Comments on Boelaert et al. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC–MS/MS versus ELISA. Toxins 2016, 8, 149
1. Method comparison of UPLC–MS/MS with ELISA
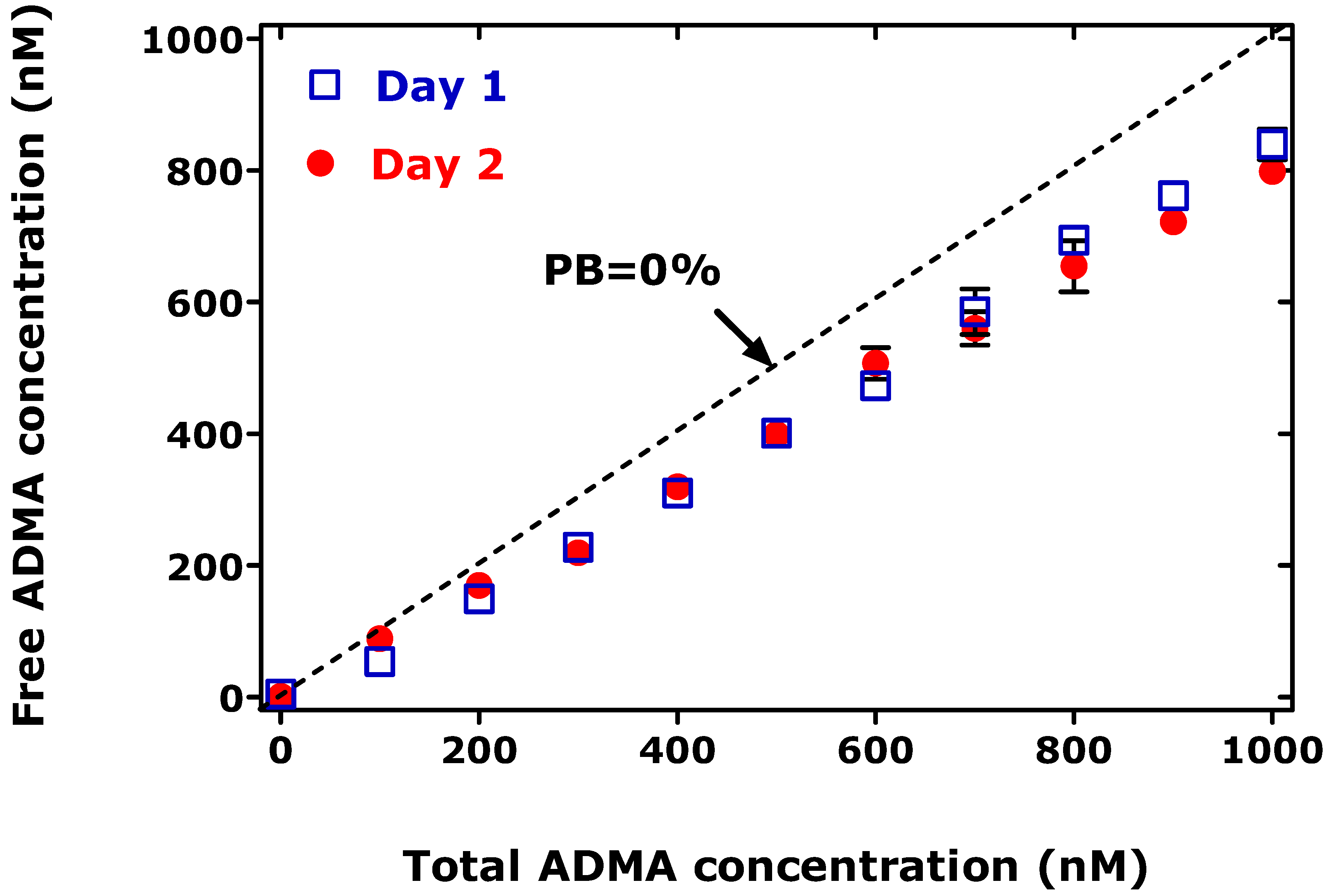
2. Protein binding of ADMA and SDMA
Conflicts of Interest
References
- Boelaert, J.; Schepers, E.; Glorieux, S.; Vanholder, R.; Lynen, F. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC–MS/MS versus ELISA. Toxins 2016, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D.; Sandmann, J.; Savva, A.; Luessen, P.; Böger, R.H.; Gutzki, F.M.; Mayer, B.; Frölich, J.C. Assessment of nitric oxide synthase activity in vitro and in vivo by gas chromatography-mass spectrometry. J. Chromatogr. B 2000, 742, 143–153. [Google Scholar] [CrossRef]
- Araujo, P. Key aspects of analytical method validation and linearity evaluation. J. Chromatogr. B 2009, 877, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Westphal, S.; Awiszus, F.; Bode-Böger, S.M.; Luley, C. Determination of asymmetric dimethylarginine: Liquid chromatography-mass spectrometry or ELISA? Clin. Chem. 2005, 51, 2188–2189. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Tan-Andresen, J.; Maas, R.; Riederer, U.; Schulze, F.; Bo, R.H. Liquid chromatography-tandem mass spectrometry method for the analysis of asymmetric dimethylarginine in human plasma. Clin. Chem. 2005, 51, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Sitar, M.E.; Kayacelebi, A.A.; Beckmann, B.; Kielstein, J.T.; Tsikas, D. Asymmetric dimethylarginine (ADMA) in human blood: Effects of extended haemodialysis in the critically ill patient with acute kidney injury, protein binding to human serum albumin and proteolysis by thermolysin. Amino Acids 2015, 47, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Trunk, H.; Weitz, B. Evaluation of methods to determine protein-binding of drugs: Equilibrium dialysis, ultrafiltration, ultracentrifugation, gel filtration. Arzneimittelforschung 1977, 27, 1373–1380. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikas, D.; Beckmann, B.; Araujo, P. Comments on Boelaert et al. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC–MS/MS versus ELISA. Toxins 2016, 8, 149. Toxins 2016, 8, 311. https://doi.org/10.3390/toxins8110311
Tsikas D, Beckmann B, Araujo P. Comments on Boelaert et al. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC–MS/MS versus ELISA. Toxins 2016, 8, 149. Toxins. 2016; 8(11):311. https://doi.org/10.3390/toxins8110311
Chicago/Turabian StyleTsikas, Dimitrios, Bibiana Beckmann, and Pedro Araujo. 2016. "Comments on Boelaert et al. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC–MS/MS versus ELISA. Toxins 2016, 8, 149" Toxins 8, no. 11: 311. https://doi.org/10.3390/toxins8110311




