Chemodenervation of the Larynx
Abstract
:1. Spasmodic Dysphonia
1.1. History of Spasmodic Dysphonia and Botulinum Neurotoxin
1.2. Spasmodic Dysphonia Classification
1.3. Spasmodic Dysphonia Diagnosis and Presentation
1.4. Spasmodic Dysphonia Treatment
2. Vocal Tremor
3. Muscle Tension Dysphonia
4. Side Effects and Complications
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| AbSD | Abductor spasmodic dysphonia |
| AdSD | Adductor spasmodic dysphonia |
| BoNT | Botulinum neurotoxin |
| BoNT-A | Botulinum neurotoxin type A |
| BoNT-B | Botulinum neurotoxin type B |
| FDA | Food and Drug Administration |
| EMG | Electromyography |
| EP | Evoked potential |
| LEMG | Laryngeal electromyography |
| MSD | Mixed spasmodic dysphonia |
| MUP | Motor unit potential |
| SD | Spasmodic dysphonia |
| TA | Thyroarytenoid |
References
- Lorch, M.P.; Whurr, R. Tracing Spasmodic Dysphonia: The Source of Ludwig Traube’s Priority. Ann. Otol. Rhinol. Laryngol. 2016, 125, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H. Ein Beitrag sur Lehre von den Larynzaffectionen beim Typhus. Abgedr. Berl. Klin. Wochenschr. 1864, 1, 60–61. [Google Scholar]
- Traube, L. Gesammelte Beiträge zur Pathologie und Physiologie; Hirschwald: Berlin, Germany, 1871. [Google Scholar]
- Mackenzie, M. Hoarseness, Loss of Voice, and Stridulous Breathings in Relation to Nervo-Muscular Affectations of the Larynx, 2nd ed.; John Churchill & Sons: London, UK, 1868; Volume 4, 74p. [Google Scholar]
- Lorch, M.P.; Whurr, R. Morell Mackenzie’s Contribution to the Description of Spasmodic Dysphonia. Ann. Otol. Rhinol. Laryngol. 2016, 125, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J. Aphonia Spastica; Wien Med Presse: Vienna, Austria, 1875; Volume 16, pp. 429–432, 477–479. [Google Scholar]
- Luchsinger, R.; Arnold, G.E. Voice, Speech, Language: Clinical Communicology: Its Physiology and Pathology, 1st ed.; Wadsworth Pub.: Belmont, CA, USA, 1965; Volume 17, 812p. [Google Scholar]
- Bicknell, J.M.; Greenhouse, A.H.; Pesch, R.N. Spastic dysphonia. J. Neurol. Neurosurg. Psychiatry 1968, 31, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D. Principles of Internal Medicine, 5th ed.; Harrison, T.R., Ed.; Blakiston Division: New York, NY, USA, 1966. [Google Scholar]
- Blitzer, A.; Brin, M.F.; Fahn, S. Clinical and laboratory characteristics of focal laryngeal dystonia: Study of 110 cases. Laryngoscope 1988, 98 Pt 1, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Lovelace, R.E.; Brin, M.F. Electromyographic findings in focal laryngeal dystonia (spastic dysphonia). Ann. Otol. Rhinol. Laryngol. 1985, 94 Pt 1, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.B.; Rosenbaum, A.; Collins, C.C. Pharmacologic weakening of extraocular muscles. Investig. Ophthalmol. 1973, 12, 924–927. [Google Scholar]
