystin toxins in blue-green algae dietary supplements. Environ. Health Perspect. 2000, 108, 435–439. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 541. [Google Scholar]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-n-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Rapala, J.; Sivonen, K.; Luukkainen, R.; Niemelä, S.I. Anatoxin-a concentration in anabaena and aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxic anabaena-strains—A laboratory study. J. Appl. Phycol. 1993, 5, 581–591. [Google Scholar] [CrossRef]
- Sivonen, K.; Niemelä, S.; Niemi, R.; Lepistö, L.; Luoma, T.; Räsänen, L. Toxic cyanobacteria (blue-green algae) in finnish fresh and coastal waters. Hydrobiologia 1990, 190, 267–275. [Google Scholar] [CrossRef]
- Scott, P.M.; Niedzwiadek, B.; Rawn, D.F.K.; Lau, B.P.Y. Liquid chromatographic determination of the cyanobacterial toxin β-N-methylamino-l-alanine in algae food supplements, freshwater fish, and bottled water. J. Food Prot. 2009, 72, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Glover, W.; Baker, T.C.; Murch, S.J. Determination of β-N-methylamino-l-alanine, N-(2-aminoethyl) glycine, and 2, 4-diaminobutyric acid in food products containing cyanobacteria by ultra-performance liquid chromatography and tandem mass spectrometry: Single-laboratory validation. J. Aoac. Int. 2015, 98, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.R.; Fischer, A.; Michel, C.; Hoeger, S.J. Toxin mixture in cyanobacterial blooms—A critical comparison of reality with current procedures employed in human health risk assessment. Adv. Exp. Med. Biol. 2008, 619, 885–912. [Google Scholar] [PubMed]
- Roy-Lachapelle, A.; Solliec, M.; Sinotte, M.; Deblois, C.; Sauvé, S. High resolution/accurate mass (HRMS) detection of anatoxin-a in lake water using LDTD-APCI coupled to a Q-Exactive mass spectrometer. Talanta 2015, 132, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Roy-Lachapelle, A.; Solliec, M.; Sinotte, M.; Deblois, C.; Sauve, S. Total analysis of microcystins in fish tissue using laser thermal desorption-atmospheric pressure chemical ionization-high-resolution mass spectrometry (LDTD-APCI-HRMS). J. Agric. Food Chem. 2015, 63, 7440–7449. [Google Scholar] [CrossRef] [PubMed]
- Roy-Lachapelle, A.; Fayad, P.B.; Sinotte, M.; Deblois, C.; Sauve, S. Total microcystins analysis in water using laser diode thermal desorption-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal. Chim. Acta 2014, 820, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, K.L.; Harada, K.; Namikoshi, M.; Chen, C.; Harvis, C.A.; Munro, M.H.G.; Blunt, J.W.; Mulligan, P.E.; Beasley, V.R.; Dahlem, A.M.; et al. Nodularin, microcystin, and the configuration of ADDA. J. Am. Chem. Soc. 1988, 110, 8557–8558. [Google Scholar] [CrossRef]
- Fayad, P.B.; Roy-Lachapelle, A.; Duy, S.V.; Prévost, M.; Sauvé, S. On-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry for the analysis of cyanotoxins in algal blooms. Toxicon 2015, 108, 167–175. [Google Scholar] [CrossRef] [PubMed]
- James, K.J.; Furey, A.; Sherlock, I.R.; Stack, M.A.; Twohig, M.; Caudwell, F.B.; Skulberg, O.M. Sensitive determination of anatoxin-a, homoanatoxin-a and their degradation products by liquid chromatography with fluorimetric detection. J. Chromatogr. A 1998, 798, 147–157. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Sauve, S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5487–5501. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.; Erler, K.; Hiller, S.; Christian, B.; Luckas, B. Determination of paralytic shellfish poisoning (PSP) toxins in dietary supplements by application of a new HPLC/FD method. Eur. Food Res. Technol. 2006, 224, 147–151. [Google Scholar] [CrossRef]
