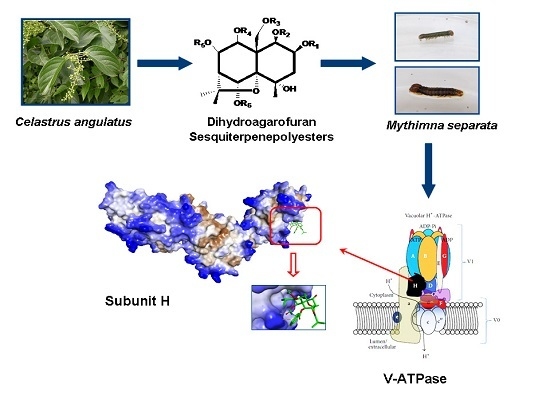
Validation of the Target Protein of Insecticidal Dihydroagarofuran Sesquiterpene Polyesters
Abstract
:1. Introduction
2. Results
2.1. Insecticidal Activity
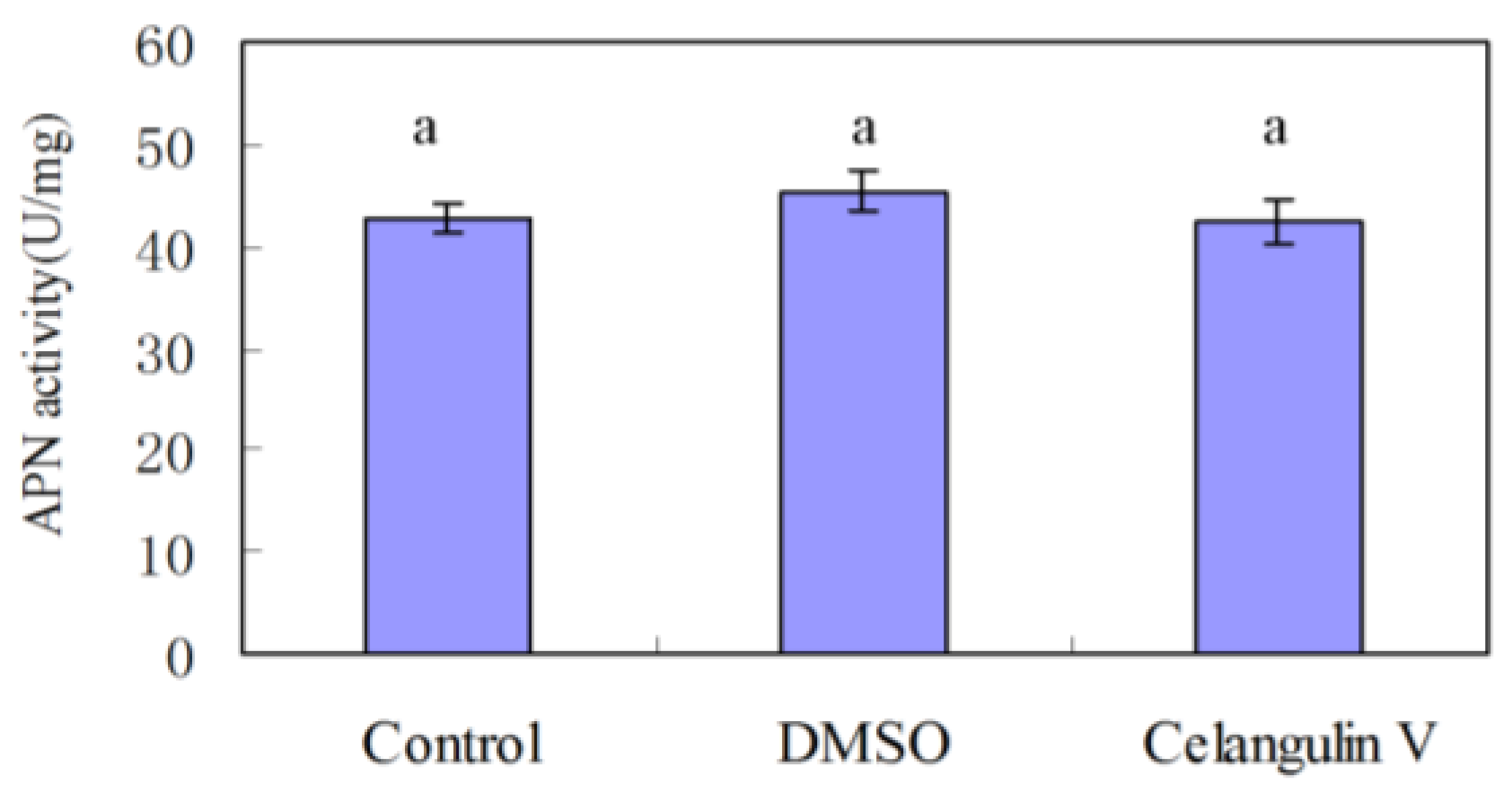
2.2. Effects on the Activity of APN and V-ATPase Activity
2.2.1. Effects on the Activity of APN
2.2.2. Effects on the Activity of V-ATPase
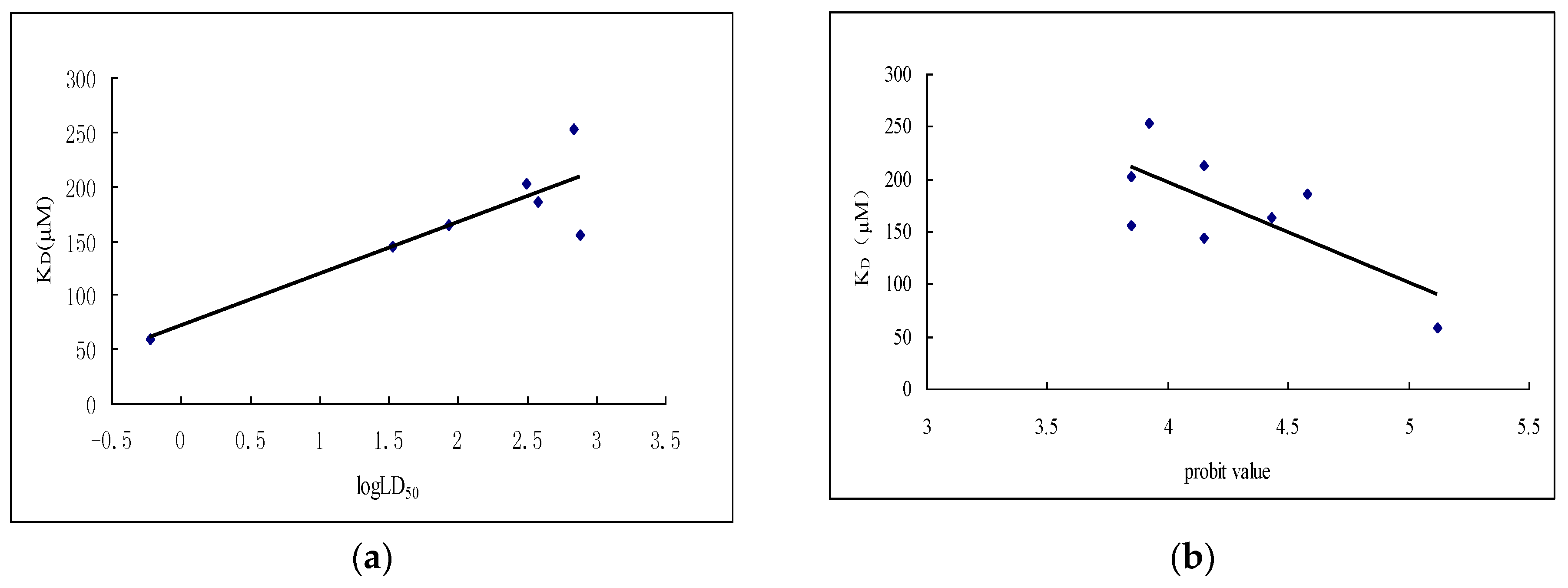
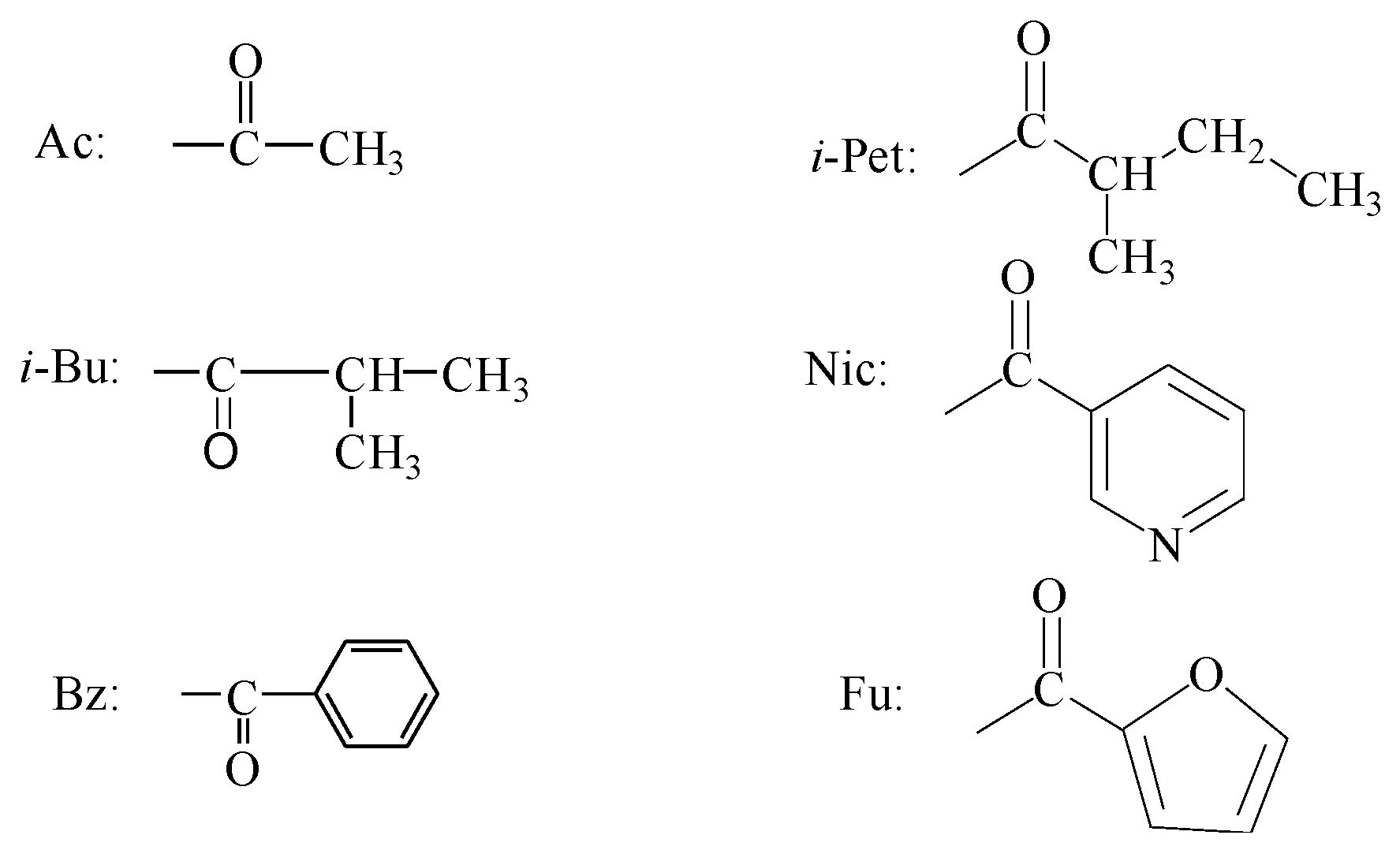
2.3. Interaction between Subunit H and Dihydroagarofuran Sesquiterpene Polyesters
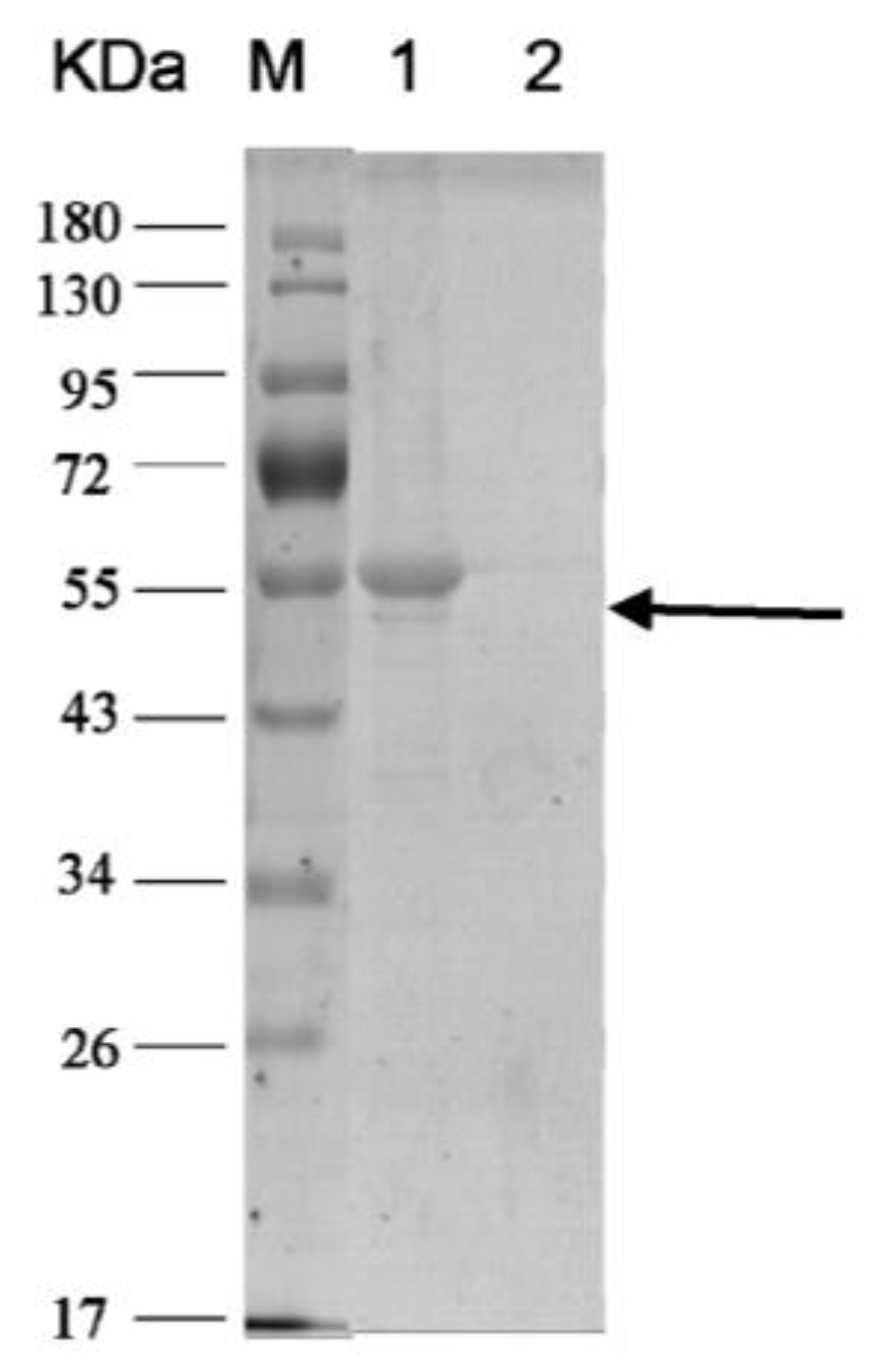
2.3.1. Expression and Purification of Subunit H of V-ATPase
2.3.2. Interaction between Subunit H and Small Molecules
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insects
5.2. Chemicals
5.3. Bioassay of Insecticidal Activity
5.4. APN and V-ATPase Activity Assays
5.4.1. APN Activity Assays
5.4.2. V-ATPase Activity Assays
5.5. Binding Affinity Measurements
5.5.1. Expression and Purification of Subunit H of V-ATPase
5.5.2. Interaction between Subunit H and Small Molecules
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RACE | Rapid-amplification of cDNA ends |
| CDS | Coding DNA Sequence |
| DMSO | dimethylsulfoxide |
| anova | Analysis of Variance |
References
