1. Introduction
Deoxynivalenol (DON) is a common contaminant of pig feed mainly originating from
Fusarium-infected cereal grains. Under practical feeding conditions, most prominent toxic effects of DON include a decrease in feed intake causing a reduced live weight gain and contributing to the DON-associated immune-modulation [
1]. Besides, DON effects on stomach weight and pathology have been reported [
2,
3,
4,
5,
6]. Gross macroscopical findings of the
pars nonglandularis (
seu pars oesophagea,
pars proventricularis) were described as a less extended area, a thicker appearance and a smaller degree of folding [
4,
6]. These alterations were suggested to reduce the susceptibility of pigs to ulceration [
6].
Porcine stomach ulcers predominantly affecting the
pars nonglandularis are a common organ pathology observed in modern pig production systems. Evaluations from slaughterhouses suggest that 20% of pigs show erosions and further 60% pre-ulcerative parakeratotic lesions [
7]. Amongst others, feed particle size was described as an important predisposing factor for gastric ulcera [
7]. Feed particle size influences mixing of gastric chyme and stomach filling whereby finely ground feed accelerates stomach emptying and increases the time in which
pars nonglandularis remains unprotected from hydrochloric acid and fundus and duodenum originating digestive juices [
7]. Not only an accelerated gastric emptying due to feeding finely ground feed but also the gastric filling itself has been identified as main risk factors for the development of gastric lesions [
7]. As DON contamination of feed decreases feed intake, it might be assumed that gastric filling is also reduced. Taking into consideration the anorectic effect of DON and the mentioned DON-associated gross-macroscopical alterations of the
pars nonglandularis it seems reasonable to hypothesize that DON might interfere with the feed particle size in developing stomach lesions in pigs. Besides possible implications of grinding fineness and DON contamination on stomach integrity, other nutritional consequences also have to be considered. For example, due to coarse ground feed a higher proportion of undigested nutrients might be shifted to the hindgut [
8] giving rise to an altered fermentation pattern in this segment. Moreover,
Fusarium infection associated physico-chemical alterations, including cell-wall pre-digestion and altered enzymatic activities of grain, could also contribute to regional changes in nutrient digestion and fermentation [
9,
10]. Therefore, main endproducts of microbial fermentation such as volatile fatty acids (VFA) in chyme of small and large intestine might reflect alterations in the sites of nutrient utilization.
To test these hypotheses, two diet types differing in particle size distribution, either in the absence or presence of a Fusarium toxin contaminated wheat containing mainly DON, were fed to rearing piglets. As endpoints we investigated growth performance, fermentative pattern in intestinal chyme and health parameters, including gross-macroscopic appearance and histological lesions of stomach.
3. Discussion
DON is well known for its anorectic effects in pigs. Literature compilation revealed a decrease in feed intake by approximately 5% per each 1 mg increase in dietary DON concentration exceeding the critical dietary concentration of 0.9 mg/kg [
1,
11]. In the present experiment the piglet groups fed the DON-contaminated diets (FUS-fine and FUS-coarse) consumed approximately 20% less feed than their control counterparts over the entire experimental period of 35 days and roughly approached the predicted decrease.
If a lower feed consumption is associated with a lower gut fill, then it seems reasonable to assume that general stomach filling would also be reduced in DON-fed piglets. Taking further into account that finely ground feed additionally contributes to an accelerated gastric emptying, group FUS-fine would be at a higher risk to develop gastric lesions than all other groups as gastric filling is generally considered as a risk factor [
7]. This might be due to a disproportion between gastric acid production and a parallel depletion of the gastric buffering ingesta [
12]. Stomach filling might be associated to the weight of this organ. In the present experiment, the observed higher relative stomach weights due to feeding coarsely ground compared to finely ground feed agree with literature reports (e.g., [
13,
14]). They are discussed as a reactive muscle hypertrophy resulting from the higher physical efforts to transport a chyme with a higher dry matter content as it has been observed after feeding coarsely ground feed [
14,
15]. The increased relative stomach weight due to feeding coarsely ground feed occurred at a higher level when the diet contained the DON-contaminated wheat. An increased relative stomach weight due to feeding of DON contaminated diets was also previously reported [
2,
6]. This effect might be due to the fact that piglets fed DON-contaminated diets consumed less feed, had lower live weight gain and consequently lower body weights at the end of the experiment. As visceral organs grow faster than the rest of the body, especially after weaning, the apparently increased relative stomach weights might simply reflect an allometric effect.