- Liu, H.; Scott, P. Determination of the cyanobacterial toxin cylindrospermopsin in algal food supplements. Food Addit. Contam. 2011, 28, 786–790. [Google Scholar] [CrossRef] [PubMed]
- McCarron, P.; Logan, A.C.; Giddings, S.D.; Quilliam, M.A. Analysis of β-N-methylamino-l-alanine (BMAA) in Spirulina-containing supplements by liquid chromatography-tandem mass spectrometry. Aquat. Biosyst. 2014, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suchy, P.; Berry, J. Detection of total microcystin in fish tissues based on Lemieux oxidation and recovery of 2-methyl-3-methoxy-4-phenylbutanoic acid (MMPB) by solid-phase microextraction gas chromatography-mass spectrometry (SPME-GC/MS). Int. J. Environ. Anal. Chem. 2012, 92, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Neffling, M.R.; Lance, E.; Meriluoto, J. Detection of free and covalently bound microcystins in animal tissues by liquid chromatography-tandem mass spectrometry. Environ. Pollut. 2010, 158, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Balasubramanian, R. Methods and approaches used for detection of cyanotoxins in environmental samples: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1349–1383. [Google Scholar] [CrossRef]
- Moreira, C.; Ramos, V.; Azevedo, J.; Vasconcelos, V. Methods to detect cyanobacteria and their toxins in the environment. Appl. Microbiol. Biot. 2014, 98, 8073–8082. [Google Scholar] [CrossRef] [PubMed]
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Mazija, L.; Fastner, J.; Dietrich, D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shimamatsu, H. Mass production of spirulina, an edible microalga. In Asian Pacific Phycology in the 21st Century: Prospects and Challenges; Springer: Berlin, Germany, 2004; pp. 39–44. [Google Scholar]
- Costa, J.A.V.; de Morais, M.G.; Dalcanton, F.; Reichert, C.d.C.; Durante, A.J. Simultaneous cultivation of Spirulina platensis and the toxigenic cyanobacteria Microcystis aeruginosa. Z Naturforsch C 2006, 61, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. B 2016, 283. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A.; Otero, P.; Alfonso, A.; Ramos, V.; Vasconcelos, V.; Aráoz, R.; Molgó, J.; Vieytes, M.R.; Botana, L.M. Detection of anatoxin-a and three analogs in Anabaena spp. Cultures: New fluorescence polarization assay and toxin profile by lc-ms/ms. Toxins 2014, 6, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Chan, E. The role of complementary and alternative medicine in attention-deficit hyperactivity disorder. J. Dev. Behav. Pediatr. 2002, 23, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Mehta, U. Supplementary effect of spirulina on hematological status of rats during pregnancy and lactation. Plant Foods Hum. Nutr. 1998, 52, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Karlsson, O.; Banack, S.A.; Brandt, I. Transfer of developmental neurotoxin β-N-methylamino-l-alanine (BMAA) via milk to nursed offspring: Studies by mass spectrometry and image analysis. Toxicol. Lett. 2016, 258, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Ernst, B.; Day, B. Human consumer death and algal supplement consumption: A post mortem assessment of potential microcystin-intoxication via microcystin immunoistochemical (mcich) analyses. In Proceedings of the 7th International Conference on Toxic Cyanobacteria, Rio de Janeiro, Brazil, 5–10 August 2007; p. 132.