- Scott, A.B. Botulinum toxin injection of eye muscles to correct strabismus. Trans. Am. Ophthalmol. Soc. 1981, 79, 734–770. [Google Scholar] [PubMed]
- Ting, P.T.; Freiman, A. The story of Clostridium botulinum: From food poisoning to Botox. Clin. Med. 2004, 4, 258–261. [Google Scholar] [CrossRef]
- Shogan, A.N.; Rogers, D.J.; Hartnick, C.J. Use of botulinum toxin in pediatric otolaryngology and laryngology. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.F.; Stewart, C.F. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): A 12-year experience in more than 900 patients. Laryngoscope 1998, 108, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Cannito, M.P.; Johnson, J.P. Spastic dysphonia: A continuum disorder. J. Commun. Disord. 1981, 14, 215–233. [Google Scholar] [CrossRef]
- Davis, P.J.; Boone, D.R.; Carroll, R.L. Adductor spastic dysphonia: Heterogeneity of physiologic and phonatory characteristics. Ann. Otol. Rhinol. Laryngol. 1988, 97 Pt 1, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.E.; Brown, J.R.; Litin, E.M. Spastic dysphonia. II. Comparison with essential (voice) tremor and other neurologic and psychogenic dysphonias. J. Speech Hear. Disord. 1968, 33, 219–231. [Google Scholar] [PubMed]
- Brin, M.; Blitzer, A.; Velickovic, M. Movement Disorders of the Larynx. In Neurologic Disorders of the Larynx; Blitzer, A., Brin, M., Ramig, L., Eds.; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 160–195. [Google Scholar]
- Brin, M.F.; Fahn, S.; Blitzer, A. Movement Disorders of the Larynx. In Neurological Disorders of the Larynx; Blitzer, A., Ed.; Thieme: New York, NY, USA, 1992; pp. 240–248. [Google Scholar]
- Blitzer, A.; Brin, M.F. Laryngeal dystonia: A series with botulinum toxin therapy. Ann. Otol. Rhinol. Laryngol. 1991, 100, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.; Kendall, K. Differentiation of spasmodic and psychogenic dysphonias with phonoscopic evaluation. Laryngoscope 1999, 109 Pt 1, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Shahangian, A.; Sung, C.K. Laryngology. In ENT Board Prep: High Yield Review for the Otolaryngology in-Service and Board Exams; Lin, F.Y., Patel, Z.M., Eds.; Springer: New York, NY, USA, 2014; pp. 299–307. [Google Scholar]