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids-insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Wu, W.J.; Zhang, J.W.; Konishi, Y. The dihydro-β-agarofuran sesquiterpenoids. Nat. Prod. Rep. 2007, 5, 1153–1189. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhang, Q.; Shi, B.; Wei, S.; Wang, M.; Wu, W. Three new insecticidal sesquiterpene pyridine alkaloids from Celastrus angulatus. Nat. Prod. Res. 2009, 23, 470–478. [Google Scholar]
- Ji, Z.; Wu, W.; Yang, H.; Shi, B.; Wang, M. Four novel insecticidal sesquiterpene esters from Celastrus angulatus. Nat. Prod. Res. 2007, 21, 334–342. [Google Scholar]
- Yang, R.Y.; Liu, H.X.; Wu, W.J.; Wang, J.L. Study on the functioning mechanism of celangulin V. J. Northwest Sci. Technol. Univ. Agric. For. 2001, 29, 77–79. [Google Scholar]
- Qi, Z.; Shi, B.; Hu, Z.; Zhang, Y.; Wu, W. Ultrastructural effects of Celangulin V on midgut cells of the oriental armyworm, Mythimna separata walker (Lepidoptera: Noctuidae). Ecotoxicol. Environ. Saf. 2011, 74, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Ji, Z.Q.; Hu, Z.N. Natural products and digestive poisons. Pesticides 1997, 36, 6–9. [Google Scholar]
- Lu, L.; Qi, Z.; Zhang, J.; Wu, W. Separation of binding protein of Celangulin V from the Midgut of Mythimna separata Walker by affinity chromatography. Toxins 2015, 7, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Tronci, L.; Liori, B.; Tocco, G.; Sasanelli, N.; Diana, A. Tulipaline A: Structure-activity aspects as a nematicide and V-ATPase inhibitor. Pestic. Biochem. Physiol. 