- Iwasa, M.; Yamamoto, M.; Tanaka, Y.; Kaito, M.; Adachi, Y. Spirulina-associated hepatotoxicity. Am. J. Gastroenterol. 2002, 97, 3212–3213. [Google Scholar] [CrossRef] [PubMed]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. CRC Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular aspects of microcystin-induced hepatotoxicity and hepatocarcinogenesis. J. Environ. Sci. Health C 2010, 28, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M. Carcinogenic aspects of protein phosphatase 1 and 2a inhibitors. In Marine Toxins as Research Tools; Fusetani, N., Kem, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Lesley, V.; D’Anglada, J.S. Drinking Water Health Advisory for the Cyanobacterial Microcystin Toxins; USEPA: Washington, DC, USA, 2015. [Google Scholar]
- Solliec, M.; Massé, D.; Sauvé, S. Analysis of trimethoprim, lincomycin, sulfadoxin and tylosin in swine manure using laser diode thermal desorption-atmospheric pressure chemical ionization-tandem mass spectrometry. Talanta 2014, 128, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Roy-Lachapelle, A.; Prévost, M.; Tremblay, P.; Solliec, M.; Sauvé, S. Ultra-fast analysis of anatoxin-a using laser diode thermal desorption-atmospheric pressure chemical ionization-tandem mass spectrometry: Validation and resolution from phenylalanine. Toxicon 2013, 61, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, M.; Fayad, P.B.; Sauvé, S. Development of a new multi-residue laser diode thermal desorption atmospheric pressure chemical ionization tandem mass spectrometry method for the detection and quantification of pesticides and pharmaceuticals in wastewater samples. Anal. Chim. Acta 2012, 754, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Viglino, L.; Prévost, M.; Sauvé, S. High throughput analysis of solid-bound endocrine disruptors by LDTD-APCI-MS/MS. J. Environ. Monit. 