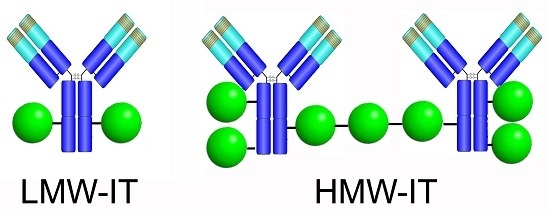
High in Vitro Anti-Tumor Efficacy of Dimeric Rituximab/Saporin-S6 Immunotoxin
Abstract
:1. Introduction
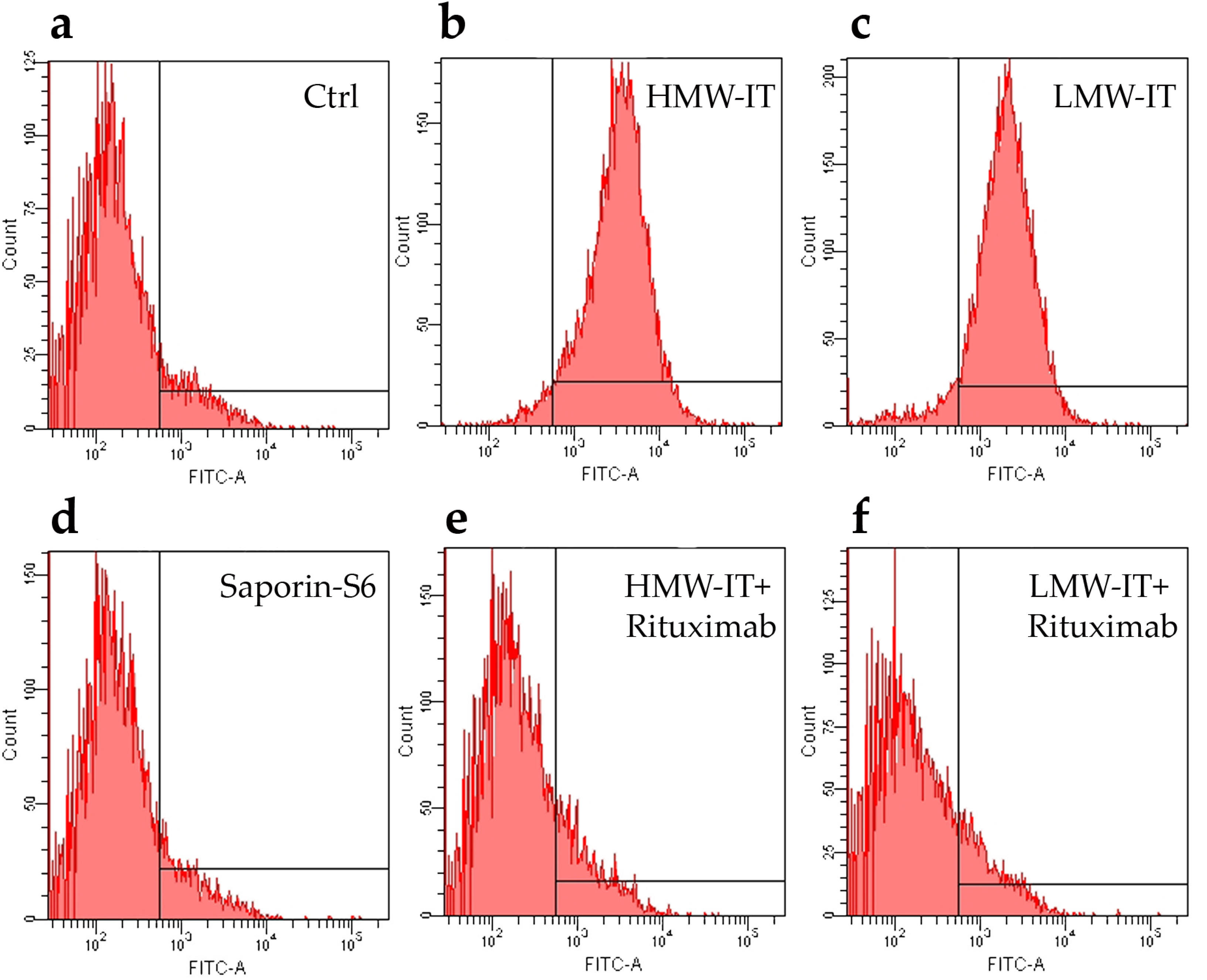
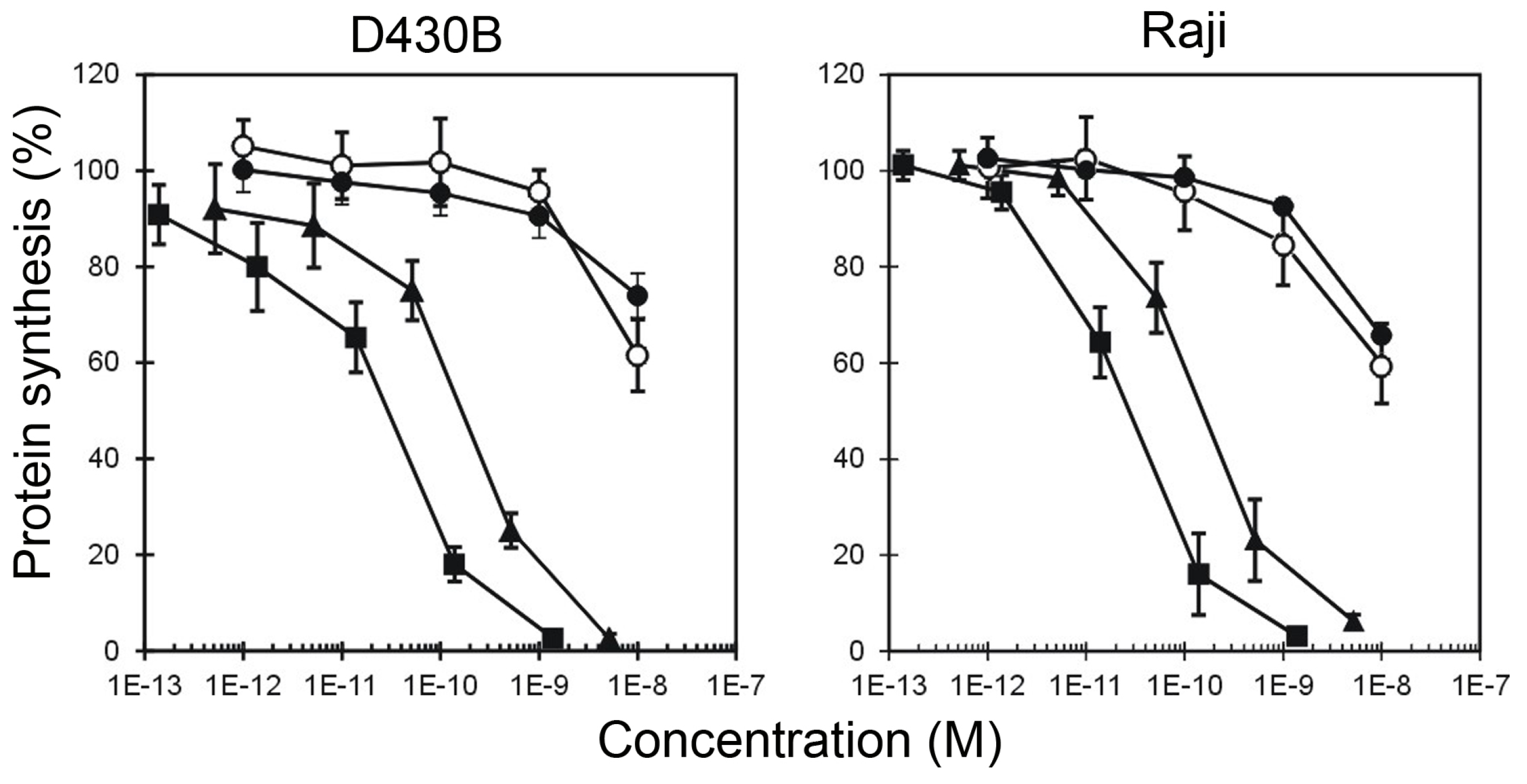
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Immunotoxin Preparation
5.2. Cell Lines
5.3. Protein Synthesis Inhibition Assays
5.4. CD20 Affinity Cytofluorimetric Experiments
5.5. Statistical Analyses
Author Contributions
Conflicts of Interest
Abbreviations
| mAb | monoclonal antibody |
| NHL | non-Hodgkin’s lymphoma |
| CDC | complement-dependent cytotoxicity |
| ADCC | antibody-dependent cellular cytotoxicity |
| RIP | ribosome-inactivating protein |
| HMW-IT | high-molecular-weight immunotoxin |
| LMW-IT | low-molecular-weight immunotoxin |
| PBS | phosphate-buffered saline |
| FBS | fetal bovine serum |
References
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Jaglowski, S.M.; Alinari, L.; Lapalombella, R.; Muthusamy, N.; Byrd, J.C. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood 2010, 116, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Mancuso, R.; Mercatelli, D.; Bortolotti, M.; Bolognesi, A. mAbs targeting CD20 and other lymphocyte CD markers in lymphoma treatment. In Monoclonal Antibodies in Oncology; Uckun, F.M., Ed.; Future Medicine: London, UK, 2013; pp. 6–19. [Google Scholar]
- Polito, L.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Bolognesi, A. Rituximab and other new anti-CD20 mAbs for non-Hodgkin’s lymphoma treatment. EMJ Oncol. 2014, 2, 63–69. [Google Scholar]
- Rezvani, A.R.; Maloney, D.G. Rituximab resistance. Best Pract. Res. Clin. Haematol. 2011, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.L.; Cuccurullo, V.; Moody, T.S.; Mansi, L. Radionuclide antibody-conjugates, a targeted therapy towards cancer. Curr. Radiopharm. 2013, 6, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L. Immunotoxins and other conjugates: Pre-clinical studies. Mini Rev. Med. Chem. 2004, 4, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, J.M.; Citores, L.; Iglesias, R.; Jiménez, P.; Girbés, T. Use of ribosome-inactivating proteins from Sambucus for the construction of immunotoxins and conjugates for cancer therapy. Toxins (Basel) 2011, 3, 420–441. [Google Scholar] [CrossRef] [PubMed]