- Hillel, A.D. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope 2001, 111 Pt 2 (Suppl. 97), 1–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Han, D.M.; Hou, L.Z. Patterns of spasmodic dysphonia and botulinum toxin injections. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2005, 40, 253–257. [Google Scholar] [PubMed]
- Yang, Q.; Xu, W.; Li, Y. Value of Laryngeal Electromyography in Spasmodic Dysphonia Diagnosis and Therapy. Ann. Otol. Rhinol. Laryngol. 2015, 124, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L.; Adler, C.H.; Berke, G.S. Research priorities in spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008, 139, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Samuels, M.A.; Klein, J.P. Chapter 23. Disorders of Speech and Language. In Adams and Victor’s Principles of Neurology; Ropper, A.H., Samuels, M.A., Klein, J., Eds.; McGraw-Hill Education Medical: New York, NY, USA, 2014. [Google Scholar]
- Dedo, H.H. Recurrent laryngeal nerve section for spastic dysphonia. Ann. Otol. Rhinol. Laryngol. 1976, 85 Pt 1, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.E.; de Santo, L.W. Adductor spastic dysphonia: Three years after recurrent laryngeal nerve resection. Laryngoscope 1983, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, P.C.; Sulica, L.; Fink, D. Spasmodic Dysphonia. In Botulinum Neurotoxin for Head and Neck Disorders; Blitzer, A., Benson, B.E., Guss, J., Eds.; Thieme Medical Publishers: New York, NY, USA, 2012; pp. 49–71. [Google Scholar]
- Blitzer, A.; Brin, M.F.; Fahn, S. Botulinum toxin (BOTOX) for the treatment of “spastic dysphonia” as part of a trial of toxin injections for the treatment of other cranial dystonias. Laryngoscope 1986, 96, 1300–1301. [Google Scholar] [PubMed]
- Brin, M.F.; Fahn, S.; Moskowitz, C. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov. Disord. 1987, 2, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L.; Naunton, R.F.; Sedory, S.E. Effects of botulinum toxin injections on speech in adductor spasmodic dysphonia. Neurology 1988, 38, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L.; Naunton, R.F.; Terada, S. Successful treatment of selected cases of abductor spasmodic dysphonia using botulinum toxin injection. Otolaryngol. Head Neck Surg. 1991, 104, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A. Spasmodic dysphonia and botulinum toxin: Experience from the largest treatment series. Eur. J. Neurol. 2010, 17 (Suppl. 1), 28–30. