The First Cry2Ac-Type Protein Toxic to Helicoverpa armigera: Cloning and Overexpression of Cry2ac7 Gene from SBS-BT1 Strain of Bacillus thuringiensis
Abstract
:1. Introduction
2. Results
2.1. Genotyping for cry2 Gene
2.2. Sequencing of the cry2ac7 Gene
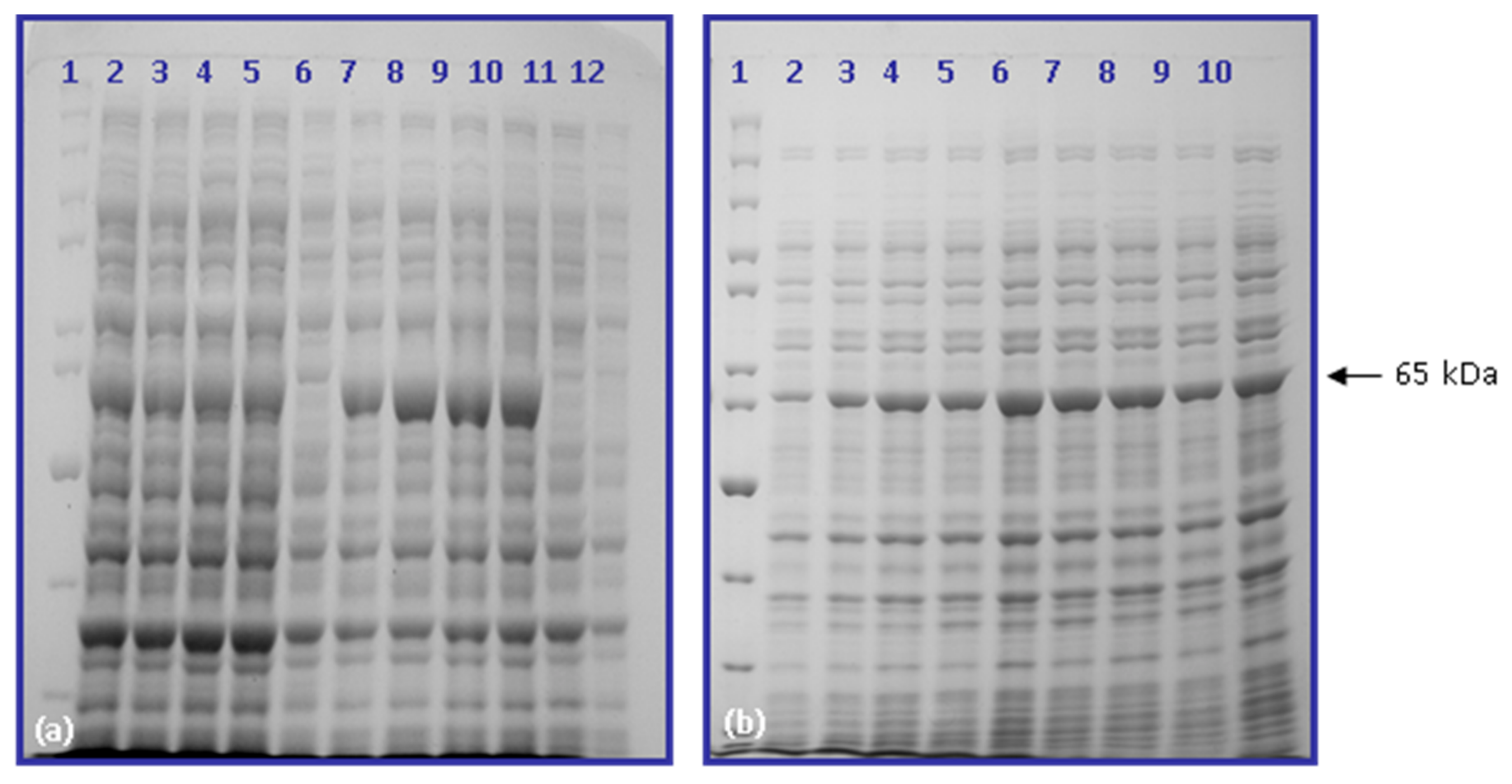
2.3. Hyperexpression of cry2ac7 Gene in Escherichia coli (BL21 Codon Plus)
2.4. Partial Purification of Cry2Ac7 Inclusion Bodies
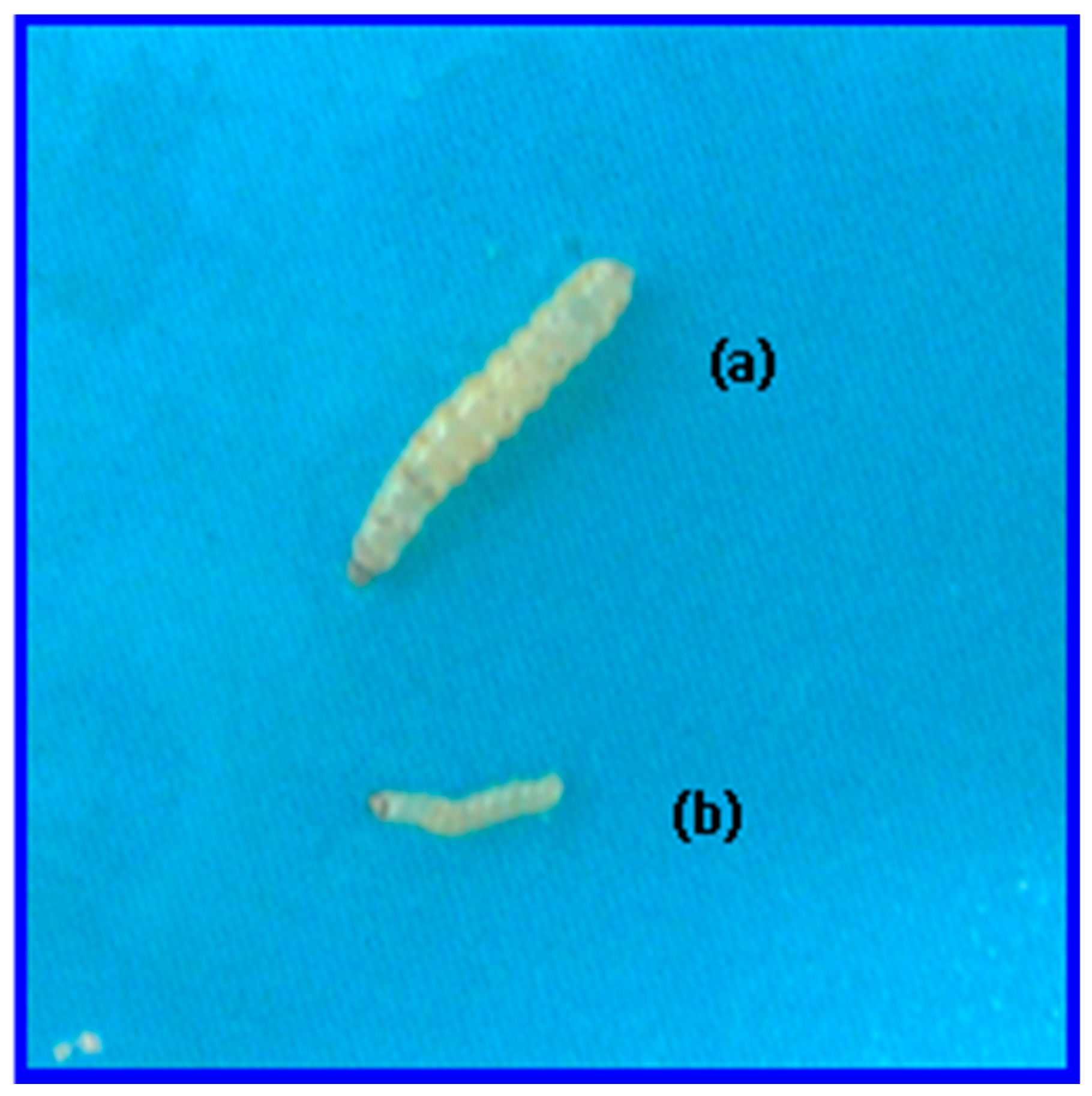
2.5. Toxicity Assay of Cry2Ac7 Toxin against Helicoverpa armigera
3. Discussion
3.1. Profile of Cry2A Subtypes and Toxicity
3.2. Sequence Homology of Cry2Ac7 Protein with Other Related Proteins
4. Materials and Methods
4.1. Sampling and Isolation of Bacillus thuringiensis Strains
4.2. Ribotyping
4.3. DNA Isolation and Genotyping
4.4. Cloning and Sequencing of the cry2ac7 Gene
- pET21a-N 5′ GCTCGGTACCTCGCTGAATGCATCTAGCATATGAATAC 3′
- M13 R 5′-d(GAGCGGATAACAATTTCACACAGG)-3′)
4.5. Sub Cloning in Expression Vector pET22b
4.6. Expression of cry2ac7 Gene in Escherichia coli (BL21C+)
4.7. Purification of Cry2Ac7
4.8. Toxicity Assay of Cloned Toxin against H. armigera and M. domestica
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Johnson, D.E.; Oppert, B.; McGaughey, W.H. Spore coat protein synergizes Bacillus thuringiensis crystal toxicity for the indianmeal moth. Curr. Microbiol. 1998, 36, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.; Weiser, J.; Mikes, L.; Kolarova, L. The effect of Bacillus thuringiensis M-exotoxin on trematode cercariae. J. Invertebr. Pathol. 1996, 68, 41–49. [Google Scholar] [CrossRef] [PubMed]
- De Maagd, R.A.; Bravo, A.; Crickmore, N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001, 17, 193–199. [Google Scholar] [CrossRef]
- Ben-Dov, E.; Zaritsky, A.; Dahan, E.; Barak, Z.; Sinai, R.; Manasherob, R.; Khamraev, A.; Troitskaya, E.; Dubitsky, A.; Berezina, N.; et al. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl. Environ. Microbiol. 1997, 63, 4883–4890. [Google Scholar] [PubMed]
- Crickmore, N.; Zeigler, D.R.; Feitelson, J.; Schnepf, E.; Van Rie, J.; Lereclus, D.; Baum, J.; Dean, D.H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 807–813. [Google Scholar] [PubMed]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [PubMed]