- Dijoseph, J.F.; Dougher, M.M.; Armellino, D.C.; Kalyandrug, L.; Kunz, A.; Boghaert, E.R.; Hamann, P.R.; Damle, N.K. CD20-specific antibody-targeted chemotherapy of non-Hodgkin’s B-cell lymphoma using calicheamicin-conjugated rituximab. Cancer Immunol. Immunother. 2007, 56, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zheng, S.; Shan, Y.; Li, L.; Zhang, X.; Wang, C. Rituximab-conjugated and doxorubicin-loaded microbubbles combined with ultrasound irradiation inhibits proliferation and induces apoptosis in Raji cell lines. Oncol. Rep. 2016, 35, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Shapira, A.; Benhar, I. Toxin-based therapeutic approaches. Toxins 2010, 2, 2519–2583. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Pedrazzi, M.; Bolognesi, A. Immunotoxins and other conjugates containing saporin-s6 for cancer therapy. Toxins (Basel) 2011, 3, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Saporin-S6: A useful tool in cancer therapy. Toxins (Basel) 2013, 5, 1698–1722. [Google Scholar] [CrossRef] [PubMed]
- Alewine, C.; Hassan, R.; Pastan, I. Advances in anticancer immunotoxin therapy. Oncologist 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Kaur, I.; Perugini, M.A.; Gupta, R.C. Ribosome-inactivating proteins: Current status and biomedical applications. Drug Discov. Today 2012, 17, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide: Adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly(A). Nucleic Acids Res. 1997, 25, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L.; Lubelli, C.; Barbieri, L.; Parente, A.; Stirpe, F. Ribosome-inactivating and adenine polynucleotide glycosylase activities in Mirabilis jalapa L. tissues. J. Biol. Chem. 2002, 277, 13709–13716. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Barbieri, L.; Bolognesi, A.; Buonamici, L.; Valbonesi, P.; Polito, L.; Van Damme, E.J.; Peumans, W.J.; Stirpe, F. Ribosome-inactivating lectins with polynucleotide: Adenosine glycosidase activity. FEBS Lett. 1997, 408, 355–359. [Google Scholar] [CrossRef]
- Barbieri, L.; Bolognesi, A.; Valbonesi, P.; Polito, L.; Olivieri, F.; Stirpe, F. Polynucleotide: Adenosine glycosidase activity of immunotoxins containing ribosome-inactivating proteins. J. Drug Target. 2000, 8, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Farini, V.; Battelli, M.G.; Barbieri, L.; Bolognesi, A. Saporin induces multiple death pathways in lymphoma cells with different intensity and timing as compared to ricin. Int. J. Biochem. Cell Biol. 2009, 41, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Djemil, A.; Bortolotti, M. Plant Toxin-Based Immunotoxins for Cancer Therapy: A Short Overview. Biomedicines 2016, 4, 12. