Acute Oral Toxicity of Tetrodotoxin in Mice: Determination of Lethal Dose 50 (LD50) and No Observed Adverse Effect Level (NOAEL)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yotsu-Yamashita, M.; Sugimoto, A.; Takai, A.; Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999, 289, 1688–1696. [Google Scholar] [PubMed]
- Suehiro, M. Historical review on chemical and medical studies of globefish toxin before world war II. Yakushigaku Zasshi 1994, 29, 428–434. [Google Scholar] [PubMed]
- Jal, S.; Khora, S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015, 119, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Ma, T.; Gong, X.; Zhang, N.; Bao, B. Identification of tetrodotoxin-producing bacteria from goby yongeichthys criniger. Toxicon 2015, 104, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, M.N.; Stokes, A.N.; Rossler, D.C.; Lotters, S. Tetrodotoxin. Curr. Biol. 2016, 26, R870–R872. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Takatani, T. Toxicity of pufferfish takifugu rubripes cultured in netcages at sea or aquaria on land. Comp. Biochem. Phys. Part D Genom. Proteom. 2006, 1, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Levinson, S.R. Tetrodotoxin, saxitoxin, and the molecular biology of the sodium channel. Ann. N. Y. Acad. Sci. 1986, 479, 1–445. [Google Scholar]
- Catterall, W.A. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 1992, 72 (Suppl. 4), S15–S48. [Google Scholar] [PubMed]
- Lee, C.H.; Ruben, P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Noguchi, T. Tetrodotoxin poisoning. Adv. Food Nutr. Res. 2007, 52, 141–236. [Google Scholar] [PubMed]
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Euro Surveill. 2015, 20, 1–33. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.H.; Zhao, X.N.; Wei, C.H.; Rong, K.T. Immunologic protection of anti-tetrodotoxin vaccines against lethal activities of oral tetrodotoxin challenge in mice. Int. Immunopharmacol. 2005, 5, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Rivera, V.R.; Poli, M.A.; Bignami, G.S. Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice. Toxicon 1995, 33, 1231–1237. [Google Scholar] [CrossRef]
- Kao, C.Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol. Rev. 1966, 18, 997–1049. [Google Scholar] [PubMed]