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, D.; Waters, H.H.; D’Elia, J.B. Botulinum toxin treatment of adductor spasmodic dysphonia: Longitudinal functional outcomes. Laryngoscope 2011, 121, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Van Esch, B.F.; Wegner, I.; Stegeman, I. Effect of Botulinum Toxin and Surgery among Spasmodic Dysphonia Patients. Otolaryngol. Head Neck Surg. 2017, 156, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Remacle, M.; Mishra, P. Vocal outcome after endoscopic thyroarytenoid myoneurectomy in patients with adductor spasmodic dysphonia. Eur. Arch. Otorhinolaryngol. 2014, 271, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Dedo, H.H.; Behlau, M.S. Recurrent laryngeal nerve section for spastic dysphonia: 5- to 14-year preliminary results in the first 300 patients. Ann. Otol. Rhinol. Laryngol. 1991, 100 Pt 1, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.K.; Mendelsohn, A.H.; Blumin, J.H. Long-term follow-up results of selective laryngeal adductor denervation-reinnervation surgery for adductor spasmodic dysphonia. Laryngoscope 2006, 116, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Stong, B.C.; Wise, J. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008, 139, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, M.; Greenberg, D.; Simon, R. (Eds.) Movement Disorders. In Clinical Neurology, 9th ed.; McGra-Hill: New York, NY, USA, 2015. [Google Scholar]
- Sulica, L.; Louis, E.D. Clinical characteristics of essential voice tremor: A study of 34 cases. Laryngoscope 2010, 120, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Gurey, L.E.; Sinclair, C.F.; Blitzer, A. A new paradigm for the management of essential vocal tremor with botulinum toxin. Laryngoscope 2013, 123, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Bansberg, S.F.; Hentz, J.G. Botulinum toxin type A for treating voice tremor. Arch. Neurol. 2004, 61, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Hertegard, S.; Granqvist, S.; Lindestad, P.A. Botulinum toxin injections for essential voice tremor. Ann. Otol. Rhinol. Laryngol. 2000, 109, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Nida, A.; Alston, J.; Schweinfurth, J. Primidone Therapy for Essential Vocal Tremor. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hartman, D.E.; Vishwanat, B. Spastic dysphonia and essential (voice) tremor treated with primidone. Arch. Otolaryngol. 1984, 110, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Justicz, N.; Hapner, E.R.; Josephs, J.S. Comparative effectiveness of propranolol and botulinum for the treatment of essential voice tremor. Laryngoscope 2016, 126, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.C.; Karatayli-Ozgursoy, S.; Best, S. False vocal cord botulinum toxin injection for refractory muscle tension dysphonia: Our experience with seven patients. Clin. Otolaryngol. 2015, 40, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.A.; Murry, T. Botox for hyperadduction of the false vocal folds: A case report. J. Voice 1999, 13, 234–239. [Google Scholar] [CrossRef]