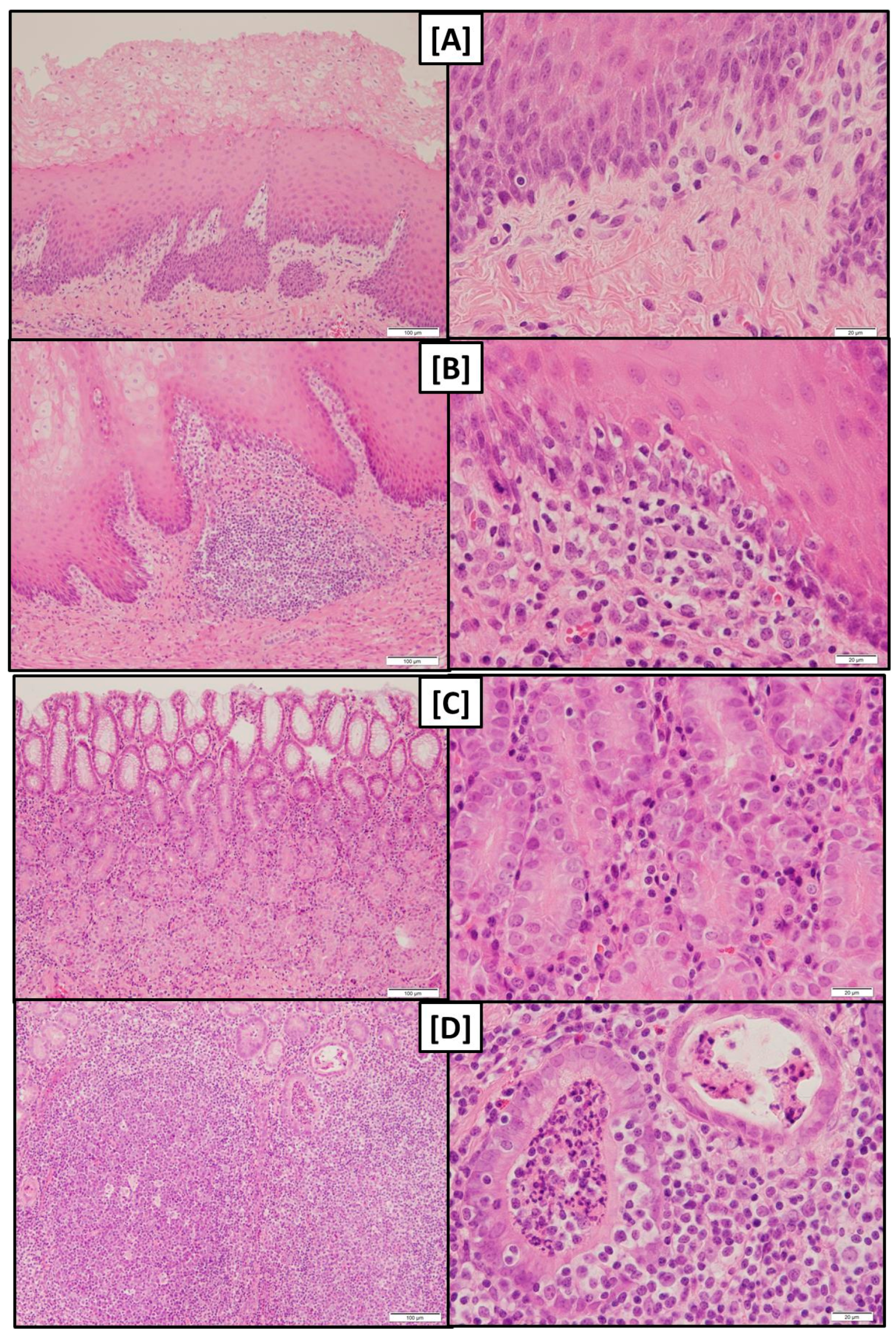
Neither grinding fineness nor DON contamination of the diet influenced the mild gross macroscopical lesions (Score 1). These results are in contrast to other findings where finely ground feedstuffs caused pronounced gross-macroscopical stomach lesions including ulcera (e.g., [
13,
16,
17,
18,
19,
20,
21]). The failure to induce overt ulcers in the present experiment might be due to several variables known or discussed as triggering factors, such as duration of the study, age of the pigs, diet composition and feeding technique, exposure to pathogens and other stressors [
7]. The incidence of gastric ulcers might also include a genetic component as suggested by Flatlandsmo and Slagsvold [
22] who observed differences between sire groups used in their experiments.
While gross macroscopical findings revealed no differences amongst the groups of the present experiment the histological evaluation showed that both pars nonglandularis and pars glandularis were characterized by a more pronounced lymphoplasmacytic infiltration in groups FUS-fine and CON-fine when compared to group FUS-coarse.
