Occurrence of Fusarium Mycotoxins in Cereal Crops and Processed Products (Ogi) from Nigeria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fusarium Mycotoxins Contamination in Cereals (Maize, Sorghum, and Millet) and Processed Products (Ogi) from Nigeria
2.2. Fumonisins and Hidden Fumonisins Contamination in Cereals (Maize, Sorghum and Millet) and Processed Products (Ogi) from Nigeria
2.3. Distribution of Fusarium Mycotoxins in Major Cereals across the Different Agro-Ecological Zones of Nigeria
2.4. Co-Occurrence of Fusarium Mycotoxins in Cereals and Processed Products (Ogi) from Nigeria
3. Conclusions
4. Materials and Methods
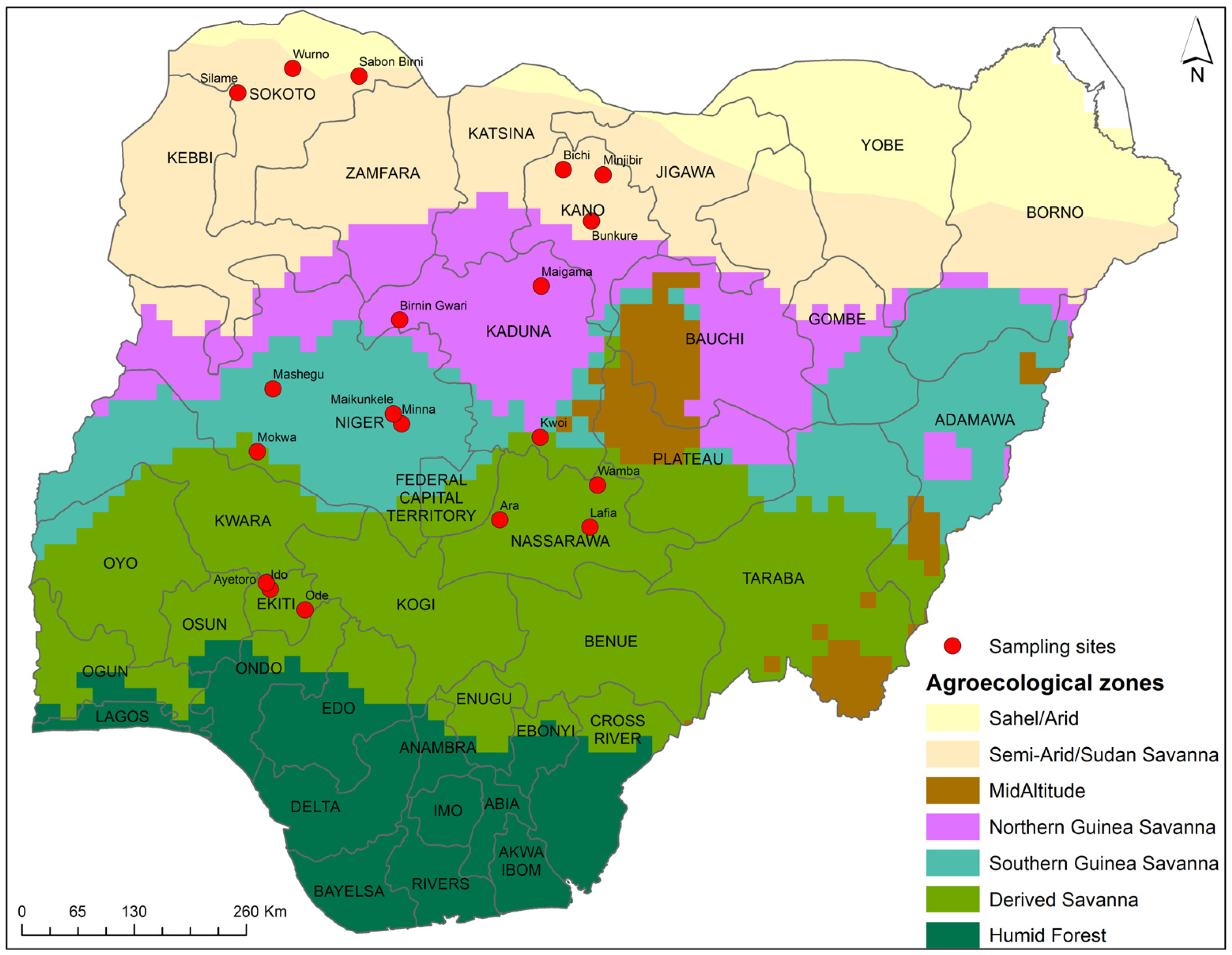
4.1. Sampling
4.2. Chemicals and Reagents
4.3. Sample Extraction
4.4. Preparation of the Hydrolysed FB1, FB2, and FB3 Standard
4.5. Sample Preparation for Hydrolysed Fumonisins
4.6. Liquid Chromatography-Tandem Mass Spectrometry
4.7. Method Validation
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Council for Agricultural Science and Technology (CAST). Mycotoxins: Risks in Plant, Animal, and Human Systems; Task force Report No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Yazar, S.; Omurtag, G.Z. Fumonisins, trichothecenes and zearalenone in cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risk to humans. IARC 1993, 56, 1–521. [Google Scholar]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Rodriguez-Carrasco, Y.; Fattore, M.; Albrizio, S.; Berrada, H.; Manes, J. Occurrence of Fusarium mycotoxins and their dietary intake through beer consumption by the European population. Food Chem. 2015, 178, 149–155. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; Monaci, L.; Pascale, M.; Visconti, A. Fate of deoxynivalenol, T-2 and HT-2 toxins and their glucoside conjugates from flour to bread: An investigation by high-performance liquid chromatography high-resolution mass spectrometry. Food Addit. Contam. Part A 2012, 30, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control 2016, in press. [Google Scholar] [CrossRef]
- Speijers, G.J.A.; Speijers, M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, J.H.; Dano, S.D.; Moukha, S.; Mobio, T.A.; Creppy, E.E. Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, {DNA} methylation and fragmentation, and viability in Caco-2 cells. Toxicon 2007, 49, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Luongo, D.; De Luna, R.; Russo, R.; Severino, L. Effects of four Fusarium toxins (fumonisin B1, α-zearalenol, nivalenol and deoxynivalenol) on porcine whole-blood cellular proliferation. Toxicon 2008, 52, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, R.B.; Edrington, T.S.; Kubena, L.F.; Elissalde, M.H.; Casper, H.H.; Rottinghaus, G.E.; Turk, J.R. Effects of dietary fumonisin B1-containing culture material, deoxynivalenol-contaminated wheat, or their combination on growing barrows. Am. J. Vet. Res. 1996, 57, 1790–1794. [Google Scholar] [PubMed]