- Ben-Dov, E.; Manasherob, R.; Zaritsky, A.; Barak, Z.; Margalith, Y. PCR analysis of cry7 genes in Bacillus thuringiensis by the five conserved blocks of toxins. Curr. Microbiol. 2001, 42, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, E.; Einav, M.; Peleg, N.; Boussiba, S.; Zaritsky, A. Restriction map of the 125-kilobase plasmid of Bacillus thuringiensis subsp. Israelensis carrying the genes that encode delta-endotoxins active against mosquito larvae. Appl. Environ. Microbiol. 1996, 62, 3140–3145. [Google Scholar] [PubMed]
- Thomas, D.J.; Morgan, J.A.; Whipps, J.M.; Saunders, J.R. Plasmid transfer between Bacillus thuringiensis subsp. Israelensis strains in laboratory culture, river water, and dipteran larvae. Appl. Environ. Microbiol. 2001, 67, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Ellar, D.J.; Bishop, A.; Johnson, C.; Lin, S.; Hart, E.R. Characterization of a Bacillus thuringiensis delta-endotoxin which is toxic to insects in three orders. J. Invertebr. Pathol. 2000, 76, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Widner, W.R.; Whiteley, H.R. Two highly related insecticidal crystal proteins of Bacillus thuringiensis subsp. Kurstaki possess different host range specificities. J. Bacteriol. 1989, 171, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; McLaughlin, R.E. Isolation of a protein from the parasporal crystal of Bacillus thuringiensis var. Kurstaki toxic to the mosquito larva, aedes taeniorhynchus. Biochem. Biophys. Res. Commun. 1981, 103, 414–421. [Google Scholar] [CrossRef]
- Donovan, W.P.; Dankocsik, C.; Gilbert, M.P. Molecular characterization of a gene encoding a 72-kilodalton mosquito-toxic crystal protein from Bacillus thuringiensis subsp. Israelensis. J. Bacteriol. 1988, 170, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Dankocsik, C.; Donovan, W.P.; Jany, C.S. Activation of a cryptic crystal protein gene of Bacillus thuringiensis subspecies kurstaki by gene fusion and determination of the crystal protein insecticidal specificity. Mol. Microbiol. 1990, 4, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cao, X.L.; Bai, Y.Y.; Aronson, A.I. Sequence of an operon containing a novel delta-endotoxin gene from Bacillus thuringiensis. FEMS Microbiol. Lett. 1991, 65, 31–35. [Google Scholar] [PubMed]