Regarding
pars glandularis, adequate regulation of the mucus layer is an important part of the first-line defense mechanisms against pathogens and antigens potentially harmful for the epithelium and the organism. Therefore, any imbalances in mucus turnover could trigger a local immune response resulting in recruitment and proliferation of immune cells. Feeding of coarse meal compared to pelleted feed was shown to enhance the proportion of acid mucins in the intestines which was discussed to have consequences for the adherence capability of bacteria [
14]. Although this effect was not demonstrated for stomach mucus, it seems reasonable to assume that grinding fineness might have consequences for the mucus composition and thus protective properties in this part of the digestive tract. Moreover, different particle sizes were suggested to result in different epitope patterns which could contribute to local immune reactions [
23].
At least the more pronounced lymphoplasmacytic infiltration of the gastric mucosa along with a higher abundance of lymphocyte follicles in groups fed the finely ground feed suggest that the local immune system had to tackle with invaded antigens.
The detritus filled foveolae and tubes observed in these groups could be interpreted as necrotic lesions of epithelial cells giving rise to a facilitated entry of antigens and the triggering of the local immune response.
An increased incidence of lymphoplasmacytic infiltration of the
pars nonglandularis without gross-macroscopical alterations was also observed by Madson, et al. [
24] after feeding a diet with a DON concentration of 5 mg/kg whereby grinding fineness was not addressed in this study. DON is known to modulate immune responses in pigs [
1,
25], whereat a stimulation or a depression might evolve, depending on dose and duration of exposure [
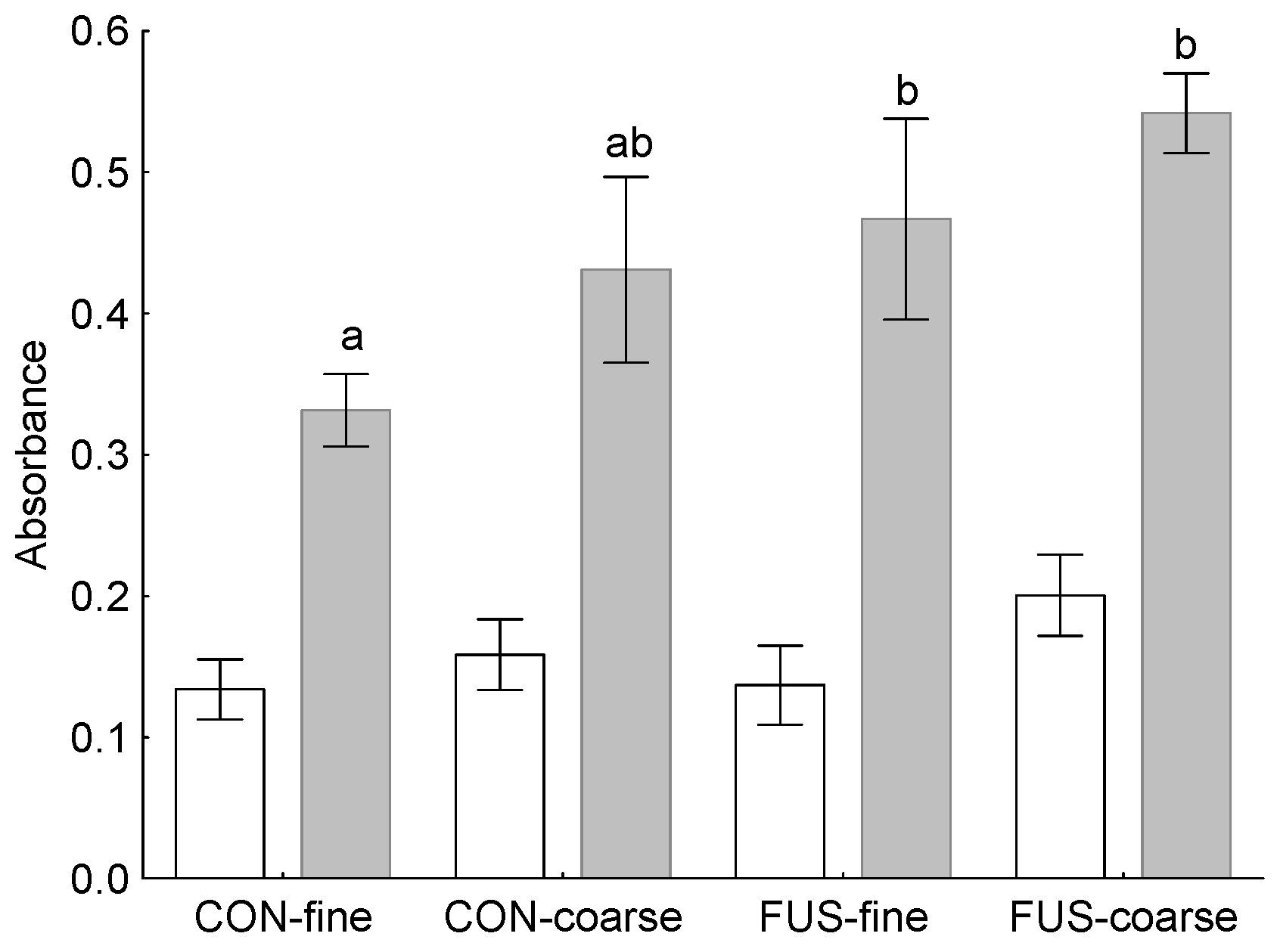
26]. In the present experiment, we examined the responsiveness of PBMC to a mitogenic stimulus
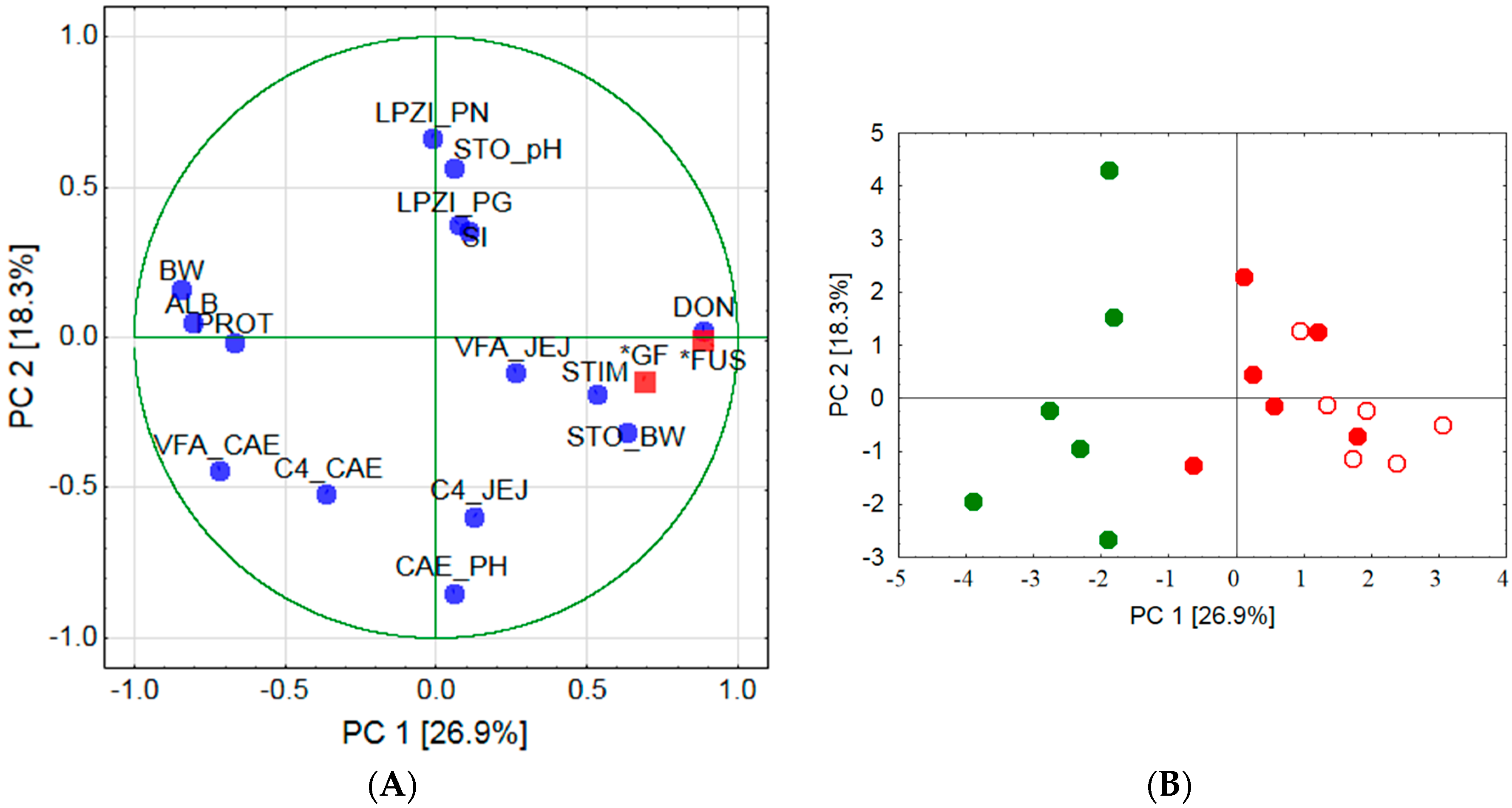
ex vivo and found these circulating immune cells to react with a higher proliferation when DON-contaminated wheat was fed. Therefore, the question remains whether this DON-associated immune-stimulating effect in circulating PBMC also occurred locally in the stomach mucosa and was responsible for the higher incidence of lymphocyte follicles observed in group FUS-fine. Additionally, the results of the PCA would support an association between local immune response and stimulation ability of circulating PBMC as indicated by clustering together with lymphoplasmacytic infiltration of
pars nonglandularis, lymphoplasmacytic infiltration of
pars glandularis and pH-value in stomach chyme. The involvement of the pH-value in stomach chyme is pathogenetically interesting as no significant treatment effects were observed for this parameter.