- Kubena, L.F.; Edrington, T.S.; Harvey, R.B.; Phillips, T.D.; Sarr, A.B.; Rottinghaus, G.E. Individual and combined effects of fumonisin B1 present in Fusarium moniliforme culture material and diacetoxyscirpenol or ochratoxin A in turkey poults. Poult. Sci. 1997, 76, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015, 89, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Humpf, H.U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekaert, N.; Devreese, M.; De Baere, S.; De Backer, P.; Croubels, S. Modified Fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food Chem. Toxicol. 2015, 80, 17–31. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M; Jacxsens, L.; Lachat, C.; Eeckhout, M.; Di Mavungu, D.M.; Audenaert, K.; Maene, P.; Haesaert, G.; Kolsteren, P.; De Meulenaer, B.; et al. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol. Lett. 2013, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Diel, A.C.L.; Rauber, R.H.; Fontoura, F.P.; Mallmann, A.; Dilkin, P.; Mallmann, C.A. Free and hidden fumonisins in Brazilian raw maize samples. Food Control 2015, 53, 217–221. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Galaverna, G.; Mangia, M.; Sforza, S.; Dossena, A.; Marchelli, R. Free and bound fumonisins in gluten-free food products. Mol. Nutr. Food Res. 2009, 53, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Mangia, M.; Berthiller, F.; Molinelli, A.; Sulyok, M.; Schuhmacher, R.; Krska, R.; Galaverna, G.; Dossena, A.; Marchelli, R. Difficulties in fumonisin determination: The issue of hidden fumonisins. Anal. Bioanal. Chem. 2009, 395, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, G.; Dallsta, C.; Mangia, M.A.; Dossena, A.; Marchelli, R. Masked mycotoxins: An emerging issue for food safety. Czech J. Food Sci. 2009, 27, 89–92. [Google Scholar]
- Dall’Asta, C.; Galaverna, G.; Aureli, G.; Dossena, A.; Marchelli, R. A LC/MS/MS method for the simultaneous quantification of free and masked fumonisins in maize and maize-based products. World Mycotoxin J. 2008, 1, 237–246. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Odebode, A.C.; Fapohunda, S.O. Zearalenone production by naturally occurring Fusarium species on maize, wheat and soybeans from Nigeria. J. Biol. Environ. Sci. 2008, 2, 77–82. [Google Scholar]
- Adejumo, T.O.; Hettwer, U.; Karlovsky, P. Occurrence of Fusarium species and trichothecenes in Nigerian maize. Int. J. Food Microbiol. 2007, 116, 350–357. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Comission regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L 255, 14–17. [Google Scholar]
- Kpodo, K.; Thrane, U.; Hald, B. Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int. J. Food Microbiol. 2000, 61, 147–157. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from burkina faso and mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef] [PubMed]