- Crickmore, N.; Ellar, D.J. Involvement of a possible chaperonin in the efficient expression of a cloned cryiia delta-endotoxin gene in Bacillus thuringiensis. Mol. Microbiol. 1992, 6, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Wheeler, V.C.; Ellar, D.J. Use of an operon fusion to induce expression and crystallisation of a Bacillus thuringiensis delta-endotoxin encoded by a cryptic gene. Mol. Gen. Genet. 1994, 242, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Dennehy, T.J.; Sims, M.A.; Larkin, K.; Head, G.P.; Moar, W.J.; Carriere, Y. Control of resistant pink bollworm (pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin cry2Ab. Appl. Environ. Microbiol. 2002, 68, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
- Kota, M.; Daniell, H.; Varma, S.; Garczynski, S.F.; Gould, F.; Moar, W.J. Overexpression of the Bacillus thuringiensis (Bt) cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc. Natl. Acad. Sci. USA 1999, 96, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; James, W.; Bird, L.J.; Beard, C. Resistance to the cry1Ac delta-endotoxin of Bacillus thuringiensis in the cotton bollworm, helicoverpa armigera (lepidoptera: Noctuidae). J. Econ. Entomol. 2003, 96, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, S.B.; Husnain, T.; Riazuddin, S.; Masson, L.; Christou, P. Effective control of yellow stem borer and rice leaf folder in transgenic rice indica varieties basmati 370 and m 7 using the novel δ-endotoxin cry2A Bacillus thuringiensis gene. Mol. Breed. 1998, 4, 7. [Google Scholar] [CrossRef]
- Zaidi, M.A.; Mohammadi, M.; Postel, S.; Masson, L.; Altosaar, I. The Bt gene cry2Aa2 driven by a tissue specific ST-LS1 promoter from potato effectively controls heliothis virescens. Transgenic Res. 2005, 14, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Shakoori, A.R. Characterization of cry2A-type gene(s) from pakistani isolates of Bacillus thuringiensis toxic to lepidopteran and dipteran insects. Pak. J. Zool. 2010, 42, 181–193. [Google Scholar]
- Finney, D.J. Probit Analysis; University Press: London, UK, 1952. [Google Scholar]
- Hansen, B.M.; Salamitou, S. Virulence of Bacillus thuringiensis. In Entomopathogenic Bacteria: From Laboratory to Field Applications; Charles, J.F., Delecluse, A., Nielsen-LeRoux, C., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2000; pp. 41–64. [Google Scholar]