2014, 112, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Mahmood, T.; Riazuddin, S. The receptor for Bacillus thuringiensis Cry 1Ac Delta-endotoxin in the brush border membrane of the Lepidopteran Helicoverpa armigera is aminopeptidase N. J. Biol. Sci. 2001, 1, 782–784. [Google Scholar]
- Knight, P.J.K.; Crickmore, N.; Ellar, D.J. The receptor for Bacillus thuringiensis CrylA(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol. Microbiol. 1994, 11, 429–436. [Google Scholar] [CrossRef] [PubMed]
- McNall, R.J.; Adang, M.J. Identification of novel Bacillus thuringiensis Cry1Ac binding proteins in Manduca sexta midgut through proteomic analysis. Insect Biochem. Mol. Biol. 2003, 33, 999–1010. [Google Scholar] [CrossRef]
- Liu, M. Structural and functional separation of the N- and C-terminal domains of the yeast V-ATPase subunit H. J. Biol. Chem. 2005, 280, 36978–36985. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.; Fackler, O.T.; Peterlin, B.M. Subunit H of the V-ATPase involved in endocytosis shows homology to β-adaptins. Mol. Biol. Cell 2002, 13, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Margaret, N.H.; Stevens, T.H.; Matthews, B.W.; Ohya, Y.; Takatsukill, A.; Stevens, T.H.; Anraku, Y. VlMA13 Encodes a 54-kDa Vacuolar H+-ATPase subunit required for activity but not assembly of the enzyme complex in Saccharomyces cerevisiae. J. Biol. Chem. 1993, 268, 18286–18292. [Google Scholar]