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Pedrazzi, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Apoptosis and necroptosis induced by stenodactylin in neuroblastoma cells can be completely prevented through caspase inhibition plus catalase or necrostatin-1. Phytomedicine 2016, 23, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L.; Scicchitano, V.; Orrico, C.; Pasquinelli, G.; Musiani, S.; Santi, S.; Riccio, M.; Bortolotti, M.; Battelli, M.G. Endocytosis and intracellular localisation of the type 1 ribosome-inactivating protein saporin-S6. J. Biol. Regul. Homeost. Agents 2012, 26, 97–109. [Google Scholar] [PubMed]
- Polito, L.; Bolognesi, A.; Tazzari, P.L.; Farini, V.; Lubelli, C.; Zinzani, P.L.; Ricci, F.; Stirpe, F. The conjugate Rituximab/saporin-S6 completely inhibits clonogenic growth of CD20-expressing cells and produces a synergistic toxic effect with Fludarabine. Leukemia 2004, 18, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Flavell, D.J.; Warnes, S.L.; Bryson, C.J.; Field, S.A.; Noss, A.L.; Packham, G.; Flavell, S.U. The anti-CD20 antibody rituximab augments the immunospecific therapeutic effectiveness of an anti-CD19 immunotoxin directed against human B-cell lymphoma. Br. J. Haematol. 2006, 134, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Aggarwal, C.; Smith, M.R. Impact of Rituximab (Rituxan) on the Treatment of B-Cell Non-Hodgkin’s Lymphoma. P T 2010, 35, 148–157. [Google Scholar] [PubMed]
- Maloney, D.G.; Smith, B.; Rose, A. Rituximab: Mechanism of action and resistance. Semin. Oncol. 2002, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Callejo, D.; González-Rincón, J.; Sánchez, A.; Provencio, M.; Sánchez-Beato, M. Action and resistance of monoclonal CD20 antibodies therapy in B-cell Non-Hodgkin Lymphomas. Cancer Treat. Rev. 2015, 41, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R. Rituximab (monoclonal anti-CD20 antibody): Mechanisms of action and resistance. Oncogene 2003, 22, 7359–7368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Khawli, L.A.; Hu, P.; Epstein, A.L. Generation of rituximab polymer may cause hyper-cross-linking-induced apoptosis in non-Hodgkin’s lymphomas. Clin. Cancer Res. 2005, 11, 5971–5980. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Ledbetter, J.A.; Press, O.W. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood 1998, 91, 1644–1652. [Google Scholar] [PubMed]