- Widner, W.R.; Whiteley, H.R. Location of the dipteran specificity region in a lepidopteran-dipteran crystal protein from Bacillus thuringiensis. J. Bacteriol. 1990, 172, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lin, J.; Luo, L.; Guan, C.Y.; Zhang, Q.L.; Guan, Y.; Zhang, Y.; Ji, J.T.; Huang, Z.P.; Guan, X. A novel Bacillus thuringiensis strain LLB6, isolated from bryophytes, and its new cry2Ac-type gene. Lett. Appl. Microbiol. 2007, 44, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Sauka, D.H.; Cozzi, J.G.; Benintende, G.B. Screening of cry2 genes in Bacillus thuringiensis isolates from Argentina. Antonie Leeuwenhoek 2005, 88, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Almond, B.D.; Dean, D.H. Suppression of protein structure destabilizing mutations in Bacillus thuringiensis δ-endotoxin by second site mutations. Biochemistry 1993, 32, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Ejiofor, A.O.; Johnson, T. Physiological and molecular detection of crystalliferous Bacillus thuringiensis strains from habitats in the south central United States. J. Ind. Microbiol. Biotechnol. 2002, 28, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.G. Bergey’s Manual of Derterminative Bacteriology, 9th ed.; Lippincott Williams & Wilkins: London, UK, 1994. [Google Scholar]
- Sacchi, C.T.; Whitney, A.M.; Mayer, L.W.; Morey, R.; Steigerwalt, A.; Boras, A.; Weyant, R.S.; Popovic, T. Sequencing of 16 s RNA gene: A rapid tool for identification of Bacillus anthracis. Emerg. Infect. Dis. 2002, 8, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Kronstad, J.W.; Schnepf, H.E.; Whiteley, H.R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J. Bacteriol. 1983, 154, 419–428. [Google Scholar] [PubMed]
- Alberola, T.M.; Aptosoglou, S.; Arsenakis, M.; Bel, Y.; Delrio, G.; Ellar, D.J.; Ferre, J.; Granero, F.; Guttmann, D.M.; Koliais, S.; et al. Insecticidal activity of Bacillus thuringiensis on larvae and adults of Bactrocera oleae Gmelin (Diptera:Tephritidae). J. Invertebr. Pathol. 1999, 74, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, A.; Weinstein, D. Assay of proteins in the presence of interfering materials. Anal. Biochem. 1976, 70, 241–250. [Google Scholar] [CrossRef]
- Ahmed, M.; McCaffery, A.R. Elucidation of detoxicsation mechanisms involved in resistance to insecticides against third instar larvae of field selected strain of Helicoverpa armigera with the use of synergists. Pestic. Biochem. Physiol. 1991, 41, 41–52. [Google Scholar] [CrossRef]