While our findings did not reveal any effect of treatments on chyme pH-values others found a significantly higher pH-value in caecal content of pigs fed coarsely ground feed [
27]. This effect was associated with a lower dry matter content whilst the starch content remained unaltered. In contrast, an increased starch inflow into the caecum has been deduced from higher starch contents in caecal chyme of piglets fed coarsely ground feed [
8]. This was associated with a significant increase in lactobacilli and Gram-positive cocci and proposed to alter the fermentative profile in the hindgut in favor of propionic and butyric acid. Our results suggest that the effect of grinding fineness on the fermentative pattern in caecum is generally less pronounced and further influenced by
Fusarium infection of the wheat. The increase in butyric acid in caecum of group CON-coarse compared to group CON-fine resulted both from a rise in its proportion and from an enhanced total VFA concentration. The decrease in group FUS-coarse compared to its finely ground counterpart, however, was solely due to a reduced butyric acid proportion. As similar relationships for butyric acid were observed in jejunal chyme it might be deduced that dietary treatments affected the microbial community in small and large intestine similarly.
The markedly lower concentration of total VFA measured in caecal chyme of piglets fed the diets containing the DON-contaminated wheat occurred independently of grinding fineness. This strongly suggests that it might be simply a reflection of the lower substrate flow to the caecum due to feed intake reduction observed both in group FUS-fine and FUS-coarse at the same order of magnitude. Besides feed intake level and associated substrate flows to the caecum a possible involvement of physico-chemical alterations might have contributed to the lower total VFA-concentrations measured in caecal chyme of FUS-fed piglets. In particular, increased activities of non-starch-polysaccharide (NSP) hydrolyzing enzymes were detected as a result of
Fusarium infection whereby the proportion of soluble NSP and extract viscosity was enhanced [
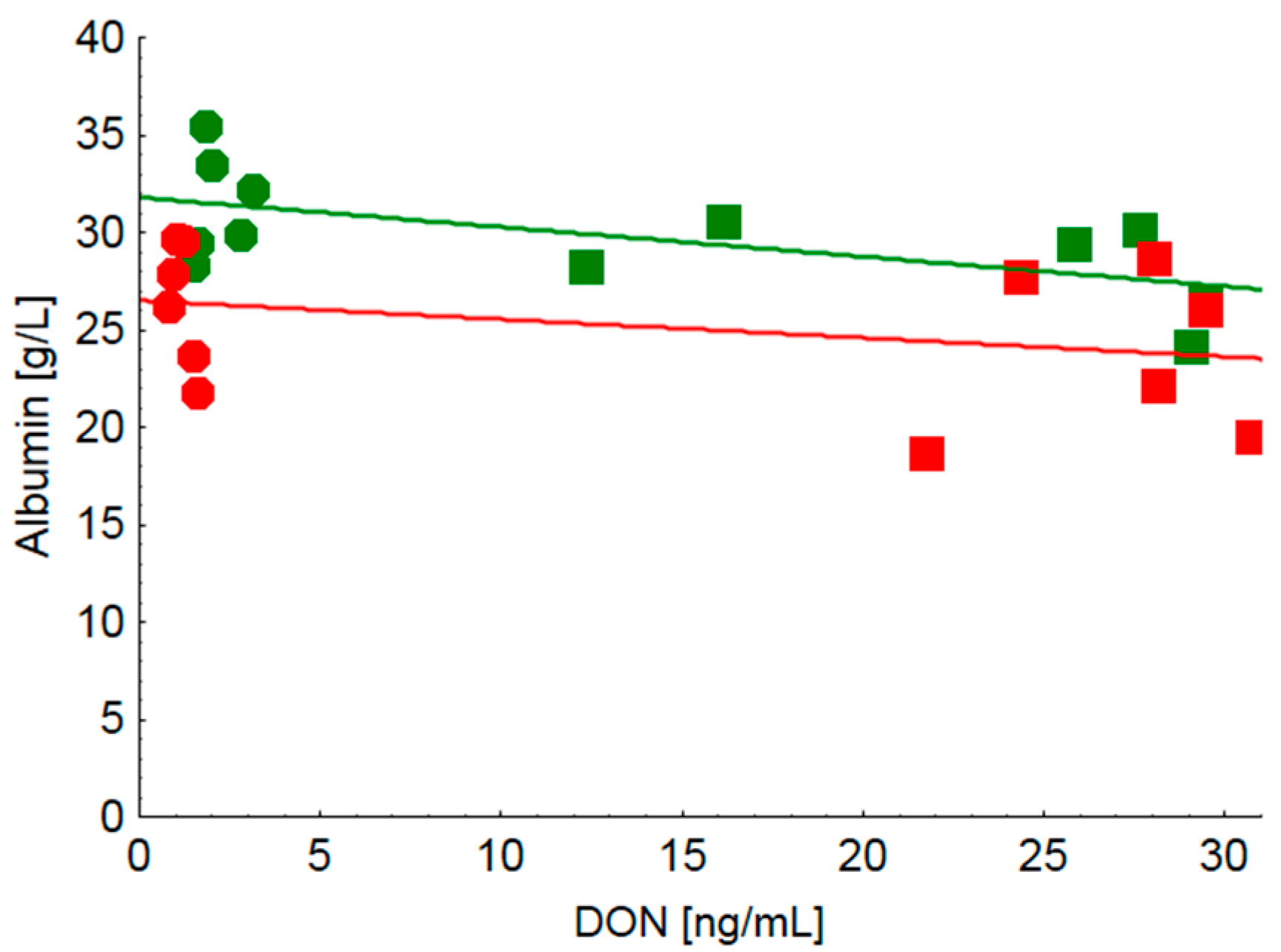
9]. Moreover, not only cell wall cleaving enzyme activities were augmented but also amylase and protease activities were increased. These effects might have consequences for both the accessibility of endogenous enzymes to their substrates within the small intestine and the proportion of nutrients escaping digestion and absorption up to the terminal ileum and thus entering the large intestine. Clinical-chemical serum characteristics revealed clear treatment effects both on total protein and albumin concentration whilst indicators of hepatocyte integrity such as GLDH, GGT, ASAT and total bilirubin