2011, 13, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.A.; Tremblay, P.; Picard, P.; Gagnon, C.; Sauvé, S. High-throughput quantitation of seven sulfonamide residues in dairy milk using laser diode thermal desorption-negative mode atmospheric pressure chemical ionization tandem mass spectrometry. J. Agric. Food Chem. 2010, 58, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Fayad, P.B.; Prévost, M.; Sauvé, S. Laser diode thermal desorption/atmospheric pressure chemical ionization tandem mass spectrometry analysis of selected steroid hormones in wastewater: Method optimization and application. Anal. Chem. 2010, 82, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Solliec, M.; Roy-Lachapelle, A.; Sauve, S. Quantitative performance of liquid chromatography coupled to q-exactive high resolution mass spectrometry (HRMS) for the analysis of tetracyclines in a complex matrix. Anal. Chim. Acta 2015, 853, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, G.; Randak, T.; Lindberg, R.H.; Grabic, R. Comparison of the quantitative performance of a q-exactive high-resolution mass spectrometer with that of a triple quadrupole tandem mass spectrometer for the analysis of illicit drugs in wastewater. Rapid. Commun. Mass Spectron. 2013, 27, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, Z.; Kellmann, M.; Huhmer, A. HR/AM targeted peptide quantitation on a Q Exactive MS: A unique combination of high selectivity, sensitivity, and throughput. Thermo Fish. Sci. Appl. Note 2012, 554, 1–12. [Google Scholar]