- Chu, T.-W.; Yang, J.; Zhang, R.; Sima, M.; Kopeček, J. Cell surface self-assembly of hybrid nanoconjugates via oligonucleotide hybridization induces apoptosis. ACS Nano 2014, 8, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.W.; Zhang, R.; Yang, J.; Chao, M.P.; Shami, P.J.; Kopeček, J. A Two-Step Pretargeted Nanotherapy for CD20 Crosslinking May Achieve Superior Anti-Lymphoma Efficacy to Rituximab. Theranostics 2015, 5, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, M.A.; Bright, H.; Vitetta, E.S. Homodimers but not monomers of rituxan (chimeric anti-CD20) induce apoptosis in human B-lymphoma cells and synergize with a chemotherapeutic agent and an immunotoxin. Blood 2001, 97, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Engert, A.; Schnell, R.; Vitetta, E.S. The in vivo anti-tumor activity of immunotoxins containing two versus one deglycosylated ricin A chains. Cancer Lett. 1995, 98, 97–101. [Google Scholar] [CrossRef]
- Flavell, D.J.; Boehm, D.A.; Noss, A.; Flavell, S.U. Comparison of the potency and therapeutic efficacy of the anti-CD7 immunotoxin HB2-saporin constructed with one or two saporin moieties per immunotoxin molecule. Br. J. Cancer 1997, 75, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.E.; Yanishevski, Y.; Masson, E.; Irvin, J.D.; Evans, W.E.; Uckun, F.M. Favorable pharmacodynamic features and superior anti-leukemic activity of B43 (anti-CD19) immunotoxins containing two pokeweed antiviral protein molecules covalently linked to each monoclonal antibody molecule. Leuk. Lymphoma 1995, 18, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Stoppa, C.; Bolognesi, A. Large-scale chromatographic purification of ribosome-inactivating proteins. J. Chromatogr. 1987, 408, 235–243. [Google Scholar] [CrossRef]
- Bolognesi, A.; Polito, L.; Farini, V.; Bortolotti, M.; Tazzari, P.L.; Ratta, M.; Ravaioli, A.; Horenstein, A.L.; Stirpe, F.; Battelli, M.G.; et al. CD38 as a target of IB4 mAb carrying saporin-S6: Design of an immunotoxin for ex vivo depletion of hematological CD38+ neoplasia. J. Biol. Regul. Homeost. Agents 2005, 19, 145–152. [Google Scholar] [PubMed]
- Vooijs, W.C.; Otten, H.G.; van Vliet, M.; van Dijk, A.J.; de Weger, R.A.; de Boer, M.; Bohlen, H.; Bolognesi, A.; Polito, L.; de Gast, G.C. B7–1 (CD80) as target for immunotoxin therapy for Hodgkin’s disease. Br. J. Cancer 1997, 76, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Bolognesi, A.; Stirpe, F. Purification and conjugation of type 1 ribosome-inactivating proteins. Methods Mol. Biol. 2001, 166, 71–85. [Google Scholar] [PubMed]