remained inconspicuous. The decreased serum protein concentration observed in FUS-fed piglets independently of grinding fineness might be caused by a compromised protein synthesis in the liver as the main site of synthesis of serum proteins. Other reasons for a reduced serum protein concentration might include a blood loss which can be observed due to gastric ulcers. Moreover, a decreased serum protein concentration might also be caused by a relative hyper-hydration induced by an excessive intake of water which, however, cannot be proved as water consumption was not recorded. Furthermore, as gross macroscopic inspection of gastric mucosa did not reveal any overt ulcers, a blood loss into the gastric lumen as a cause for the decreased protein concentration seems less probable. The DON-associated decrease in serum protein concentration which appeared to be independent of grinding fineness can only partly be explained by the lower albumin content as it further decreased in group FUS-coarse compared to group FUS-fine. This grinding fineness associated effect was also observed after feeding the CON-diets albeit at a higher level. Moreover, low background DON-exposure (CON-fine vs. CON-coarse) was associated with larger differences in serum albumin concentrations than a higher DON exposure (FUS-fine vs. FUS-coarse). Looking at the effects of grinding fineness in particular, the steeper negative slope of the regression of serum DON concentration on albumin concentration in piglets fed finely ground diets (CON-fine and FUS-fine) compared to their counterparts (CON-coarse and FUS-coarse) suggests that albumin concentration responded more sensitively to increasing DON exposure when DON containing diets were fed in a finely ground form.
While the effects of DON on albumin synthesis were inconsistent and demonstrated either a decrease [
28] or no effect [
29], grinding fineness was hardly examined with regard to albumin and protein concentration which were shown to remain uninfluenced [
27]. Similarly, feeding of fattening pigs with DON-contaminated diets up to a concentration of 1.12 mg DON/kg, either in a mash or pelleted form, did not influence serum protein concentration [
30]. Despite these controversial literature reports it seems to be necessary to examine the possible underlying mechanisms of the clear treatment effects on albumin concentration that were observed in the present experiment in more detail. Thus, direct measurement of albumin synthesis using isotope techniques could clarify whether differences in serum albumin concentration are due to a variation in synthesis. Moreover, recording of water consumption together with indicators of water balance could provide information and help explain treatment effects on serum albumin concentration.