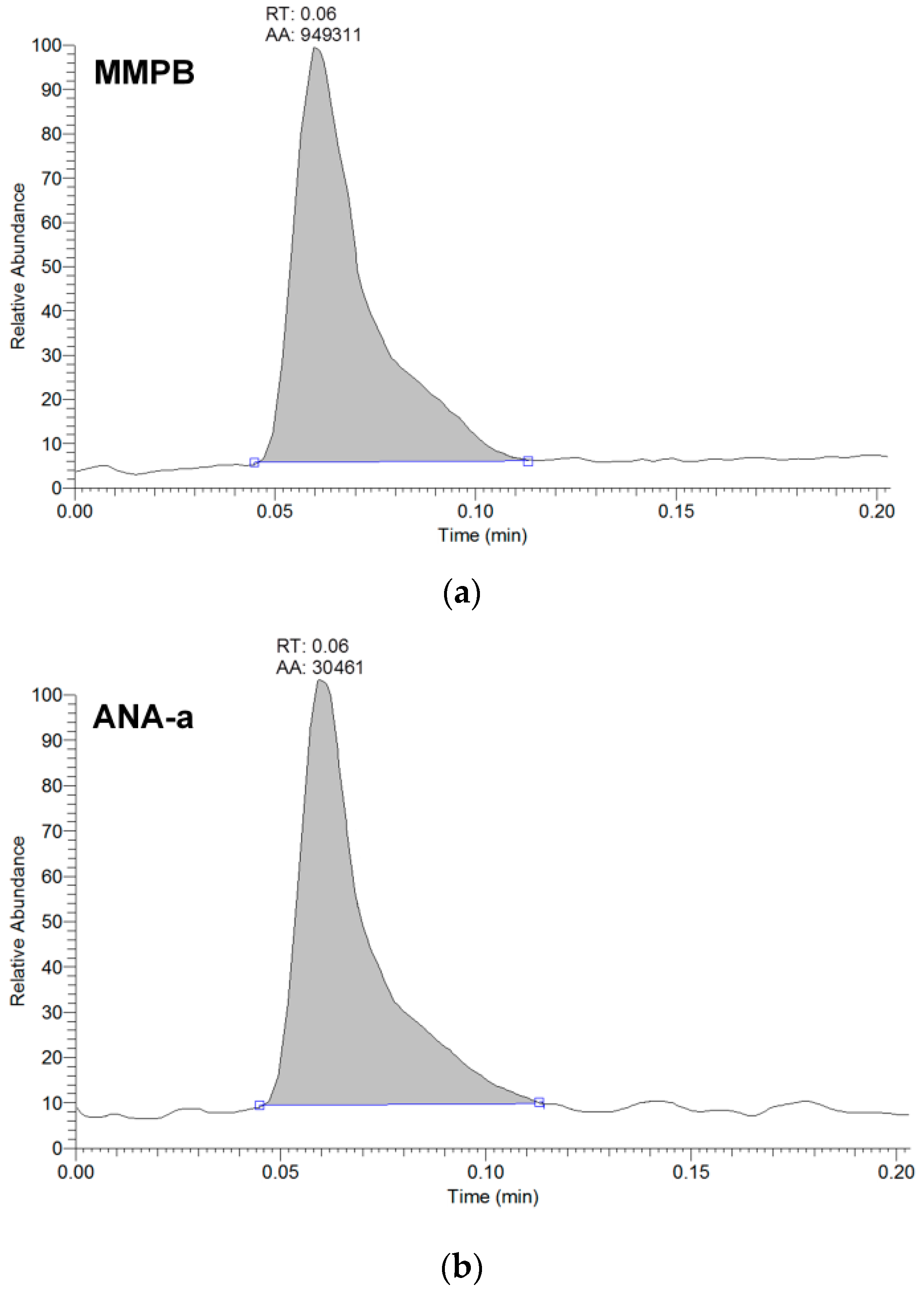

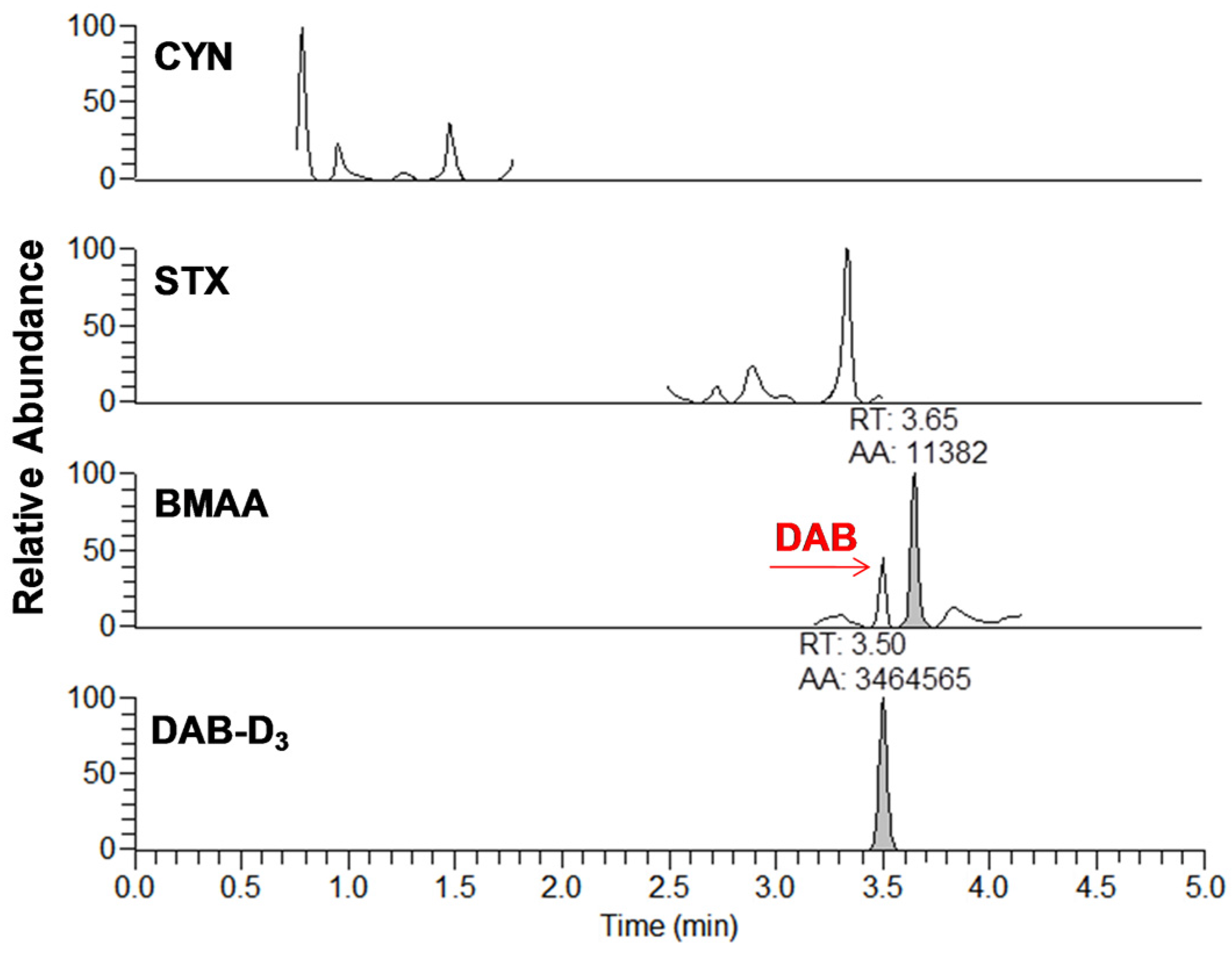
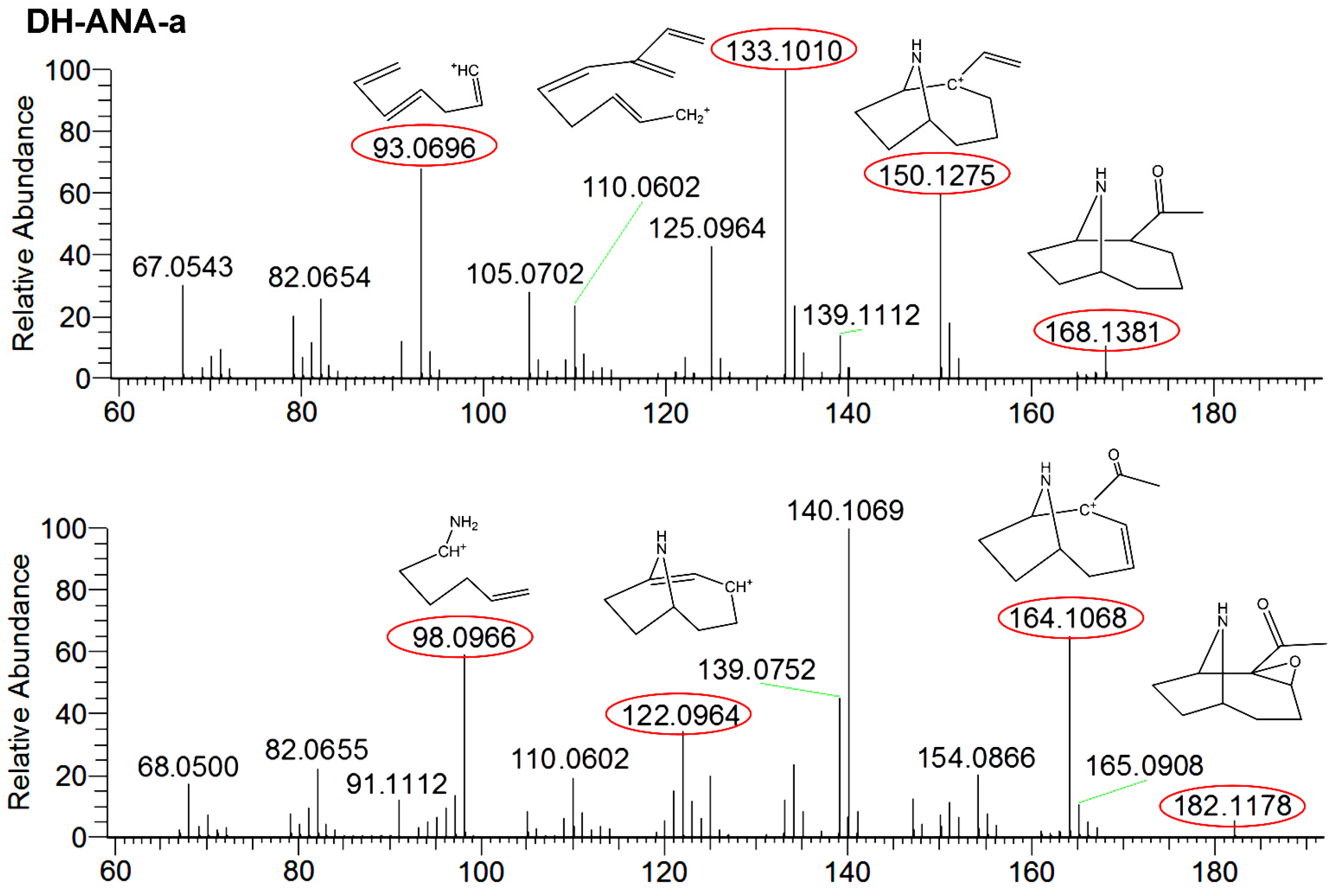
| Compounds a | Accuracy (RE %) | Intraday (RSD %) | Interday (RSD %) | Recovery (%) | R2 | Linearity Range (µg∙g−1) | MDL (µg∙g−1) | MQL (µg∙g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | H | L | H | L | H | L | H | ||||||
| 1 | MMPB b,c | 8 | 6 | 7 | 9 | 12 | 12 | 79 (9) | 85 (10) | 0.9988 | 0.6–20 | 0.2 | 0.6 |
| ANA-a b | 6 | 5 | 6 | 7 | 11 | 13 | 95 (7) | 92 (8) | 0.9987 | 0.8–20 | 0.3 | 0.8 | |
| 2 | ANA-a | 9 | 7 | 5 | 5 | 8 | 9 | 89 (7) | 90 (8) | 0.9995 | 0.1–20 | 0.04 | 0.1 |
| MC-RR | 7 | 8 | 7 | 3 | 11 | 8 | 86 (9) | 89 (8) | 0.9993 | 0.03–20 | 0.01 | 0.03 | |
| MC-YR | 8 | 4 | 5 | 2 | 12 | 9 | 85 (7) | 90 (6) | 0.9992 | 0.07–20 | 0.02 | 0.07 | |
| MC-LR | 6 | 3 | 7 | 6 | 9 | 7 | 91 (10) | 95 (10) | 0.9995 | 0.06–20 | 0.02 | 0.06 | |
| MC-LA | 6 | 4 | 5 | 1 | 9 | 11 | 92 (7) | 91 (9) | 0.9990 | 0.03–20 | 0.01 | 0.03 | |
| MC-LY | 7 | 6 | 8 | 5 | 7 | 9 | 87 (8) | 92 (8) | 0.9995 | 0.1–20 | 0.03 | 0.1 | |
| MC-LW | 10 | 7 | 9 | 4 | 9 | 10 | 96 (7) | 94 (9) | 0.9993 | 0.05–20 | 0.02 | 0.05 | |
| MC-LF | 8 | 5 | 10 | 2 | 11 | 8 | 93 (9) | 90 (7) | 0.9992 | 0.1–20 | 0.03 | 0.1 | |
| 3 | CYN | 11 | 10 | 11 | 9 | 15 | 12 | 88 (10) | 85 (7) | 0.9990 | 0.1–20 | 0.04 | 0.1 |
| STX | 12 | 10 | 12 | 8 | 13 | 13 | 83 (11) | 86 (9) | 0.9989 | 0.3–20 | 0.1 | 0.3 | |
| BMAA | 8 | 7 | 8 | 8 | 10 | 12 | 97 (6) | 94 (5) | 0.9993 | 0.08–20 | 0.02 | 0.08 | |