- Jefferies, K.C.; Forgac, M. Subunit H of the Vacuolar (H+) ATPase inhibits ATP hydrolysis by the free V1 domain by interaction with the rotary subunit F. J. Biol. Chem. 2007, 283, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Titov, D.V.; Liu, J.O. Identification and validation of protein targets of bioactive small molecules. Bioorg. Med. Chem. 2012, 20, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, H.; Beyenbach, K.W.; Huss, M.; Vitavska, O. Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 2009, 212, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, H.; Grüber, G.; Harvey, W.R.; Huss, M.; Merzendorfer, H.; Zeiske, W. Structure and regulation of insect plasma membrane H+-V-ATPase. J. Exp. Biol. 2000, 203, 127–135. [Google Scholar] [PubMed]
- Wang, Y. A Comparative Study of Digestive Poisons Periplocoside P and Celangulin V on Midgut Cell Transmembrane Potential of Mythimna Separata Larvae. Master’s Thesis, Northwest A & F University, Yangling, China, 14 May 2015. [Google Scholar]
- Zhang, S.; Wang, G.; Liu, S.; Wu, W.; Qi, Z. Silencing of V-ATPase subunit H of Mythimna separata through RNA interference. Acta Agric. Boreali Occident. Sin. 2015, 24, 170–174. [Google Scholar]