In conclusion, DON contamination of feed exacerbates the adverse effects of finely ground feed on stomach mucosal integrity of rearing piglets with possible consequences for the local and systemic immune system. Consequently, finely ground feed and DON might influence the immune system in an interactive manner. From a preventive viewpoint, it is recommended to avoid a too fine particle size distribution and to minimize DON contamination of feed for rearing piglets.
4. Materials and Methods
The piglet experiment was conducted according to the European Community regulations concerning the protection of experimental animals and the guidelines of the Regional Council of Braunschweig, Lower Saxony, Germany (File number 509.42502/09-01.03).
4.1. Experimental Design
The experiment was planned according to a complete two by two 2-factorial design and included two diets containing
Fusarium-toxin contaminated wheat (FUS) at a proportion of 20% to target a DON concentration of 5 mg/kg while the other two diets contained uncontaminated control wheat instead (CON). The intended DON concentration of 5 mg/kg was more than five times higher than the guidance value for the critical dietary DON concentration of 0.9 mg/kg [
11] in order to ensure adverse effects as a precondition for studying the interactions between dietary DON-contamination and grinding fineness.
Components of the two diet types were ground on a hammer mill either with a sieve width of 5 mm (coarse) or 3 mm (fine) with the same rotational speed. The four resulting diets and the piglet groups fed therewith were designated as CON-fine, CON-coarse, FUS-fine and FUS-coarse.
4.2. Piglet Experiment
Crossbred piglets (“Bundeshybridzuchtprogramm”, BHZP, Germany) weaned at an age of 21 days were used for the experiment. A total of 16 castrated piglets were assigned to each of the four treatment groups. Four piglets were kept in each of the 16 slatted floor pens in an air-conditioned sub-unit of the pig house. The floor pens had a width of 85 cm, a depth of 173 cm and they were equipped with two nipple drinkers and an automatic feeder for dry feed with a width of 70 cm at the front of the box. The stable was air-conditioned and temperature was adjusted to 32 °C on arrival of the piglets and thereafter continuously decreased to 20 °C. The light–dark cycle included 12 h light and 12 h dark. Piglets were clinically monitored during the experiment.
The experiment started after a period of 10 d in which piglets were fed a commercial diet for weaned piglets. The mean piglet weights of the CON-fine, CON-coarse, FUS-fine and FUS-coarse groups were 7.8 ± 1.5 kg, 7.7 ± 1.1 kg, 7.7 ± 1.2 kg, and 7.7 ± 1.1 kg, respectively, at the beginning of the experiment. The experiment lasted 5 weeks and covered the typical period for rearing piglets under practical conditions. Individual body weight and feed consumption per box were recorded weekly. Diets were provided in a dry meal form and offered for ad libitum consumption.
In addition, after 3 weeks of feeding the experimental diets, blood was collected from superficial neck vessels from 6 piglets per group. Serum and heparinized plasma were used for clinical-chemical analyses and for the ex vivo viability and activity assays performed with PBMC.
At the end of the experiment, these 6 piglets of each group sampled previously for blood were slaughtered by bleeding after electrical stunning. Stomach, duodenum, jejunum, ileum, caecum, colon and rectum were quickly localized
in situ, incised in the fundus region of the stomach and in the approximate mid third of the intestinal segments and pH-values were measured by using a pH-meter equipped with an insertion electrode (WTW pH 530, BLB, Brunswick, Germany). Ingesta was collected from the corresponding sites of the jejunum and the caecum and either used fresh for ammonia-N determination or kept frozen at −20 °C for later analysis of volatile fatty acids. Finally, the weights of the emptied stomach, of liver, kidneys, spleen and heart were recorded. Stomach mucosa was grossly inspected macroscopically and graded according to a scoring system after Betscher [
31]: score 0—intact epithelium, smooth and glistening white surface; score 1—mild changes, partially bile staining and hyperkeratosis; score 2—moderate degree of hyperkeratosis and bile staining over entire surface; score 3—high-degree hyperkeratosis; and score 4—severe lesions and scarring. Finally,
pars nonglandularis and adjacent
pars glandularis of the cardia glandular region of the stomach were excised and further processed for histology.
4.3. Analyses
4.3.1. Diets
Particle size distribution of the diets was determined by dry sieve analyses according to DIN 66165-1:1987-04 [
32] and DIN 66165-2:1987-04 [
33] employing sieves according to DIN ISO 3310/1 [
34].
DON in diet samples was analyzed by high performance liquid chromatography (HPLC) coupled to a diode array detector (DAD) after a cleanup with immuno-affinity columns (IAC) (DONprep™, R-Biopharm Rhone Ltd., Darmstadt, Germany) according to slightly modified method as recommended by R-Biopharm Rhone [
35]. The detection limit was 0.03 mg/kg and the recovery rate amounted to approximately 89%.