- Phoku, J.Z.; Dutton, M.F.; Njobeh, P.B.; Mwanza, M.; Egbuta, M.A.; Chilaka, C.A. Fusarium infection of maize and maize-based products and exposure of a rural population to fumonisin B1 in Limpopo Province, South Africa. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.N.; Njobeh, P.B.; Dutton, M.F.; Moundipa, P.F. Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Control 2013, 31, 438–453. [Google Scholar] [CrossRef]
- Adetunji, M.; Atanda, O.; Ezekiel, C.N.; Sulyok, M.; Warth, B.; Beltrán, E.; Krska, R.; Obadina, O.; Bakare, A.; Chilaka, C.A. Fungal and bacterial metabolites of stored maize (Zea mays, L.) from five agro-ecological zones of Nigeria. Mycotoxin Res. 2014, 30, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Thomas, D.; Boers, E.; De Rijk, T.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Colour-encoded paramagnetic microbead-based direct inhibition triplex flow cytometric immunoassay for ochratoxin A, fumonisins and zearalenone in cereals and cereal-based feed rapid detection in food and feed. Anal. Bioanal. Chem. 2013, 405, 7783–7794. [Google Scholar] [CrossRef] [PubMed]
- Ediage, E.N.; Hell, K.; De Saeger, S. A comprehensive study to explore differences in mycotoxin patterns from agro-ecological regions through maize, peanut, and cassava products: A case study, cameroon. J. Agric. Food Chem. 2014, 62, 4789–4797. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; de Kock, S.; Phoku, J.Z.; Mwanza, M.; Egbuta, M.A.; Dutton, M.F. Fungal and mycotoxin contamination of South African commercial maize. J. Food Agric. Environ. 2012, 10, 296–303. [Google Scholar]
- Chala, A.; Taye, W.; Ayalew, A.; Krska, R.; Sulyok, M.; Logrieco, A. Multimycotoxin analysis of sorghum (Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) from Ethiopia. Food Control 2014, 45, 29–35. [Google Scholar] [CrossRef]
- Ayalew, A.; Fehrmann, H.; Lepschy, J.; Beck, R.; Abate, D. Natural occurrence of mycotoxins in staple cereals from Ethiopia. Mycopathologia 2006, 162, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Calado, T.; Venâncio, A. Mycotoxin production by Aspergillus niger aggregate isolated from harvested maize in three Portuguese regions. Rev. Iberoam. Micol. 2012, 30, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Abia, W.A.; Ogara, I.M.; Sulyok, M.; Warth, B.; Krska, R. Fate of mycotoxins in two popular traditional cereal-based beverages (kunu-zaki and pito) from rural Nigeria. LWT Food Sci. Technol. 2015, 60, 137–141. [Google Scholar] [CrossRef]
- Makun, H.A.; Dutton, M.F.; Njobeh, P.B.; Mwanza, M.; Kabiru, A.Y. Natural multi-occurrence of mycotoxins in rice from Niger State, Nigeria. Mycotoxin Res. 2011, 27, 97–104. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. Part A 2012, 29, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Mirocha, C.J.; Abbas, H.K.; Windels, C.E.; Xie, W. Variation in deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, and zearalenone production by Fusarium graminearum isolates. Appl. Environ. Microbiol. 1989, 55, 1315–1316. [Google Scholar] [PubMed]