- Kendall, K.A.; Leonard, R.J. Treatment of ventricular dysphonia with botulinum toxin. Laryngoscope 1997, 107, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Dastolfo, C.; Hersan, R. Perceptions of voice therapy from patients diagnosed with primary muscle tension dysphonia and benign mid-membranous vocal fold lesions. J. Voice 2014, 28, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.E.; Kempster, G.B.; Sims, H.S. Patient factors related to voice therapy attendance and outcomes. J. Voice 2010, 24, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Portone, C.; Johns, M.M.; Hapner, E.R. A review of patient adherence to the recommendation for voice therapy. J. Voice 2008, 22, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Hapner, E.; Portone-Maira, C.; Johns, M.M. A study of voice therapy dropout. J. Voice 2009, 23, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.N.; Bless, D.M.; Lowery, J.D. Indirect laryngoscopic approach for injection of botulinum toxin in spasmodic dysphonia. Otolaryngol. Head Neck Surg. 1990, 103 Pt 1, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Rhew, K.; Fiedler, D.A.; Ludlow, C.L. Technique for injection of botulinum toxin through the flexible nasolaryngoscope. Otolaryngol. Head Neck Surg. 1994, 111, 787–794. [Google Scholar] [CrossRef]
- Adams, S.G.; Hunt, E.J.; Irish, J.C. Comparison of botulinum toxin injection procedures in adductor spasmodic dysphonia. J. Otolaryngol. 1995, 24, 345–351. [Google Scholar] [PubMed]
- Adams, S.G.; Hunt, E.J.; Charles, D.A. Unilateral versus bilateral botulinum toxin injections in spasmodic dysphonia: Acoustic and perceptual results. J. Otolaryngol. 1993, 22, 171–175. [Google Scholar] [PubMed]
- Upile, T.; Elmiyeh, B.; Jerjes, W. Unilateral versus bilateral thyroarytenoid Botulinum toxin injections in adductor spasmodic dysphonia: A prospective study. Head Face Med. 2009, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.Z.; Lerner, B.A.; Patel, A.A. Gender differences in onabotulinum toxin A dosing for adductor spasmodic dysphonia. Laryngoscope 2017, 127, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Lew, M.F.; Adler, C.H. Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-resistant cervical dystonia. Neurology 1999, 53. [Google Scholar] [CrossRef] [PubMed]