- Abdullah, M.A.F.; Moussa, S.; Taylor, M.D.; Adang, M.J. Manduca sexta (Lepidoptera: Sphingidae) cadherin fragments function as synergists for Cry1A and Cry1C Bacillus thuringiensistoxins against noctuid moths Helicoverpa zea, Agrotis ipsilon and Spodoptera exigua. Pest Manag. Sci. 2009, 65, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Wolfersberger, M.G.; Luthy, P.; Maurer, A.; Parenti, P.; Sacchi, V.F.; Giordana, B.; Hanozet, G.M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. A Physiol. 1987, 86, 301–308. [Google Scholar] [CrossRef]
- Ferré, J.; Real, M.D.; Rie, J.V.; Jansens, S.; Peferoen, M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Liang, G.M.; Zhang, J.; Wu, K.M.; Guo, Y.Y.; Rector, B.G. Proteomic analysis of novel Cry1Ac binding proteins in Helicoverpa armigera (Hübner). Arch. Insect Biochem. Physiol. 2010, 73, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Xu, G.; Wang, G.; Wu, K.; Guo, Y. Isolation and purification of aminopeptidase N (APNs), the main receptor proteins for Bacillus thuringiensis toxin in midgut of Helicoverpa armigera larva. J. Agric. Biotechnol. 2005, 13, 102–107. [Google Scholar]
- Watanabe, Y.; Shimamori, Y.; Ozaki, M.; Uchida, M.; Fujimoto, Y. Purification of a membrane-bound neutral endopeptidase from rat kidney and its activation by polyamines. J. Biochem. 1984, 95, 1725–1732. [Google Scholar] [PubMed]