- Shakoori, A.R.; Butt, U. Effect of thioacetamide on the develoipment of a dipterpous fly: Morphological studies. Pak. J. Zool. 1979, 11, 315–328. [Google Scholar]


| y2Ac5 | MNNVLNSGRNTTCHAHNVVAHDPFSFEHKSLNTIEKEWKEWRRTDHSLYVAPIVGTVGSF |
| Cry2Ac7 | MNTVLNNGRNTTCHAHNVVAHDPFSFEHKSLNTIEKEWKEWKRTDHSLYVAPIVGTVGSF |
| Cry2Ac1 | MNTVLNNGRNTTCHAHNVVAHDPFSFEHKSLNTIEKEWKEWKRTDHSLYVAPIVGTVGSF |
| **.***.**********************************:****************** | |
| Cry2Ac5 | LLKKVGSLVGKRILSELQNLIFPSGSIDLMQEILRATEQFINQRLNADTLGRVNAELAGL |
| Cry2Ac7 | LLKKVGSLVGKRILSELQNLIFPGGSIDLMQEILRATEQFINQRLNADTLGRVNAELAGL |
| Cry2Ac1 | LLKKVGSLVGKRILSELQNLIFPSGSIDLMQEILRATEQFINQRLNADTLGRVNAELAGL |
| ***********************.************************************ | |
| Cry2Ac5 | QANVAEFNRQVDNFLNLNQNPVPLAIIDSVNTLQQLFLSRLPQFQIQGYQLLLLPLFAQA |
| Cry2Ac7 | QANVAEFNRQVDNFLNPNQNPVPLAIIDSVNTLQQLFLSRLPQFQIQGYQLLLLPLFAQA |
| Cry2Ac1 | QANVAEFNRQVDNFLNPNQNPVPLAIIDSVNTLQQLFLSRLPQFQIQGYQLLLLPLFAQA |
| **************** ******************************************* | |
| Cry2Ac5 | ANLHLSFIRDVILNADEWGISAATVRTYRDHLRNFTRDYSNYCINTYQTAFRGLNTRLHD |
| Cry2Ac7 | ANLHLSFIRDVILNADEWGISAATVRTYRDHLRNFTRDYSNYCINTYQTAFRGLNTRLHD |
| Cry2Ac1 | ANFNLSFIRGVILNADEWGISAATVRTYRDHLRKFHRDYSNYCINPYQTAFRGLNHRLPD |
| **::*****.***********************:* *********.********* ** * | |
| Cry2Ac5 | MLEFRTYMFLNVFEYVSIWSLFKYQSLLVSSGANLYASGSGPTQSFTAQNWPFLYSLFQV |
| Cry2Ac7 | MLEFRTYMFLNVFEYVSIWSLFKYQSLLVSSGANLYASGSGPTQSFTAQNWPFLYSLFQV |
| Cry2Ac1 | MLEFRTYMFLNVFEYVSIWSLFKYQSLLVSSGANLYASGSGPTQSFTAQNWPFLYSLFQV |
| ************************************************************ | |
| Cry2Ac5 | NSNYVLNGLSGARTTITFPNIGGLPGSTTTQTLHFARINYRGGVSSSRIGQANLNQNFNI |
| Cry2Ac7 | NSNYVLNGLSGARTTITFPNIGGLPGSTTTQTLHFARINYRGGVSSSRIGQANLNQNFNI |
| Cry2Ac1 | NSNYVLNGLSGARTTITFPNIGGLP-VYHNSTLHFARINYRGGVSSSRIGQANLNQNFNI |
| ************************* ..***************************** | |
| Cry2Ac5 | STLFNPLQTPFIRSWLDSGTDREGVATSTNWQSGAFETTLLRFSIFSARGNSNFFPDYFI |
| Cry2Ac7 | STLFNPLQTPFIRSWLDSGTDREGVATSTNWQSGAFETTLLRFSIFSARGNSNFFPDYFI |
| Cry2Ac1 | STLFNPLQTPFIRSWLDSGTDREGVATSTNWQSGAFETTLLRFSIFSARGNSNFFPDYFI |
| ************************************************************ | |