- Abedi-tizaki, M.; Sabbagh, S. Detection of 3-Acetyldeoxynivalenol, 15-Acetyldeoxynivalenol and Nivalenol-Chemotypes of Fusarium graminearum from Iran using specific PCR assays a b c d. Plant Knowl. J. 2013, 2, 38–42. [Google Scholar]
- Li, H.-P.; Wu, A.-B.; Zhao, C.-S.; Scholten, O.; Löffler, H.; Liao, Y.-C. Development of a generic PCR detection of deoxynivalenol- and nivalenol-chemotypes of Fusarium graminearum. FEMS Microbiol. Lett. 2005, 243, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.-Á.; Montes, R.; Navarro, A.; Segarra, R.; Cuesta, G.; Hernández, E. Occurrence of deoxynivalenol and nivalenol in Spanish corn-based food products. J. Food Compos. Anal. 2008, 21, 423–427. [Google Scholar] [CrossRef]
- Gottschalk, C.; Barthel, J.; Engelhardt, G.; Bauer, J.; Meyer, K. Simultaneous determination of type A, B and D trichothecenes and their occurrence in cereals and cereal products. Food Addit. Contam. 2009, 26, 1273–1289. [Google Scholar] [CrossRef]
- Sokolović, M.; Šimpraga, B. Survey of trichothecene mycotoxins in grains and animal feed in Croatia by thin layer chromatography. Food Control 2006, 17, 733–740. [Google Scholar] [CrossRef]
- European Commission (EC). Commission recomendations on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 56, 12–15. [Google Scholar]
- Adejumo, T.O.; Hettwer, U.; Karlovsky, P. Survey of maize from south-western Nigeria for zearalenone, α- and β-zearalenols, fumonisin B1 and enniatins produced by Fusarium species. Food Addit. Contam. 2007, 24, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission regulation (EC) No 1881/2006 Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364/5, 1–26. [Google Scholar]
- De Boevre, M.; Graniczkowska, K.; De Saeger, S. Metabolism of modified mycotoxins studied through in vitro and in vivo models: An overview. Toxicol. Lett. 2015, 233, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Scott, P.M.; Lau, B.P.-Y.; Lewis, D.A. Analysis of heat-processed corn foods for fumonisins and bound fumonisins. Food Addit. Contam. 2004, 21, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Scott, P.M.; Lau, B.P.Y. Hidden fumonisin in corn flakes. Food Addit.Contam. 2003, 20, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Dossena, A.; Marchelli, R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010, 58, 12042–12047. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T. The fumonisin paradox: A review of research on oral bioavailability of fumonisin B1, a mycotoxin produced by Fusarium moniliforme. J. Toxicol. Toxin Rev. 2000, 19, 161–187. [Google Scholar] [CrossRef]
- Shephard, G.S.; Thiel, P.G.; Stockenstrom, S.; Sydenham, E.W. Worldwide survey of fumonisin concentration of corn and corn-based foods. J. AOAC Int. 1996, 79, 671–687. [Google Scholar] [PubMed]
- Femine Early Warning Systems Network (FEWS NET). Visualizing Trends in 1981–2015 Rainfall in Nigeria. Nigeria Special Report. Available online: www.fews.net/sites/default/files/documents/reports/FEWS NET_Nigeria Rainfall Trends Map Book_20160601 (accessed on 28 September 2016).