- Tazzari, P.L.; de Totero, D.; Bolognesi, A.; Testoni, N.; Pileri, S.; Roncella, S.; Reato, G.; Stein, H.; Gobbi, M.; Stirpe, F. An Epstein-Barr virus-infected lymphoblastoid cell line (D430B) that grows in SCID-mice with the morphologic features of a CD30+ anaplastic large cell lymphoma, and is sensitive to anti-CD30 immunotoxins. Haematologica 1999, 84, 988–995. [Google Scholar] [PubMed]
- Polito, L.; Bortolotti, M.; Mercatelli, D.; Mancuso, R.; Baruzzi, G.; Faedi, W.; Bolognesi, A. Protein synthesis inhibition activity by strawberry tissue protein extracts during plant life cycle and under biotic and abiotic stresses. Int. J. Mol. Sci. 2013, 14, 15532–15545. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L.; Tazzari, P.L.; Lemoli, R.M.; Lubelli, C.; Fogli, M.; de Boer, M.; Stirpe, F. In vitro anti-tumor activity of anti-CD80 and anti-CD86 immunotoxins containing type 1 ribosome-inactivating proteins. Br. J. Haematol. 2000, 110, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Farini, V.; Pedrazzi, M.; Tazzari, P.L.; Bolognesi, A. ATG-saporin-S6 immunotoxin: A new potent and selective drug to eliminate activated lymphocytes and lymphoma cells. Br. J. Haematol. 2009, 147, 710–718. [Google Scholar] [CrossRef] [PubMed]




| Cnjugates | RIP/mAb Molar Ratio | Average Mw (kDa) | IC50 1 (M × 10−11) | Yield (%) 2 | |
|---|---|---|---|---|---|
| mAb | RIP | ||||
| LMW | 1.93 | 210 | 7.31 | 37 | 5 |
| HMW | 3.60 | 510 | 19.9 | 16 | 4 |
| Tested substances | CD20+ Target Cells | CD20− not Target Cells | ||
|---|---|---|---|---|
| D430B | Raji | MOLT-4 | Jurkat | |
| IC50 (M) 1 | ||||
| HMW-IT | 2.93 × 10−11 | 2.26 × 10−11 | >10−8 | >10−8 |
| LMW-IT | 1.98 × 10−10 | 1.52 × 10−10 | >10−8 | >10−8 |
| Saporin-S6 | >10−8 | >10−8 | >10−8 | >10−8 |
| Saporin-S6 + Rituximab | >10−8 | >10−8 | >10−8 | >10−8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolotti, M.; Bolognesi, A.; Battelli, M.G.; Polito, L. High in Vitro Anti-Tumor Efficacy of Dimeric Rituximab/Saporin-S6 Immunotoxin. Toxins 2016, 8, 192. https://doi.org/10.3390/toxins8060192
Bortolotti M, Bolognesi A, Battelli MG, Polito L. High in Vitro Anti-Tumor Efficacy of Dimeric Rituximab/Saporin-S6 Immunotoxin. Toxins. 2016; 8(6):192. https://doi.org/10.3390/toxins8060192
Chicago/Turabian StyleBortolotti, Massimo, Andrea Bolognesi, Maria Giulia Battelli, and Letizia Polito. 2016. "High in Vitro Anti-Tumor Efficacy of Dimeric Rituximab/Saporin-S6 Immunotoxin" Toxins 8, no. 6: 192. https://doi.org/10.3390/toxins8060192