- Takesue, Y.; Yokota, K.; Nishi, Y.; Taguchi, R.; Ikezawa, H. Solubilization of trehalase from rabbit renal and intestinal brush-border membranes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1986, 201, 5–8. [Google Scholar] [CrossRef]
- Silva-Filha, M.H.; Nielsen-LeRoux, C.; Charles, J.-F. Identification of the receptor for Bacillus sphaericus crystal toxin in the brush border membrane of the mosquito Culex pipiens (Diptera Culicidae). Insect Biochem. Mol. Biol. 1999, 29, 711–721. [Google Scholar] [CrossRef]
- Tiburcy, F.; Beyenbach, K.W.; Wieczorek, H. Protein kinase A-dependent and -independent activation of the V-ATPase in Malpighian tubules of Aedes aegypti. J. Exp. Biol. 2012, 216, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, H.; Cioffi, M.; Klein, U.; Harvey, W.R.; Schweikl, H.; Wolfersberger, M.G. Isolation of goblet cell apical membrane from tobacco hornworm midgut and purification of its vacuolar-type ATPase. Methods Enzymol. 1990, 192, 608–616. [Google Scholar] [PubMed]
- Lu, L.; Qi, Z.; Wu, W. Cloning, expression and purification of subunit H of vacuolar H+-ATPase from Mythimna separata Walker (Lepidoptera: Noctuidae). Int. J. Mol. Sci. 2014, 15, 15443–15455. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, J.; Qi, Z.; Wu, W. Prokaryotic expression and purification of V-ATPase subunit H in the midgut of Mythimna separata. Acta Agric. Boreali Occident. Sin. 2014, 23, 102–106. [Google Scholar]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef] [PubMed]