Nutrients in diets were analyzed according to the methods of the VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, Germany). In particular, the following methods were applied: No. 3.1 for dry matter (DM), No. 4.1.1 for Kjeldahl Nitrogen, No. 5.1.1 for ether extract, No. 6.1.1 for crude fiber, and No. 6.5.2 for aNDF
OM [
36].
4.3.2. Chyme
Ammonia-N in fresh chyme was determined by a modified Conway-method as described in detail by Voigt and Steeger [
37], using a micro-diffusion vessel. Volatile fatty acids in chyme were determined according to Geissler, et al. [
38] by using a gas chromatograph (Hewlett Packard 5580, Avondale, PA, USA) with a flame ionization detector.
4.3.3. Blood
Plasma samples were analyzed for DON and de-epoxy-DON (de-DON) by using an HPLC method as described by Valenta, et al. [
39] with modifications. In brief, samples were incubated overnight with β-glucuronidase (Type H2, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) at a pH of 5.5 and a temperature of 37 °C. Thereafter, samples were extracted with ethyl acetate using disposable ChemElut
® columns for liquid/liquid extraction containing diatomaceous earth (Varian Deutschland GmbH, Darmstadt, Germany). Extracts were cleaned up using IAC (DONtest™ of VICAM, Klaus Ruttmann GmbH, Hamburg, Germany). HPLC was equipped with an UV-detector for detecting DON and de-DON. The detection limits for both substances were approximately 2 ng/mL with mean recoveries of 87%–98% and 88%–101% for DON and de-DON, respectively.
Total bilirubin, total protein, albumin, aspartat-aminotransferase (ASAT), glutamat-dehydrogenase (GLDH), and γ-glutamyl transferase (GGT) were determined in serum samples by using an automatic clinical chemistry analyser (Eurolyser CCA180, Eurolab, Austria).
For
ex vivo examination of basal metabolic activity and concanavalin A (ConA) stimulated metabolic activity PBMC were isolated from heparinized blood, diluted with phosphate buffered saline (PBS) at a ratio of 1:1, by density gradient centrifugation using Biocoll separation solution (Biochrome AG, Berlin, Germany). Metabolic activity was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) as described elsewhere [
40,
41]. The results of the test are reported as optical density of the ConA-stimulated and non-stimulated cells, and as ratio between both measures, defined as stimulation index (SI).
4.3.4. Histology
Tissue samples of pars nonglandularis and pars glandularis of the stomachs were fixed in a 10% formaldehyde solution buffered with CaCO3 for at least 24 h and embedded in paraffin. Tissues were then sectioned at a thickness of 5 µm and stained with hematoxylin and eosin (H & E) for histological examination.
The tissue sections were visually graded according to the occurrence of lymphoplasmacytic infiltration (score 1, 2 and 3 for low, medium and high degree, respectively) blind to the treatments. Scores are reported as cumulative scores of two evaluated slides.
For technical reasons, the samples of group CON-coarse were lost. Therefore, only groups CON-fine, FUS-coarse and FUS-fine were evaluated.
4.4. Calculations and Statistics
Performance parameters, clinical-chemical serum characteristics, results of the cell culture tests and organ weights were evaluated according to a complete two by two 2-factorial design of analysis of variance (ANOVA) with cereal type (CT) (uncontaminated control wheat, CON, Fusarium-toxin contaminated wheat, FUS) and structure, resulting from different sieve widths of the hammer mill used for feedstuff grinding, (Str) (fine, coarse) and the interactions between CT and Str as fixed factors. Chyme characteristics (pH, volatile fatty acids) were analyzed according to a complete two by two by two 3-factorial design ANOVA with the intestinal segment (Seg, jejunum, caecum) and the corresponding interactions as additional fixed factors. A “Repeated” statement was applied to account for possible dependencies of a specific parameter measured both in jejunum and caecum of the same animal.
Results are reported as LSmeans and pooled standard error of means (PSEM). Tukey test was used for post-hoc testing of differences between LSmeans in case of significance of the fixed factors.
De-DON concentrations in serum and histological lesion scores were not normally distributed and were evaluated by the non-parametric Mann–Whitney U test. Corresponding results were presented as medians and ranges (minimum–maximum).
Finally, to visualize the relationships between the large number of variables in a two-dimensional space, a principal component analysis (PCA) based on correlations was performed additionally.
The relationships between serum DON and albumin concentration were further examined by linear regressions and slope comparisons.
All statistics were performed using STATISTICA 12.0 (StatSoft, Inc. 2014, Tulsa, OK, USA).