| Cry2Ac5 | RNISGVVGTISNADLARPLHFNEIRDIGTTAVASLVTVHNRKNNIYDTHENGTMIHLAPN |
| Cry2Ac7 | RNISGVVGTISNADLARPLHFNEIRDIGTTAVASLVTVHNRKNNIYDTHENGTMIHLAPN |
| Cry2Ac1 | RNISGVVGTISNADLARPLHFNEIRDIGTTAVASLVTVHNRKNNIYDTHENGTMIHLAPN |
| ************************************************************ | |
| Cry2Ac5 | DYTGFTVSPIHATQVNNQIRTFISEKYGNQGDSLRFELSNTTARHTLRGNGNSYNLYLRV |
| Cry2Ac7 | DYTGFTVSPIHATQVNNQIRTFISEKYGNQGDSLRFELSNTTARYTLRGNGNSYNLYLRV |
| Cry2Ac1 | DYTGFTVSPIHATQVNNQIRTFISEKYGNQGDSLRFELSNPTARYTLRGNGNSYNLYLRV |
| ****************************************.***:*************** | |
| Cry2Ac5 | SSIGSSTIRVTINGRVYTANVNTTTNNDGVLDNGARFSDINIGNVVASANTNVPLDIQVT |
| Cry2Ac7 | SSIGSSTIRVTINGRVYTANVNTTTNNDGVLDNGARFSDINIGNVVASANTNVPLDIQVT |
| Cry2Ac1 | SSIGSSTIRVTINGRVYTANVNTTTNNDGVLDNGARFSDINIGNVVASANTNVPLDIQVT |
| ************************************************************ | |
| Cry2Ac5 | FNGNPQFELMNIMFVPTNIPPLY |
| Cry2Ac7 | FNGNPQFELMNIMFVPTNLPPLY |
| Cry2Ac1 | FNGNPQFELMNIMFVPTNLPPLY |
| ******************:**** |
| Amino Acid | Cry2Ac1 | Cry2Ac5 | Cry2Ac7 |
|---|---|---|---|
| 3 | Asn | Thr | |
| 7 | Ser | Asn | |
| 42 | Arg | Lys | |
| 84 | Ser | Ser | Gly |
| 137 | Leu | Pro | |
| 183 | Phe | Leu | |
| 184 | Asn | His | |
| 190 | Gly | Asp | |
| 214 | Lys | Asn | |
| 216 | His | Thr | |
| 226 | Pro | Thr | |
| 236 | His | Thr | |
| 239 | Pro | His | |
| 326 | Insertion | Gly | |
| 327 | Val | Ser | |
| 328 | Tyr | Thr | |
| 329 | His | Thr | |
| 330 | Asn | Thr | |
| 331 | Ser | Gln | |
| 521 | Pro | Thr | |
| 525 | His | Tyr | |
| 619 | Ile | Leu |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, F.; Shakoori, A.R. The First Cry2Ac-Type Protein Toxic to Helicoverpa armigera: Cloning and Overexpression of Cry2ac7 Gene from SBS-BT1 Strain of Bacillus thuringiensis. Toxins 2017, 9, 358. https://doi.org/10.3390/toxins9110358
Saleem F, Shakoori AR. The First Cry2Ac-Type Protein Toxic to Helicoverpa armigera: Cloning and Overexpression of Cry2ac7 Gene from SBS-BT1 Strain of Bacillus thuringiensis. Toxins. 2017; 9(11):358. https://doi.org/10.3390/toxins9110358
Chicago/Turabian StyleSaleem, Faiza, and Abdul Rauf Shakoori. 2017. "The First Cry2Ac-Type Protein Toxic to Helicoverpa armigera: Cloning and Overexpression of Cry2ac7 Gene from SBS-BT1 Strain of Bacillus thuringiensis" Toxins 9, no. 11: 358. https://doi.org/10.3390/toxins9110358