| No. | MCs tot a,b | ANA-a a | ANA-a | DH-ANA-a c | E-ANA-a c | MC-RR | MC-YR | MC-LR | MC-LA | MC-LY | MC-LW | MC-LF | CYN | STX | BMAA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 5 | ND | ND | ND | ND | 7.2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | 0.25 (10) | ND | ND | 0.41 | ND | ND | ND | ND | 0.3 (13) | ND | ND | ND | ND | ND | ND |
| 9 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 11 | 2.5 (104) | ND | ND | ND | 8.1 | ND | 1.6 (67) | 0.06 (3) | 0.08 (3) | ND | ND | ND | ND | ND | ND |
| 12 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 13 | 0.6 (25) | ND | ND | ND | ND | ND | ND | 0.03 (1) | ND | ND | ND | ND | ND | ND | ND |
| 14 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 15 | 16.4 (683) | ND | 0.30 (5) | 2.2 | 1.8 | 0.8 (33) | ND | 8.6 (358) | 2.2 (92) | 0.04 (17) | ND | ND | ND | ND | 0.08 |
| 16 | 4.5 (188) | ND | ND | ND | 0.28 | ND | ND | 3.6 (150) | 1.2 (50) | ND | ND | ND | ND | ND | ND |
| 17 | 3.3 (138) | 0.35 (6) | 0.32 (5) | 5.8 | ND | ND | 0.18 (8) | 0.04 (17) | 2.6 (108) | ND | ND | ND | ND | ND | 0.44 |
| 18 | 0.8 (33) | ND | ND | ND | ND | ND | ND | 0.52 (22) | ND | ND | ND | ND | ND | ND | ND |
| No. | Content | Place of Harvest/Purchased From | Exp. Date | Recommended Maximum Dosage/Day | Specifics |
|---|---|---|---|---|---|
| 1 | Spirulina | Pacific/Store | 01/2006 | 4000 mg | — |
| 2 | Spirulina | NA/Store | 02/2006 | 3000 mg | — |
| 3 | Spirulina | Hawaii/Store | 06/2006 | 2000 mg | — |
| 4 | Spirulina | NA/Store | 02/2007 | 2000 mg | — |
| 5 | Spirulina | NA/Store | 08/2007 | 4500 mg | Not recommended for children |
| 6 | Spirulina | NA/Store | 12/2007 | 1440 mg | — |
| 7 | Spirulina | NA/Store | 01/2008 | 3000 mg | Not recommended for children |