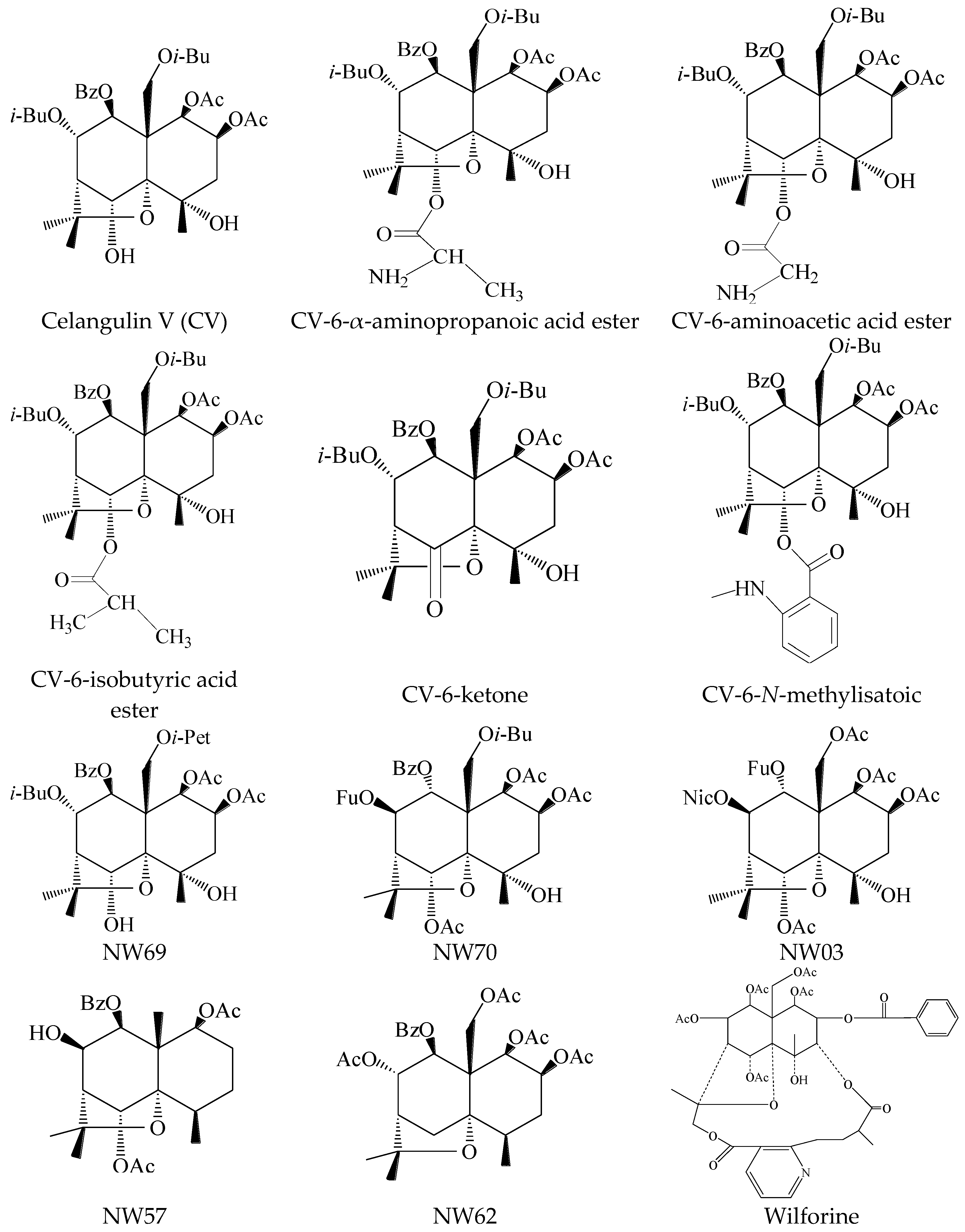
| Samples | Regression Equation | Correlation Coefficient | LD50 (μg/g) | 95% Confidence Limits (μg/g) |
|---|---|---|---|---|
| Celangulin V (CV) | Y = 3.6544 + 0.47509x | 0.9813 | 679.479 | 171.7784–2687.7183 |
| CV-6-α-aminopropanoic acid ester | Y = 1.9537 + 1.99575x | 0.9488 | 33.605 | 27.1526–41.5919 |
| NW03 | Y = 2.9385 + 0.79935x | 0.9922 | 379.286 | 173.1693–830.7353 |
| NW70 | Y = 1.3655 + 1.87692x | 0.9829 | 86.271 | 60.8162–122.3788 |
| NW69 | Y = −3.6165 + 3.45942x | 0.9912 | 309.559 | 257.2790–372.4620 |
| Wilforine | Y = 5.3414 + 1.52479x | 0.9972 | 0.597 | 0.3752–0.9506 |
| CV-6-aminoacetic acid ester | Y = 3.2465 + 0.6077x | 0.9805 | 767.811 | 189.6674–894.5342 |
| Compounds | Concentration (μM) | Specific Activity (nmol Pi/min/mg) | Inhibition Rate (%) |
|---|---|---|---|
| DMSO | - | 451.65 ± 19.2740 a | - |
| Bafilomycin A1 | 3 | 233.52 ± 14.3258 h | 48.29 |
| Celangulin V (CV) | 100 | 387.57 ± 12.6793 cde | 14.19 |
| CV-6-aminoacetic acid ester | 100 | 394.19 ± 29.1940 bcd | 12.72 |
| CV-6-α-aminopropanoic acid ester | 100 | 362.05 ± 21.6747 bcd | 19.83 |
| Wilforine | 100 | 204.25 ± 34.6747 h | 54.78 |
| NW03 | 100 | 298.19 ± 12.3455 g | 33.98 |
| NW69 | 100 | 394.61 ± 35.3014 bcd | 12.63 |
| NW70 | 100 | 317.80 ± 17.9918 fg | 29.64 |