- Greene, P.; Fahn, S.; Diamond, B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov. Disord. 1994, 9, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Zuber, M.; Sebald, M.; Bathien, N. Botulinum antibodies in dystonic patients treated with type A botulinum toxin: Frequency and significance. Neurology 1993, 43, 1715–1718. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Schwartz, K. Response and immunoresistance to botulinum toxin injections. Neurology 1995, 45, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Simpson, L.L.; Anderson, T.D. Immunologic characterization of spasmodic dysphonia patients who develop resistance to botulinum toxin. J. Voice 2003, 17, 255–264. [Google Scholar] [CrossRef]
- Smith, M.E.; Ford, C.N. Resistance to botulinum toxin injections for spasmodic dysphonia. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Bansberg, S.F.; Krein-Jones, K. Safety and efficacy of botulinum toxin type B (Myobloc) in adductor spasmodic dysphonia. Mov. Disord. 2004, 19, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A. Botulinum toxin A and B: A comparative dosing study for spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2005, 133, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, S.; Stager, S.V.; Badillo, A. Unilateral versus bilateral injections of botulinum toxin in patients with adductor spasmodic dysphonia. J. Voice 2002, 16, 117–123. [Google Scholar] [CrossRef]
- Langeveld, T.P.; Drost, H.A.; de Jong, R.J.B. Unilateral versus bilateral botulinum toxin injections in adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 1998, 107, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Maloney, A.P.; Morrison, M.D. A comparison of the efficacy of unilateral versus bilateral botulinum toxin injections in the treatment of adductor spasmodic dysphonia. J. Otolaryngol. 1994, 23, 160–164. [Google Scholar] [PubMed]
- Young, N.; Blitzer, A. Management of supraglottic squeeze in adductor spasmodic dysphonia: A new technique. Laryngoscope 2007, 117, 2082–2084. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.B.; Lee, C.T.; Hatcher, J.L. Botulinum toxin treatment of false vocal folds in adductor spasmodic dysphonia: Functional outcomes. Laryngoscope 2016, 126, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.F.; Stewart, C. Abductor laryngeal dystonia: A series treated with botulinum toxin. Laryngoscope 1992, 102, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.A.; Maronian, N.C.; Waugh, P.F. Findings of multiple muscle involvement in a study of 214 patients with laryngeal dystonia using fine-wire electromyography. Ann. Otol. Rhinol. Laryngol. 