| 8 | Spirulina | Hawaii/Store | 06/2008 | 1200 mg | — |
| 9 | Spirulina | Hawaii/Store | 05/2009 | 1200 mg | — |
| 10 | Spirulina | NA/Store | 06/2009 | 3040 mg | Not recommended for children |
| 11 | Spirulina | NA/Store | 07/2015 | 3000 mg | — |
| 12 | Spirulina | NA/Internet | 05/2015 | 3000 mg | — |
| 13 | Spirulina | NA/Internet | 06/2015 | 3000 mg | — |
| 14 | Spirulina | Hawaii/Internet | 06/2015 | 3000 mg | — |
| 15 | A. flos-aquae | NA/Store | 07/2015 | 2000 mg | Not recommended for children |
| 16 | A. flos-aquae | Klamath Lake/Internet | 05/2015 | 3000 mg | Not recommended for children |
| 17 | A. flos-aquae | Klamath Lake/Internet | 06/2015 | 800 mg | — |
| 18 | A. flos-aquae | Klamath Lake/Internet | 06/2015 | 1000 mg | — |
| MCs and ANA-a | CYN, STX and BMAA | |||||
|---|---|---|---|---|---|---|
| Time (min) | A (%) | B (%) | C (%) | Time (min) | A (%) | B (%) |
| 0.00 | 75 | 25 | 0 | 0.00 | 70 | 30 |
| 0.35 | 45 | 55 | 0 | 2.00 | 10 | 90 |
| 1.15 | 45 | 55 | 0 | 4.00 | 0 | 100 |
| 1.16 | 55 | 0 | 45 | 6.00 | 0 | 100 |
| 2.95 | 55 | 0 | 45 | 6.01 | 70 | 30 |
| 4.51 | 5 | 0 | 95 | 10.00 | 70 | 30 |
| 4.66 | 75 | 25 | 0 | |||
| 7.00 | 75 | 25 | 0 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. https://doi.org/10.3390/toxins9030076
Roy-Lachapelle A, Solliec M, Bouchard MF, Sauvé S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins. 2017; 9(3):76. https://doi.org/10.3390/toxins9030076
Chicago/Turabian StyleRoy-Lachapelle, Audrey, Morgan Solliec, Maryse F. Bouchard, and Sébastien Sauvé. 2017. "Detection of Cyanotoxins in Algae Dietary Supplements" Toxins 9, no. 3: 76. https://doi.org/10.3390/toxins9030076