- Turner, A.D.; Higgins, C.; Higman, W.; Hungerford, J. Potential threats posed by tetrodotoxins in UK waters: Examination of detection methodology used in their control. Mar. Drugs 2015, 13, 7357–7376. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H. Differences in susceptibility of mouse strains to tetrodotoxin. Toxicon 2016, 119, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Moczydlowski, E.G. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M.; Hess, P.; Munday, R.; Nathalie, A.; DeGrasse, S.L.; Feeley, M.; Suzuki, T.; Van den Berg, M.; Fattori, V.; Gamarro, E.G.; et al. Derivation of toxicity equivalency factors for marine biotoxins associated with bivalve molluscs. Trends Food Sci. Technol. 2017, 59, 15–24. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD)/OCDE. Acute Oral Toxicity-Up and Down Procedure; OECD Guidelines for The testing of Chemicals 425; Organisation for Economic Co-operation and Development: Paris, France, 2008; pp. 1–27. [Google Scholar]
- Tsujimura, K.; Yamanouchi, K. A rapid method for tetrodotoxin (TTX) determination by LC-MS/MS from small volumes of human serum, and confirmation of pufferfish poisoning by TTX monitoring. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 977–983. [Google Scholar] [CrossRef] [PubMed]
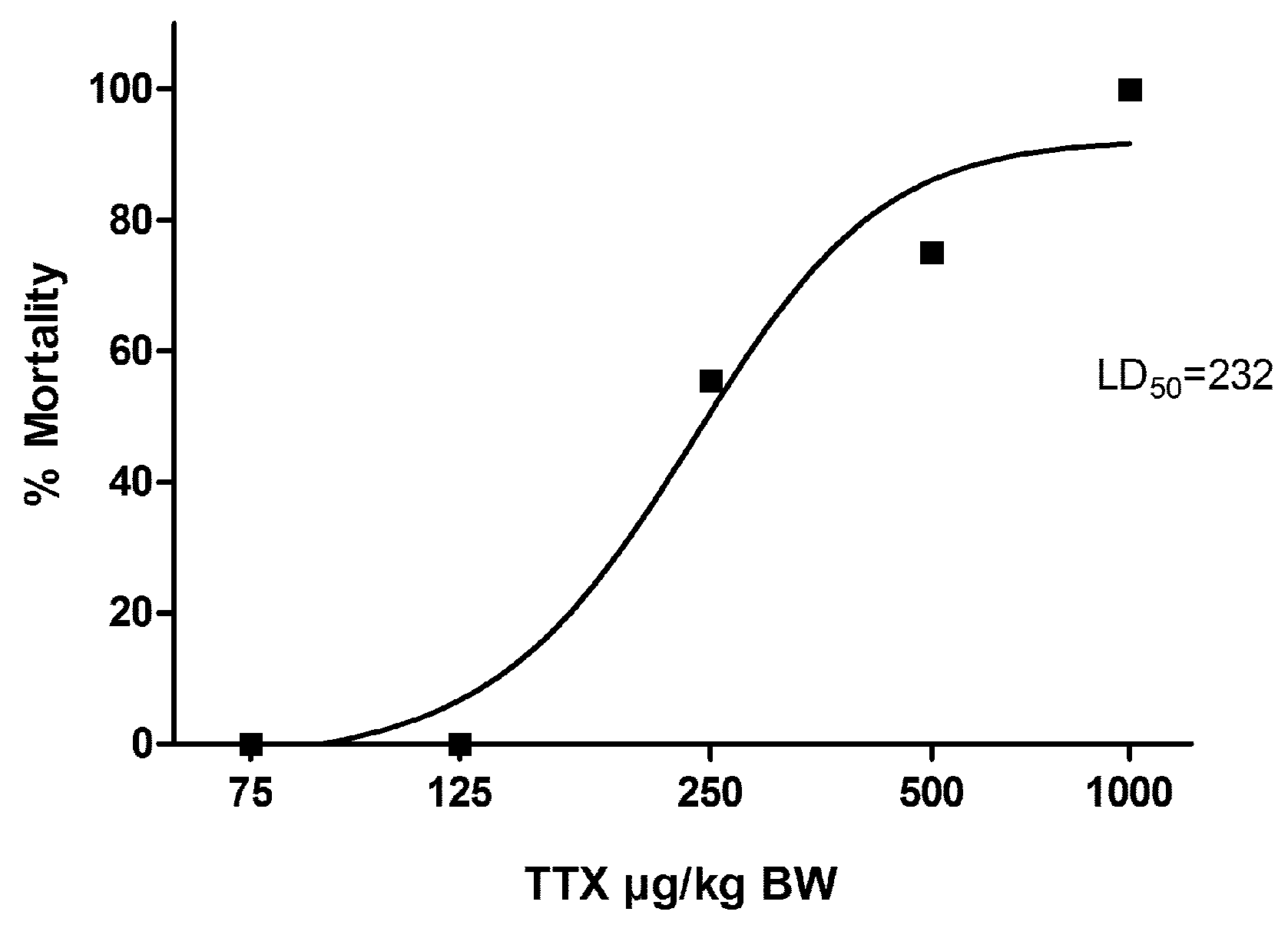
| Dose (µg/kg) | Mortality | Survival Times (min) |
|---|---|---|
| 1000 | 3/3 | 7, 18, 37 |
| 500 | 4/5 | 58, 3, 2, 1 |
| 250 | 4/7 | 100, 7, 19, 54 |
| 125 | 0/9 | >120 |
| 75 | 0/9 | >120 |
| 25 | 0/9 | >120 |
| Symptoms | TTX Dose (µg/kg) | |||||
|---|---|---|---|---|---|---|
| 1000 | 500 | 250 | 125 | 75 | 25 | |
| Apathy | 3/3 | 5/5 | 7/7 | 9/9 | 0/9 | 0/9 |
| Piloerection | 0/3 | 0/5 | 1/7 | 2/9 | 0/9 | 0/9 |
| Paralysis of extremities | 3/3 | 2/5 | 2/7 | 0/9 | 0/9 | 0/9 |
| Seizures | 3/3 | 2/5 | 2/7 | 0/9 | 0/9 | 0/9 |
| Circling | 0/3 | 2/5 | 1/7 | 0/9 | 0/9 | 0/9 |
| Squint eyes | 0/3 | 1/5 | 0/7 | 0/9 | 0/9 | 0/9 |
| Dose (µg/kg) | TTX Level (ng/mL) |
|---|---|
| 500 | 6.46 ± 1.91 |
| 250 | 1.29 ± 0.18 |
| 125 | <LOQ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abal, P.; Louzao, M.C.; Antelo, A.; Alvarez, M.; Cagide, E.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Acute Oral Toxicity of Tetrodotoxin in Mice: Determination of Lethal Dose 50 (LD50) and No Observed Adverse Effect Level (NOAEL). Toxins 2017, 9, 75. https://doi.org/10.3390/toxins9030075
Abal P, Louzao MC, Antelo A, Alvarez M, Cagide E, Vilariño N, Vieytes MR, Botana LM. Acute Oral Toxicity of Tetrodotoxin in Mice: Determination of Lethal Dose 50 (LD50) and No Observed Adverse Effect Level (NOAEL). Toxins. 2017; 9(3):75. https://doi.org/10.3390/toxins9030075
Chicago/Turabian StyleAbal, Paula, M. Carmen Louzao, Alvaro Antelo, Mercedes Alvarez, Eva Cagide, Natalia Vilariño, Mercedes R. Vieytes, and Luis M. Botana. 2017. "Acute Oral Toxicity of Tetrodotoxin in Mice: Determination of Lethal Dose 50 (LD50) and No Observed Adverse Effect Level (NOAEL)" Toxins 9, no. 3: 75. https://doi.org/10.3390/toxins9030075