- Milani, J.M. Ecological conditions affecting mycotoxin production in cereals: A review. Vet. Med. (Praha) 2013, 58, 405–411. [Google Scholar]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxin profiles in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Kim, D.-H.; Yoon, B.R.; Jeon, M.-H.; Kim, B.-C.; Cho, B.-I.; Hong, S.-Y.; Hyun, C.S. Simultaneous Determination of Multi-mycotoxins in Cereal Grains by LC-MS/MS. In International Association for Food Protection; IAFP: Portland, OR, USA, 2015; pp. P2–P65. [Google Scholar]
- Okeke, C.A.; Ezekiel, C.N.; Nwangburuka, C.C.; Sulyok, M.; Ezeamagu, C.O.; Adeleke, R.A.; Dike, S.K.; Krska, R. Bacterial diversity and mycotoxin reduction during maize fermentation (steeping) for Ogi production. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Udoh, J.M.; Cardwell, K.F.; Ikotun, T. Storage structures and aflatoxin content of maize in five agroecological zones of Nigeria. J. Stored Prod. Res. 2000, 36, 187–201. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Donner, M.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- Zill, G.; Ziegler, W.; Engelhardt, G.; Wallnöfer, P.R. Chemically and biologically synthesized zearalenone-4-β-d-glucopyranoside: Comparison and convenient determination by gradient HPLC. Chemosphere 1990, 21, 435–442. [Google Scholar] [CrossRef]
- Sofie, M.; Van Poucke, C.; Detavernier, C.; Dumoultn, F.; Van Velde, M.D.E.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar]
- Monbaliu, S.; Van Poucke, C.; Van Peteghem, C.; Van Poucke, K.; Heungens, K.; De Saeger, S. Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Commun. Mass Spectrom. 2009, 23, 3–11. [Google Scholar] [CrossRef] [PubMed]
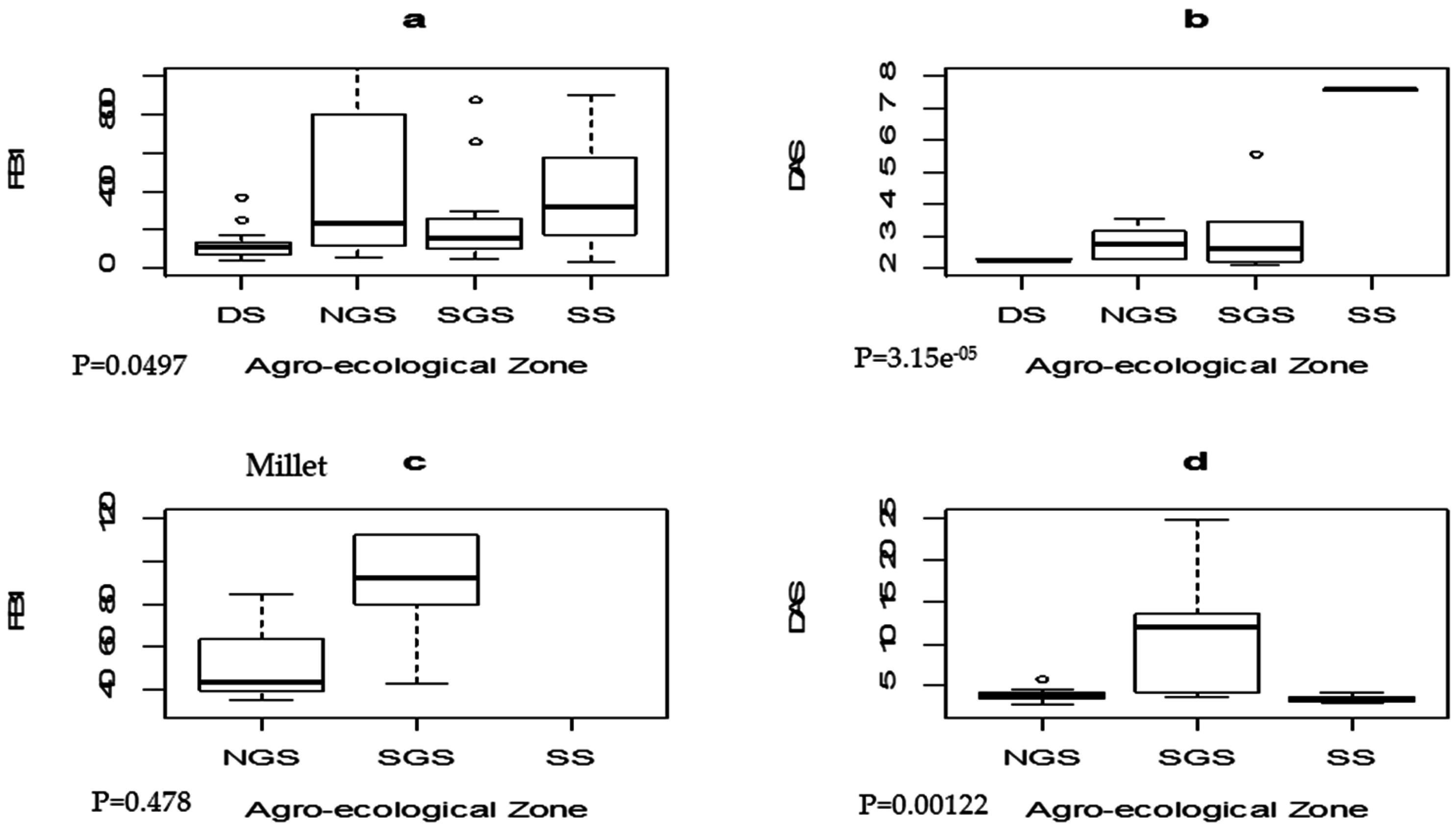

| Mycotoxin 1 | Maize (n = 136) | Sorghum (n = 110) | Millet (n = 87) | Ogi (n = 30) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % + ve Samples 2 | Mean 3 | Max 4 | % + ve Samples | Mean | Max | % + ve Samples | Mean | Max | % + ve Samples | Mean | Max | |
| FB1 | 65 | 541 | 8222 | 8 | 64 | 78 | 9 | 2333 | 18,172 | 93 | 590 | 1903 |
| FB2 | 54 | 376 | 2885 | 2 | 48 | 55 | 13 | 609 | 3892 | 87 | 472 | 1283 |
| FB3 | 43 | 117 | 445 | 2 | 38 | 46 | 0 | na | na | 77 | 121 | 371 |
| ∑FB | 65 | 935 | 8508 | 8 | 83 | 180 | 14 | 2113 | 22,064 | 93 | 1128 | 3557 |