2004, 113, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Hillel, A.D.; Maronian, N.C.; Waugh, P.F. Treatment of the interarytenoid muscle with botulinum toxin for laryngeal dystonia. Ann. Otol. Rhinol. Laryngol. 2004, 113, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.M.; Chagury, A.A.; Hachiya, A. Diffusion of aniline blue injected into the thyroarytenoid muscle as a proxy for botulinum toxin injection: An experimental study in cadaver larynges. Int. Arch. Otorhinolaryngol. 2013, 17, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, G.J.; Weiner, W.J.; Factor, S.A. Hyperkinetic movement disorders. In Neurology for the Non-Neurologist; Weiner, W.J., Goetz, C.G., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 241–286. [Google Scholar]
- Koller, W.C.; Busenbark, K.; Miner, K. The relationship of essential tremor to other movement disorders: Report on 678 patients. Essential Tremor Study Group. Ann. Neurol. 1994, 35, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Koda, J.; Ludlow, C.L. An evaluation of laryngeal muscle activation in patients with voice tremor. Otolaryngol. Head Neck Surg. 1992, 107, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Warrick, P.; Dromey, C.; Irish, J.C. Botulinum toxin for essential tremor of the voice with multiple anatomical sites of tremor: A crossover design study of unilateral versus bilateral injection. Laryngoscope 2000, 110, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Maronian, N.C.; Waugh, P.F.; Robinson, L. Tremor laryngeal dystonia: Treatment of the lateral cricoarytenoid muscle. Ann. Otol. Rhinol. Laryngol. 2004, 113, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Maryn, Y.; de Bodt, M.S.; van Cauwenberge, P. Ventricular dysphonia: Clinical aspects and therapeutic options. Laryngoscope 2003, 113, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.P.; Meleca, R.J.; Simpson, M.L. Use of topical lidocaine in the treatment of muscle tension dysphonia. J. Voice 2000, 14, 567–574. [Google Scholar] [CrossRef]
- Roy, N.; Smith, M.E.; Allen, B. Adductor spasmodic dysphonia versus muscle tension dysphonia: Examining the diagnostic value of recurrent laryngeal nerve lidocaine block. Ann. Otol. Rhinol. Laryngol. 2007, 116, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, I.; Szachowicz, E.; Hilger, P. Laser therapy of dysphonia plica ventricularis. Ann. Otol. Rhinol. Laryngol. 1987, 96 Pt 1, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Stong, B.C.; DelGaudio, J.M.; Hapner, E.R. Safety of simultaneous bilateral botulinum toxin injections for abductor spasmodic dysphonia. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 793–795. [Google Scholar] [CrossRef] [PubMed]