| NW57 | 100 | 443.07 ± 34.4936 abc | 1.90 |
| NW62 | 100 | 420.49 ± 5.5092 ab | 6.90 |
| CV-6-N-methylisatoic | 100 | 343.06 ± 11.3159 efg | 24.04 |
| CV-6-isobutyric acid ester | 100 | 396.10 ± 11.2234 cd | 12.30 |
| CV-6-ketone | 100 | 421.308 ± 27.3712 abc | 6.72 |
| Samples | Interaction | KD Value (μM) |
|---|---|---|
| Wilforine | binding | 59.0 |
| CV-6-α-aminopropanoic acid ester | binding | 144.0 |
| CV-6-aminoacetic acid ester | binding | 156.0 |
| NW70 | binding | 164.0 |
| NW69 | binding | 186.0 |
| NW03 | binding | 203.0 |
| CV-6-N-methylisatoic | binding | 213.0 |
| Celangulin V (CV) | binding | 253.0 |
| CV-6-isobutyric acid ester | no-binding | - |
| CV-6-ketone | no-binding | - |
| NW57 | no-binding | - |
| NW62 | no-binding | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Qi, Z.; Li, Q.; Wu, W. Validation of the Target Protein of Insecticidal Dihydroagarofuran Sesquiterpene Polyesters. Toxins 2016, 8, 79. https://doi.org/10.3390/toxins8030079
Lu L, Qi Z, Li Q, Wu W. Validation of the Target Protein of Insecticidal Dihydroagarofuran Sesquiterpene Polyesters. Toxins. 2016; 8(3):79. https://doi.org/10.3390/toxins8030079
Chicago/Turabian StyleLu, Lina, Zhijun Qi, Qiuli Li, and Wenjun Wu. 2016. "Validation of the Target Protein of Insecticidal Dihydroagarofuran Sesquiterpene Polyesters" Toxins 8, no. 3: 79. https://doi.org/10.3390/toxins8030079