| DON | 16 | 99 | 225 | 3 | 100 | 119 | 13 | 151 | 543 | 13 | 61 | 74 |
| 15 ADON | 0 | na 5 | na | 2 | 39 | 44 | 1 | 11 | 11 | 3 | 60 | 60 |
| DON-3G | 0 | na | na | 23 | 24 | 63 | 0 | na | na | 17 | 30 | 44 |
| ZEN | 1 | 65 | 65 | 1 | 38 | 38 | 14 | 419 | 1399 | 3 | 39 | 39 |
| ZEN-14G | 9 | 21 | 24 | 3 | 19 | 22 | 6 | 23 | 34 | 3 | 31 | 31 |
| α-ZEL | 1 | 20 | 20 | 3 | 33 | 33 | 0 | na | na | 7 | 20 | 22 |
| β-ZEL | 2 | 20 | 21 | 1 | 21 | 21 | 1 | 39 | 39 | 10 | 19 | 20 |
| HT-2 | 1 | 20 | 20 | 8 | 20 | 31 | 5 | 36 | 36 | 3 | 13 | 13 |
| NIV | 2 | 206 | 271 | 0 | na | na | 0 | na | na | 7 | 148 | 160 |
| FUS-X | 1 | 154 | 154 | 0 | na | na | 0 | na | na | 7 | 133 | 137 |
| DAS | 13 | 3 | 8 | 18 | 5 | 16 | 29 | 5 | 25 | 0 | na | na |
| Food Type | FB (μg/kg) | Total FB (μg/kg) | Hidden FB (μg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | Maximum | Median | Mean | Maximum | Median | Mean | Maximum | |
| Maize (n = 10) | 358 | 835 | 3514 | 543 | 1636 | 4568 | 144 | 801 | 2923 |
| Sorghum (n = 10) | 41 | 61 | 180 | 95 | 182 | 502 | 50 | 120 | 323 |
| Millet (n = 10) | 118 | 277 | 840 | 302 | 776 | 3059 | 179 | 499 | 2254 |
| Ogi (n = 10) | 247 | 531 | 1496 | 391 | 672 | 1795 | 117 | 141 | 313 |
| Mycotoxin 1 | Maize (μg/kg) | Sorghum (μg/kg) | Millet (μg/kg) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS (n = 30) | SGS (n = 36) | NGS (n = 40) | SS (n = 30) | SGS (n = 30) | NGS (n = 40) | SS (n = 40) | SGS (n = 30) | NGS (n = 30) | SS (n = 27) | |||||||||||
| Mean 2 (% + ve) 3 | Max 4 | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | Mean (% + ve) | Max | |
| FB1 | 117 (67) | 366 | 249 (33) | 876 | 928 (83) | 8222 | 505 (77) | 2443 | 70 (7) | 71 | 59 (10) | 76 | 67 (8) | 78 | 3700 (17) | 18,172 | 54 (10) | 84 | na | na |
| FB2 | 289 (57) | 1011 | 350 (28) | 677 | 508 (65) | 2885 | 295 (70) | 1107 | na | na | 41 (3) | 41 | 55 (3) | 55 | 417 (37) | 3892 | 56 (13) | 103 | 44 (7) | 47 |
| FB3 | 114 (47) | 353 | 147 (25) | 445 | 126 (53) | 441 | 91 (50) | 213 | na | na | 31 (3) | 31 | 46 (3) | 46 | na | na | na | na | na | na |
| DON | 78 (27) | 147 | 99 (17) | 180 | 98 (10) | 151 | 140 (13) | 225 | 119 (3) | 119 | 91 (5) | 92 | na | na | 140 (7) | 200 | 171 (20) | 543 | 118 (11) | 118 |
| 15 ADON | na 5 | na | na | na | na | na | na | na | 34 (3) | 34 | 44 (3) | 44 | na | na | na | na | na | na | 11 (4) | 11 |