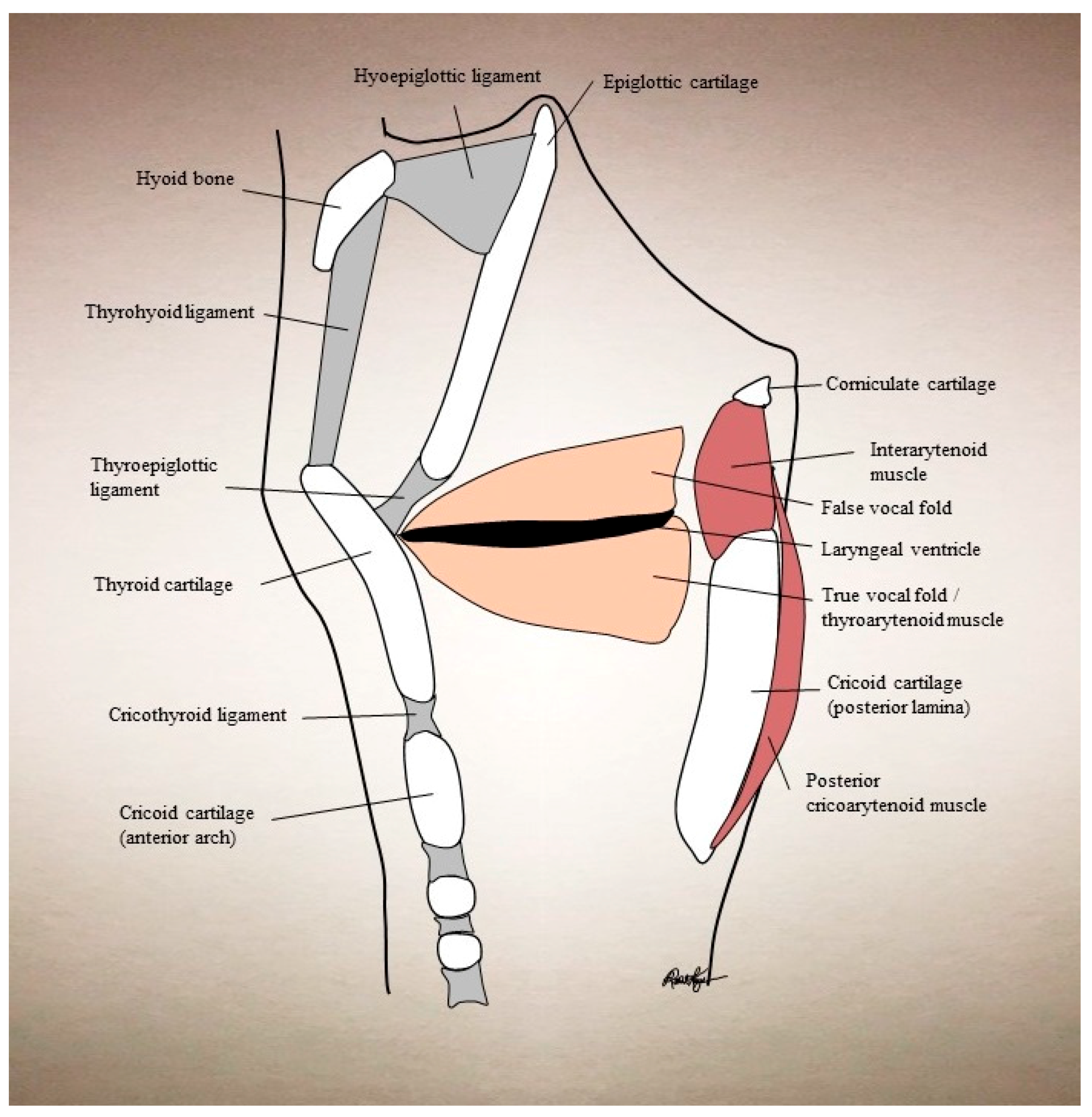
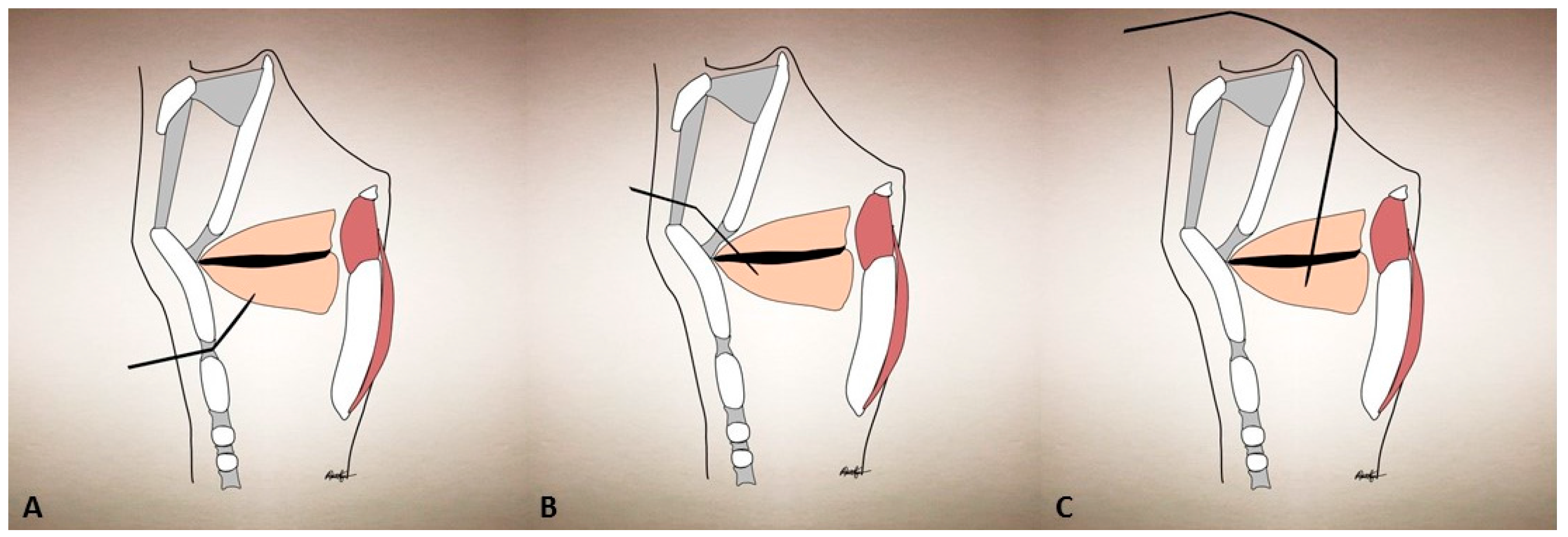

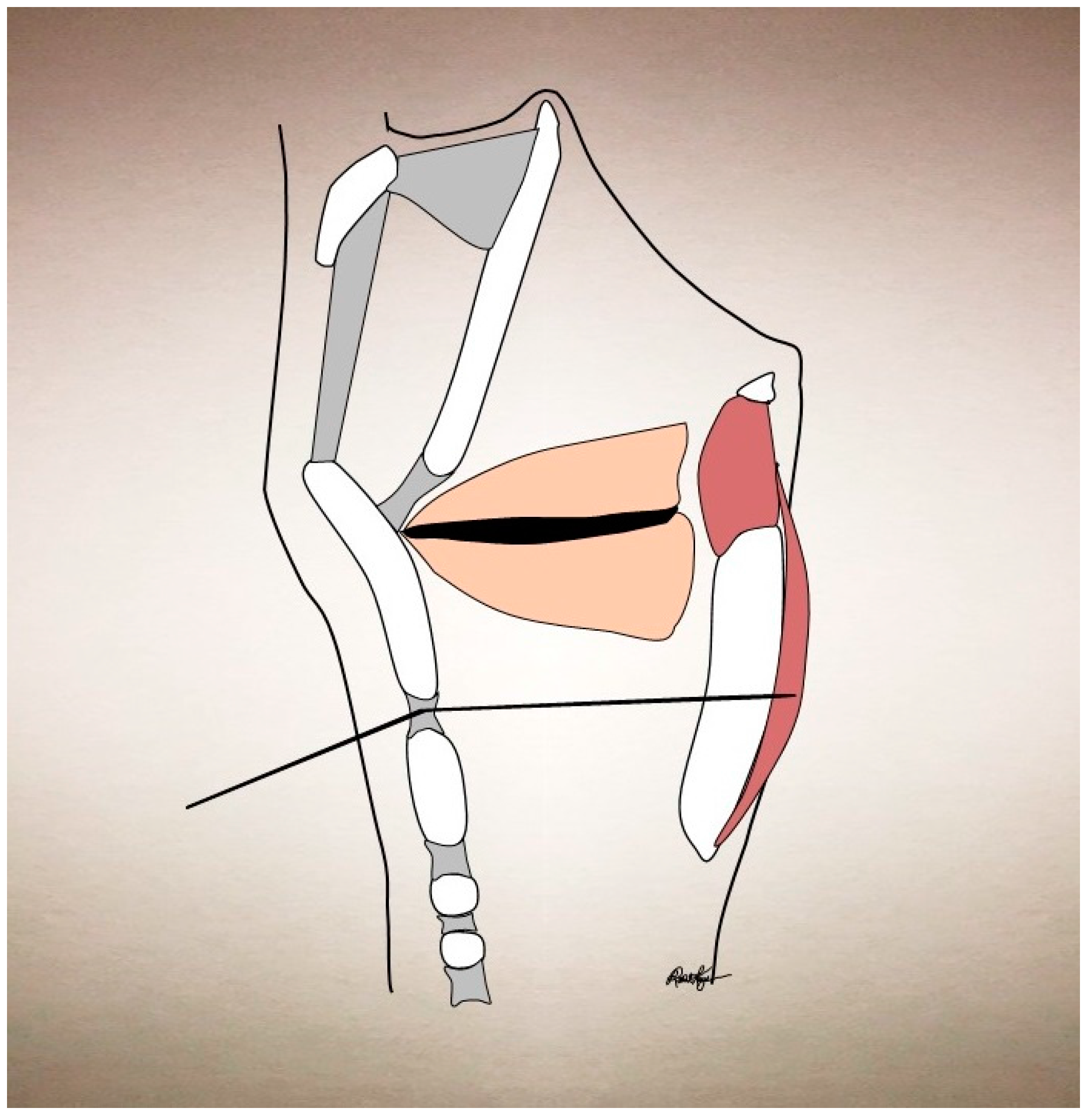
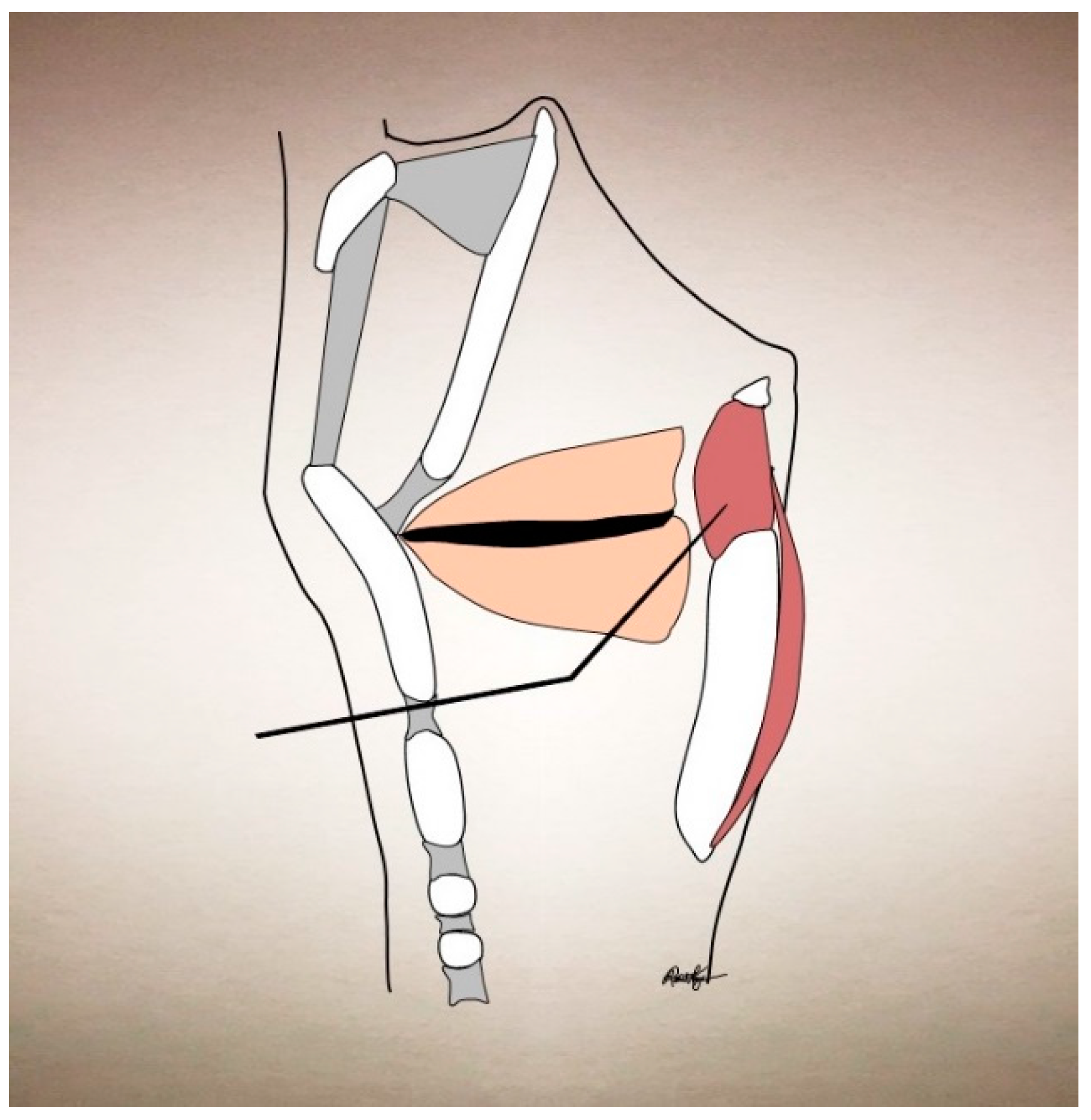
| Condition | Treatment | Success | Side Effects |
|---|---|---|---|
| AdSD | BoNT | 90% [16,22,35,36] | 25–28.5% Breathy dysphonia |
| 10–14.2% Dysphagia to liquids | |||
| 9.4% Dry Mouth (BoNT-B only) | |||
| 2% Dyspnea or breathlessness | |||
| <1% Local pain, bruising, or itch [37,38] | |||
| Surgery | 59–69% [39,40,41,42] | Relapse of symptoms [39] | |
| Pharmacotherapy | Anecdotally low [29] | Not reported | |
| AbSD | BoNT | 89% [43] | 6% Dysphagia to solids (mild) |
| 2% Exertional wheezing [37] | |||
| Pharmacotherapy (Anticholinergics) | 33% [10] | Dry mouth, constipation, urinary retention, defective pupillary accommodation, confusion [44] | |
| Vocal Tremor | BoNT | 56–100% [45,46,47,48] | 53% Mild hoarse dysphonia (TA injection) |
| 0% Strap muscle injection [46] | |||
| Pharmacologic (Primidone) | 25–54% [49,50] | 73% Overall: | |
| 30% Fatigue | |||
| 13% Nausea | |||
| 10% Unspecified | |||
| 7% Dizziness | |||
| 7% Headache | |||
| 7% Disequilibrium [49] | |||
| Pharmacologic | 55.6% (any improvement) | 25% Dizziness or gastrointestinal distress [51] | |
| (Beta Blocker) | 33% (significant improvement) [51] | ||
| Muscle Tension Dysphonia | BoNT | 83–100% [52,53,54] | 50% Dysphagia to liquids (mild) |
| 32% Breathy dysphonia | |||
| 16% Tongue paresthesia [52] | |||
| Speech therapy | 100% [55] | 44–65% Noncompliance rate [55,56,57,58] |
| BoNT Serotype | Conversion Factor | Onset of Action * | Duration of Benefit * | Autonomic Side Effects | Mean Self-Reported Symptom Improvement (0–100%) |
|---|---|---|---|---|---|
| Type A | 1 U | 3.2 days | 17 weeks | None | 89% |
| Type B | 52.3 U | 2.09 days | 10.8 weeks | Dry mouth | 85.4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaye, R.; Blitzer, A. Chemodenervation of the Larynx. Toxins 2017, 9, 356. https://doi.org/10.3390/toxins9110356
Kaye R, Blitzer A. Chemodenervation of the Larynx. Toxins. 2017; 9(11):356. https://doi.org/10.3390/toxins9110356
Chicago/Turabian StyleKaye, Rachel, and Andrew Blitzer. 2017. "Chemodenervation of the Larynx" Toxins 9, no. 11: 356. https://doi.org/10.3390/toxins9110356