| DON-3G | na | na | na | na | na | na | na | na | 12 (27) | 16 | 30 (40) | 63 | 22 (3) | 22 | na | na | na | na | na | na |
| ZEN | na | na | na | na | 65 (3) | 65 | na | na | 38 (3) | 38 | na | na | na | na | 481 (33) | 1399 | 109 (7) | 198 | na | na |
| ZEN-14G | 20 (23) | 24 | 21 (6) | 22 | 23 (8) | 23 | na | na | 20 (3) | 20 | 19 (5) | 22 | na | na | 29 (7) | 34 | 19(7) | 20 | 23 (4) | 23 |
| α-ZEL | na | na | 20 (3) | 20 | na | na | na | na | na | na | 33 (8) | 33 | na | na | na | na | na | na | na | na |
| β-ZEL | 20 (3) | 20 | na | na | 21 (3) | 21 | na | na | 21 (3) | 21 | na | na | na | na | na | na | 39 | 39 | na | na |
| HT-2 | 20 (3) | 20 | na | na | na | na | na | na | 19 (17) | 19 | 24 (8) | 31 | 11 (3) | 11 | 35 (7) | 35 | 36 (7) | 36 | na | na |
| NIV | 228 (7) | 271 | na | na | na | na | 163 (3) | 163 | na | na | na | na | na | na | na | na | na | na | na | na |
| FUS-X | 154 (3) | 154 | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na | na |
| DAS | 2 (10) | 2 | 3 (17) | 6 | 3 (18) | 4 | 8 (7) | 8 | 5 (27) | 13 | 4 (13) | 5 | 5 (18) | 16 | 12 (17) | 25 | 4 (37) | 6 | 3 (33) | 4 |
| Product Type | AEZ | State | No. of Markets | No. of Samples |
|---|---|---|---|---|
| Maize | SS | Kano | 6 | 30 |
| NGS | Kaduna | 6 | 36 | |
| SGS | Niger | 8 | 40 | |
| DS | Nasarawa | 6 | 30 | |
| Total number = 136 | ||||
| Sorghum | SS | Kano | 6 | 30 |
| NGS | Kaduna | 2 | 40 | |
| SGS | Niger | 8 | 40 | |
| Total number = 110 | ||||
| Millet | SS | Sokoto | 6 | 30 |
| NGS | Kaduna | 6 | 30 | |
| SGS | Niger | 8 | 27 | |
| Total number = 87 | ||||
| Ogi | DS | Ekiti | 5 | 30 |
| Total number = 30 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Occurrence of Fusarium Mycotoxins in Cereal Crops and Processed Products (Ogi) from Nigeria. Toxins 2016, 8, 342. https://doi.org/10.3390/toxins8110342
Chilaka CA, De Boevre M, Atanda OO, De Saeger S. Occurrence of Fusarium Mycotoxins in Cereal Crops and Processed Products (Ogi) from Nigeria. Toxins. 2016; 8(11):342. https://doi.org/10.3390/toxins8110342
Chicago/Turabian StyleChilaka, Cynthia Adaku, Marthe De Boevre, Olusegun Oladimeji Atanda, and Sarah De Saeger. 2016. "Occurrence of Fusarium Mycotoxins in Cereal Crops and Processed Products (Ogi) from Nigeria" Toxins 8, no. 11: 342. https://doi.org/10.3390/toxins8110342






